ABSTRACT
Background: Uterine malformation is a rare deformity in woman, and only a few cases concerning endometrial cancer arising in patients with congenital uterine anomalies have been reported. Herein, we present 3 cases of endometrial cancer with different congenital uterine anomalies, and review studies involving congenital uterine anomalies associated with endometrial cancer in the past 25 years, to identify similarities and differences in clinicopathologic characteristics and prognosis between endometrial cancer associated with uterine anomalies, and normal uterus. Cases: Case 1 was a 75-year-old gravida 1, para 0, woman with carcinosarcoma (mixed well-differentiated endometrial adenocarcinoma and undifferentiated sarcoma) of the right cavity (grade III, and at least stage II ) of a uterus didelphys. The tumor recurred within 7 months after surgery, salvage radiotherapy was unsuccessful; the patient died 8 months after the surgery. Case 2 was a 63-year-old gravida 5, para 3, woman with a bicornuate uterus and uterus papillary serous carcinoma of the right horn (grade III, stage IIIC). She did not respond to the chemotherapy post surgery and died within 4 months. Case 3 was a 60-year-old gravida 0, para 0, woman with a complete septate uterus and an oblique vaginal septum of the upper region of the vagina with endometrioid adenocarchcinoma of the left cavity (grade II, stage IA). No adjuvant therapy was administered and the patient had recovered 2 y after the surgery. Conclusion: Clinicians should be aware of the coexistence of uterine malignancies and uterine anomalies in patients presenting with persistent abnormal uterine bleeding, but with negative endometrial biopsy or failed in the operation of endometrial biopsy. In such cases, magnetic resonance imaging has an important role in the diagnosis of both malformation and malignancy, and an exploratory laparotomy should be performed to avoid delaying the diagnosis and treatment of cancers.
Introduction
Congenital malformations of the female genital tract are defined as deviations from the normal anatomy resulting from embryological maldevelopment of the Müllerian or paramesonephric ducts. Congenital uterine malformations are common deviations from normal anatomy with an estimated prevalence of 4–7% in the general population and an even greater prevalence in high-risk populations including those with a history of miscarriage, infertility, and recurrent miscarriage.Citation1-3 The incidence of Müllerian anomalies is underestimated because many are clinical silence. The clinical presentations of Müllerian duct anomalies are diverse. The obstructed reproductive tract anomaly usually presents in 2 distinct ways: in teenagers who present with primary amenorrhea and pain, and those with menstrual periods and progressive dysmenorrhea.Citation4. Women with non-obstructive reproductive tract anomalies are often asymptomatic and have often been referred to a gynecologist for infertility, miscarriage, or recurrent miscarriage.
Endometrial cancer is the most common female genital tract neoplasm in developed countries, in the US, it is estimated that 60,050 women will be diagnosed with uterine corpus cancer, and 10,470 women will die from the disease in 2016.Citation5 Around 75% of patients with endometrial cancer are diagnosed in the early stages (FIGO stages I or II), and 5-year overall survival is 74–91%.Citation5,6
Endometrial cancer associated with uterine anomalies are extremely rare, with only 18 reports, including 22 cases reported for the past 25years. Of the 18 reports, 15 presented only a single case, 2 presented 2 cases,Citation7,8 and the other one presented 3 cases.Citation9 Herein, we present 3 cases of endometrial cancers with different types of congenital uterine anomalies, and review the literature concerning congenital uterine anomalies associated with endometrial cancer since 1990, including a list of all 25 cases in ,Citation7-24 to discuss the similarities and differences in clinicopathologic characteristics between endometrial cancer associated with uterine anomalies, compared with normal uterus.
Table 1. List of reported Endometrial Cancer with Congenital Uterine Anomalies since 1990.
Cases report
Case 1 A 75-year-old, gravida 1, para 0 woman with a body mass index (BMI) 25.7kg/m2, was referred to our department because of postmenopausal vaginal bleeding of 4 y and pelvic pain for half a year, having been menopausal since the age of 49. She was aware of her double uterus because of the history of miscarriage 50 y ago. The patient had a 30-year history of hypertension. The vaginal examination showed double vagina, both of the cervixes could not be exposed which led to the failure of hysteroscopy and endometrial biopsy. Transvaginal ultrasonography indicated a uterus didelphys associated with a large mass of the right isthmus uteri. Contrast-enhanced Magnatic Resonance Imaging (MRI) revealed a uterus didelphys and double cervix with a malignant tumor of the right uterine cavity (). The diagnosis of uterus didelphys with a malignant tumor was considered, and an exploratory laparotomy was performed. Upon surgical inspection, the right uterus was enlarged and the tumor was 10cm; also, the left atrophic uterus was pushed to the left pelvic wall. Both fallopian tubes and ovaries were normal. A total abdominal hysterectomy and bilateral salpingo-oophorectomy was performed, an intra-surgical frozen section evaluation confirmed the diagnosis of carcinosarcoma. Pelvic and para-aortic lymph nodes dissection was not performed owing to old age and hypertension. Final histological diagnosis confirmed carcinosarcoma (well-differentiated endometrial adenocarcinoma and undifferentiated sarcoma) of the right cavity with invasion to the deep myometrium (> 2/3) and cervical canal. The left uterus and both ovaries and fallopian tubes were not invaded. Immunohistochemical staining indicated that the sarcoma component was positive for P53 and the Ki-67 index was approximately 70%. The tumor was classified as stage II according to FIGO (International Federation of Gynecology and Obstetrics) 2009. The patient refused a adjuvant treatment following surgery and did not receive regular examination post-surgery. The patient experienced tumor recurrence in the pelvic after 7 months and received 23 rounds of whole pelvic radiotherapy and the whole dose was 46 Gy; however, it was not effective and the patient died 8 months after surgery.
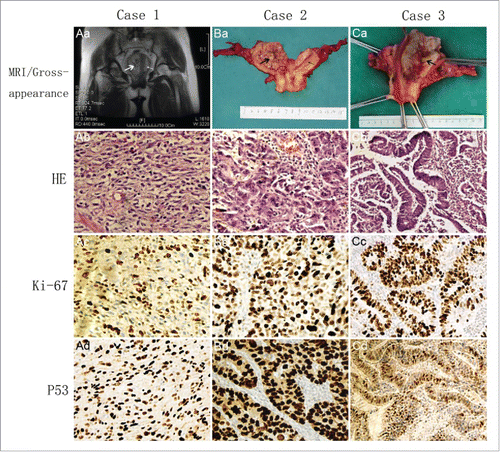
Figure 1. (Aa) MRI of Case 1 showing the right cavity with tumor and normal left cavity. (Ba) Bicornuate uterus of Case 2 with grossly visible tumor in the right cavity and normal endometrium of the left cavity. (Ca) Complete septate uterus of Case 3 with grossly visible tumor in the left cavity and normal endometrium of the right cavity. (Ab) Undifferentiated endometrial sarcoma (HE × 100). (Bb) Poorly differentiated uterus serous papillary carcinoma (HE × 100). (Cb) Moderately differentiated endometrioid adenocarcinoma (HE × 100). The index of ki-67 of (Ac,Bc,Cc) were 70%, 90%, 80% (Ki67 × 100). (Ad,Bd,Cd) P53 overexpressed in all these 3 cases (P53 × 100).
Case 2 A 63-year-old gravida 5, para 3, woman BMI of 22.9kg/m2 consulted to the gynecologist following a month of postmenopausal vaginal bleeding, having been menopausal since the age of 48 y. The patient was unaware of her uterine anomaly. She had a history of hypertension for 7 y. Vaginal examination indicated a normal vagina and cervix. Ultrasonography suggested double uterus, with the right uterus enlarged by the mass and cavity effusion, in addition to the presence of ascites. Contrast-enhanced MRI revealed bicornuate uterus and a strong indication of endometrial carcinoma of the right cavity. Positron emission tomography-computed tomography (PET-CT) demonstrated a metabolically active tumor with intense metabolic activity in the right uterine cavity, and was considered as malignant lesions associated with peritoneal multiple metastases. The patient underwent exploratory hysteroscopy that revealed that the left cavity was normal and the right cavity was inaccessible. Endometrial curettage and biopsy of the left cavity showed negative results. As the bleeding from the uterine cavity continued and the tumor of the right cavity was highly suspected to be cancer, we recommended that the patient undergo exploratory laparotomy. During the surgery, a moderate amount of ascites was observed and bicornuate uterus was ascertained. Furthermore, the right uterine corpus was larger than the left one, and both the bilateral fallopian tubes and ovaries were normal. Multiple metastatic lesions of omentum and peritoneum and the surface of the intestinal canal was detected. The frozen sections of the lesions of the omentum revealed poorly differentiated metastatic carcinoma. Total hysterectomy and bilateral salpingo-oophorectomy and partial omentectomy were performed. Gross examination showed a bicornuate uterus with a single cervix, the right cavity of which was filled with a mass, while the left was normal (). Pathology confirmed poorly differentiated uterus serous papillary carcinoma (UPSC) in the right cavity, which invasion to the right fallopian tube and ovary. Additionally, the peritoneum, sigmoid colon, and omentum were positive for metastases. Cytology of the ascites was also positive. Immunohistochemical analysis revealed positive staining for P53, and the Ki-67 index was approximately 90%. The final diagnosis was a FIGO stage IIIC, grade III tumor. A combined chemotherapy of paclitaxel and cisplatinum was administered post-surgery; however, the therapy was not effective and the side effects were intolerable. The ascites recurred shortly, and the patient died within 4 months.
Case 3 A 60-year-old gravida 0, para 0 woman with a BMI 28.6kg/m2 presented with postmenopausal bleeding for one and a half years, having been menopausal since the age of 52 y. The patient was unaware of her uterine anomaly. She had a 3-year history of hypertension. Pervaginal examination revealed the oblique vaginal septum of the upper region of the vagina, and the right cervix was atrophic and the left one was covered by the septum. Autocyte prep cytologic test results of the right cervix was negative. Ultrasonography revealed an enlarged uterus and 2 masses were seen in the cavity. Contrast-enhanced MRI revealed the complete septate uterus, associated with a soft tissue mass of the left cavity, which was highly indicative of endometrial carcinoma. Hysteroscopy was attempted and failed because of the oblique vaginal septum. The diagnosis of complete septate uterus with endometrial cancer of the left cavity was highly suspected, and an exploratory laparotomy was performed. Total abdominal hysterectomy with bilateral salpingo-oophorectomy was performed initially; gross examination indicated that 2 horns of the uterus were not symmetric, and the left one was enlarged, while both of the fallopian tubes and ovaries were normal. A complete septum from fundus to cervix was observed, and the left cavity was filled with irregular polypoidal mass, while the right cavity was relatively normal (). The frozen sections of the uterus revealed endometrial adenocarcinoma of the left cavity, and subsequently, bilateral pelvic and para-aortic lymph node dissection were performed. The final pathologic diagnosis confirmed endometrioid adenocarcinoma in the left cavity, grade II, invading less than half of the myometrium. The right cavity was normal. Immunohistochemical staining showed P53 was positive and Ki-67 index was approximately 80%. FIGO stage IA, grade II-III tumor was diagnosed. No adjuvant therapy was administered and the patient has been healthy without evidence of disease 2 y after surgery.
Discussion
Epidemiologic and clinical characteristics of endometrial cancer associated with uterine anomalies
Different kinds of classification for female genital anomalies has been published, including the AFS(American Fertility Society) classification systemCitation25 in 1988, the Clinical and Embryological ClassificationCitation26 in 2004, the VCUAM (Vagina, Cervix, Uterus, Adnexae and associated Malformations) systemCitation27 in 2005, the Acién systemCitation28 in 2011 and the recent ESHRE/ESGE (European Society of Human Reproduction and Embryology and European Society for Gynecological Endoscopy) classificationCitation29 in 2013. The AFS system described by the American Society of Reproductive Medicine remains the most widely used and involves the division of uterine anomalies into hypoplasis/agenesis uterus, unicornuate uterus, bicornuate uterus, uterus didelphus, septate uterus, arcuate uterus and drug-related uterus (DES)(25). Uterine anomalies is the most common conditions of congenital malformations of the female genital tract. The prevalence of these anomalies may be higher than reported due to the asymptomatic nature. Of the 25 patients ,the ratio of uterus didelphy was as high as 52% (13/25), bicornuate uterus was 36% (9/25), and septate uterus was 8% (2/25). Patient No.1 had a unique type of double uterus involving a normal sized uterus with a rudimentary uterus attached.
Urinary tract anomalies are the most common abnormality associated with congenital anomalies of the female reproductive tract, because the development of a normal Müllerian duct occurs in association with the normal development of the mesonephric duct. The incidence of associated genital abnormalities in women with renal anomalies is estimated to be between 25% and 89%.Citation30 Unilateral renal agenesis was the most common abnormality (31.8%), and most frequent associated with uterus didelphys and lower frequency with other anomaly types.Citation31 For the 25 patients in , all of our 3 patients and patient No.21 had normal kidneys, and patient No.1 had double uterus with bifid left ureter and double right ureter, patients No.5 and No.18 had uterus dideiphys with absence of the right kidney. Conditions regarding the other patients' urinary system were not available.
Women with Mullerian duct anomalies appear to have an increased rate of unexplained infertility, endometriosis, spontaneous abortion, breech presentation, and premature delivery.Citation30 The incidence of uterine anomalies was found to be significantly higher in women with a history of miscarriage and miscarriage in association with infertility than in the unselected population.Citation32 Of all these 25 cases, 9 of 21 were infertility, excluding 4 patients whose fertility situation were not disclosed. Five of these patients had primary infertility and 2 patients had a history of miscarriage; the gravidity of the remaining 2 patients were undisclosed. The patients with fertility problems may have been made aware of their uterus anomalies earlier when they were referred to their gynecologist because of infertility or miscarriage. However, most patients had normal deliveries and were unaware of their anomalies.
Endometrial carcinoma is the most frequently observed gynecologic malignancy in developed countries. More than 90% of endometrial cancer cases occur in women older than 50 y of age, with a median age of 63 y. The majority of cases of endometrial cancer are diagnosed in early stages, as abnormal uterine bleeding is the presenting symptom in 90% of cases.Citation33 The 25 patients presented ranged in age from 33 to 80 y old, 68% (17/25) were postmenopausal women over 50 years-of-age, and 92% (22/24) presented abnormal uterine bleeding as the initial symptom. The bleeding duration ranged from 1 week to 4 years, and several patient did not consider the condition severe and thus did not seek treatment, delaying the diagnosis and subsequent treatment. Only 2 patients (Nos.18 and 23) reported pelvic pain, and both had enlarged pelvic masses that were finally diagnosed as carcinosarcoma.
Multiple risk factors of endometrial carcinoma have been identified, including early onset of menstruation, late menopause, obesity, nulliparity , diabetes mellitus, hypertension, infertility, unopposed estrogen exposure, and tamoxifen. With regard to the 25 patients, 7 had a history of hypertension, 8 had a history of obesity, 2 had a history of diabetes mellitus, 2 had a history of breast cancer and were treated with tamoxifen for 5 y ,1 had polycystic ovarian syndrome (PCOS), 1 had a family history of endometrial cancer, and 1 had a history of adenocarcinoma of the cecum. There were also 8 patients' whose medical histories have not been mentioned.
With regard to the epidemiology, clinical characteristics and risk factors between endometrial cancer with or without uterine anomalies are quite similar and no obvious differentiations.
Diagnosis of endometrial cancer associated with uterine anomalies
The diagnostic evaluation for endometrial cancer includes pelvic ultrasonography, CT (CT) or MRI, hysteroscopy, and dilatation and curettage (D&C) or endometrial biopsy. Biopsy under hysteroscopic guidance remains the gold standard in the diagnostic evaluation for endometrial cancer. Compared to blind D&C, D&C with hysteroscopy guidance has a greater level of accuracy and superior diagnostic yield.Citation34,35
Contrast-enhanced MRI serves an important role in the diagnosis of congenital malformations of the female genital tract, and there is excellent agreement between MRI diagnosis and clinical diagnosis.Citation36 In addition, it is useful in revealing malignant tumors of the uterus and valuable imaging modality for pre-surgical locoregional staging of endometrial cancer and the negative predictive value were high.Citation37
All of our 3 cases underwent ultrasound and MRI examination, and MRI revealed all the uterine anomalies correctly and also revealed the highly suspect of uterine tumor as endometrial cancer, whereas the complete septate uterus of case 3 didn't detect by ultrasonography. Three patients (Nos.19,20, and 22) underwent CT, which revealed all the anomalies. Nine patients (Nos. 12–15,20,21, and 23–25) underwent MRI, and the results of 8 patients revealed the uterus malformation correctly except patient No.12 with septate uterus. Eleven patients (Nos. 11,13–18,21, and 23–25) underwent ultrasound, and only the results of 3 patients (Nos.11, 21, and 23) unequivocally revealed didelphys uterus, No.24 revealed bicornuate uterus, 3 patients (Nos.14–16 ) only revealed double uterus and could not recognize bicornuate or didelphys uterus, while the remaining 4 patients (Nos.13,17,18, and 25) failed detect the uterine anomalies.
Previously, the principal method of investigation for endometrial cancer has been diagnostic D&C. There is a trend toward minimally invasive exams using endometrial biopsy, vaginal ultrasound scan and hysteroscopy.Citation33 It was reported that less than half of the uterine cavity was curetted in 60 % of cases in which D&C was performed as the method for endometrial sampling, the sample size-weighted sensitivities of D&C were 78%; the pipelle was the superior device in both postmenopausal and premenopausal women, with detection rates of 99.6% and 91%, respectively.Citation38 According to a systematic quantitative review, the overall accuracy of outpatient endometrial biopsy in diagnosing endometrial cancer was as high as 81.7% for a positive test when an adequate specimen is obtained.Citation39 For the accuracy of hysteroscopy in diagnosing endometrial cancer, the estimated sensitivity was 82.6% and the specificity was 99.7%.Citation40
The pathological diagnosis rate of the 25 cases before surgery was only 68% (17/25). The remaining 8 patients (Nos.6–8,11,12 and 23–25) did not receive pathological diagnosis of cancer pre-operatively and can be divided into 2 groups: those with failed D&C or hysteroscopy (4 patients; Nos.8,11,23, and 25) ,and those with the negative results of endometrial biopsy (4 patients; Nos.6,7,12, and 24) because all of these tumors involved only one cavity or horn. Only 6 of the 25 patients (Nos.1,12–14,17, and 19) described endometrial carcinomas of double uteri involved both of the cavities or horns, implying that there is a 50% chance of obtaining endometrial sampling of the negative cavity at the time of initial examination, and resulting false-negative diagnosis.
For endometrial cancer associated with uterus anomalies, the anomalies rendered the performance of D&C more difficult, and hysteroscopy also failed or missed the involved cavity, thus, the pathology diagnosis rate of endometrial biopsy or D&C are relatively lower than normal uterus, and delayed the diagnosis and treatment. Consequently, the use of imaging studies to assist in diagnosis, especially MRI, served a more important role in the diagnosis of uterus anomalies with malignancies requiring further diagnostic evaluation and treatment.
Pathologic characteristics of endometrial cancer associated with uterine anomalies
Twelve (Nos.2,5,9,10,12,15–17,21,22,24, and 25) of the 25 patients underwent complete staging surgery, and only 2 patient was stage Ⅲ disease and the remaining 10 had stage I disease. The remaining 13 patients who only underwent total hysterectomy and bilateral or unilateral salpingo-oophorectomy had more advanced stages of disease: 1 patients were stage disease, 3 had stage III disease, 3 had stage II disease, and 6 had stage I disease. We restaged the 25 cases reported over 25 y according to FIGO 2009. Overall, 64% had stage I disease (16/25), 12% had stage II (3 /25), 20% had stage III (5 /25) and 4% had stage IV (1/25).
For the patients with endometrial cancer with uterine anomalies, the failure of diagnostic D&C with or without hysteroscopy and the negative results of endometrial biopsy delays the diagnose, as high as 32% of the 25 cases did not have pre-surgical confirmation of their endometrial cancer, and the tumor was usually larger at the time of diagnosis. For our 3 cases, all the tumors occupied the entire cancerous endometrial cavity. The size of the tumor was available in 17 of the 25 cases, and 14 of these 17 patients' tumors occupied more than half of the cavity or horn. The uterus malformation that was enlarged by the tumor and the anatomic problems of the pelvic associated with the Müllerian anomalies made the surgery more difficult compared with that for endometrial cancer in a normal uterus.
Endometrial carcinomas have been recognized as 2 main clinicopathological types corresponding to estrogen-dependent endometrioid (type 1) and estrogen- independent non-endometrioid carcinomas (type 2). Endometrioid adenocarcinomas represent 80–85% of endometrial carcinomas. Serous carcinomas are considered the prototype of Type 2. Of all 25 cases, 20 patients had endometrioid adenocarcinomas, 2 patient (Nos.11 and 24) had UPSC and 3 patients (Nos. 18,20,and 23) had carcinosarcoma. The ratio of special type in these cases accounted for 20% (5/25).
UPSC is more aggressive even when it is only a component of a mixed-type carcinoma. It often presents with advanced disease and follows a pattern of spread that resembles the serous carcinoma of the ovary. The patient described in the current study's Case 2 presented with stage IIIC and did not respond to the chemotherapy post-surgery, and died within 4 months. Patient No.9 had mixed endometrioid (80% component) and serous papillary (20% component) carcinoma of the endometrium with stage IA Grade II disease. The patient's post-surgical treatment included paclitaxel and carboplatin chemotherapy for 6 cycles followed by vaginal cuff brachytherapy, and the survival time was not mentioned.Citation13 Carcinosarcomas are also called mixed Müllerian tumors, which are one of the most aggressive uterine malignancies, and their typical characteristic include a typical biphasic pattern with carcinomatous and sarcomatous elements. To the best of our knowledge, our Case 1 is the third described in the literature concerning carcinosarcoma in a patient with didelphys uterus. The patient's tumor recurred in 7 months in the pelvic, and salvage radiotherapy were not effective and she died in 8 months after the surgery. The first case (No.18) described in the literature was 72-year-old patient with a history of breast cancer who had been and administered with tamoxifen for 5 y. The histological diagnosis was carcinosarcoma that arose in the left horn, and the tumor recurred in 7 weeks, and the salvage radiotherapy was unsuccessful and the patient died within 5 months.Citation20 The second case (No.20) involved a 73-year-old patient with didelphys uterus, Para 5, and final histology confirmed the diagnosis of high-grade carcinosarcoma stage IIIb. She received palliative radiotherapy; however, the survival time was not mentioned.Citation22 All 3 cases presented women over 70 y of age with considerably large pelvic tumors, which almost occupied the entire pelvic region.
The expression of Ki-67 is strongly associated with tumor cell proliferation and growth, and the Ki-67 labeling index is an independent prognostic factor for survival(41). The ki-67 labeling index of the present 3 cases was 70%, 90% and 80% respectively (). P53 overexpressed is observed in most Type 2 endometrial carcinomas and is also observed in several Type 1 cases. P53 overexpression is associated with adverse clinicalpathologic variables and unfavorable clinical outcome, and it represents an independent prognostic factor for poor survival.(42) P53 was overexpressed in all these 3 cases ( ).
Adjuvant therapy and follow up of endometrial cancer associated with uterine anomalies
Adjuvant therapy strategies usually depend on the stage, grade, and type of tumor and the physical condition of the patients. Five patients (Nos.6, 9, 10,19, and 22) received combined chemo-radiotherapy. Six patients (Nos.2,17,18,20,21,and 23) only received radiotherapy include 2 patients (Nos.18 and 23) had carcinosarcoma and received salvage radiotherapy; however, both failed. Patient No.17 received intracavitatory radiation before surgery. Two patient (Nos.12 and 24) only received chemotherapy and No.24 received chemotherapy due to USPC, stage IIIC, grade III, but did not respond positively. Patient No.1 with endometrial adenocarcinoma of both cavities and metastasized to supraclavicular lympth nodes, and was treated with tamoxifen plus progestin. The patient died of lung metastasis after 14 months.Citation10
All these cases ranged over 25 y and quite unique. For the majority of cases, the reported time was just after operation, their condition and survival rates were unavailable. Most of the patients' prognosis was not mentioned in reports, as these cases were rare and hard to assess their prognosis in comparison to that of normally uterine cancers. The follow-up conditions were mentioned only in 11of the 25 patients, and the follow-up time ranged from 4 months to 7 y. Four of the 11 patients died of advanced stage or more aggressive type of tumors (1 was stage IV, 2 were carcinosarcoma, and 1 was UPSC), the period of survival time ranged from 4 to 14 months.
Conclusion
In conclusion, uterine anomalies are rare, and the diagnosis of uterine malformations may go undetected because of their asymptomatic nature; also, they may have no effect on fertility, resulting in diagnostic delay. Compared with endometrial cancer of a normal uterus, their epidemiological characteristics, risk factors, and histological features are similar. However, as many as 32% of the 25 cases did not involve confirmation of cancer by histological diagnosis as a result of failed of endometrial biopsy, D&C or hysteroscopy pre-operation, and the fault negative result from the normal cavity. The majority of the cancers (76%) only involved one of the cavities or horns, and the malformations rendered it difficult to enter the cancerous cavity, thus delaying the diagnosis; several patients presented with larger tumors and advanced cancer. Contrast-enhanced MRI of the pelvic before surgery as a further diagnostic evaluation served an important role in the diagnosis of both uterine anomalies and endometrial cancer. When the coexistence of uterine malformation and endometrial carcinoma is highly suspected, as revealed by these assistance examinations, exploratory laparotomy should be performed actively even if the histological examinations is negative or fails, to avoid a delay in treatment.
Disclosure potential conflicts of interest
No potential conflicts of interest were disclosed.
Informed consent
We have obtained the informed consent from all individual participants included in the cases report.
Funding
This study was supported by the Natural Science Fund of China under Grant No.81272863 and No.81572568; the Natural Science Fund of Tianjin Municipal Science and Technology Commission, China under Grant No.12JCYBJC17900; and the Fund of Tianjin Health Authority Science and Technology Commission, China under Grant No.2015kz116.
References
- Chan YY, Jayaprakasan K, Zamora J, Thornton JG, Raine-Fenning N, Coomarasamy A. The prevalence of congenital uterine anomalies in unselected and high-risk populations: a systematic review. Hum Reprod Update 2011; 17(6):761-71; PMID:21705770; http://dx.doi.org/10.1093/humupd/dmr028
- Saravelos SH, Cocksedge KA, Li TC. Prevalence and diagnosis of congenital uterine anomalies in women with reproductive failure: a critical appraisal. Hum Reprod Update 2008; 14(5):415-29; PMID:18539641; http://dx.doi.org/10.1093/humupd/dmn018
- Grimbizis GF, Camus M, Tarlatzis BC, Bontis JN, Devroey P. Clinical implications of uterine malformations and hysteroscopic treatment results. Hum Reprod Update 2001; 7(2):161-74; PMID:11284660; http://dx.doi.org/10.1093/humupd/7.2.161
- Dietrich JE, Millar DM, Quint EH. Obstructive reproductive tract anomalies. J Pediatr Adolesc Gynecol 2014; 27(6):396-402; PMID:25438708; http://dx.doi.org/10.1016/j.jpag.2014.09.001
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016; 66(1):7-30; PMID:26742998; http://dx.doi.org/10.3322/caac.21332
- Creasman WT, Odicino F, Maisonneuve P, Quinn MA, Beller U, Benedet JL, Heintz AP, Ngan HY, Pecorelli S. Carcinoma of the corpus uteri. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet 2006; 95(Suppl 1):S105-43; PMID:17161155; http://dx.doi.org/10.1016/S0020-7292(06)60031-3
- Molpus KL, Puleo JG, Williams AM, Bernal KL, Remmenga SW. Endometrial adenocarcinoma within a single horn of a didelphic uterus: a report of 2 cases. J Reprod Med 2004; 49(2):123-5; PMID:15018442
- Kosinski A, Dini M. Endometrial cancer in a double uterus. A report of two cases. J Reprod Med 1994; 39(11):926-7; PMID:7853290
- Kondi-Pafiti A, Spanidou-Carvouni H, Dimopoulou C, Kontogianni CI. Endometrioid adenocarcinoma arising in uteri with incomplete fusion of Mullerian ducts. Report of three cases. Eur J Gynaecol Oncol 2003; 24(1):83-4; PMID:12691326
- Tsukahara Y, Fukamatsu Y, Tomita K, Shiozawa T, Iinuma H, Fukuta T. Endometrial carcinoma arising from a double uterus. Gynecol Obstet Invest 1990; 29(4):311-2; PMID:2361641; http://dx.doi.org/10.1159/000293344
- Herzog TJ, Fry R, Husseinzadeh N. Adenocarcinoma in a single horn of a bicornuate uterus. A case report. J Reprod Med 1991; 36(8):619-21; PMID:1941806
- Holub Z, Shomani A. Uterine reduplication, unilateral ureteral and renal aplasia syndrome associated with endometrial cancer: a case report. Eur J Gynaecol Oncol 1998; 19(6):575-6; PMID:10215445
- Moore JR, Davidson SA, Singh M. Endometrial carcinoma in one horn of a bicornuate uterus. Gynecol Oncol 2004; 95(3):729-32; PMID:15581992; http://dx.doi.org/10.1016/j.ygyno.2004.08.003
- Itoh K, Shiozawa T, Shiohara S, Ashida T, Konishi I. Endometrial carcinoma in septate uterus detected 6 months after full-term delivery: case report and review of the literature. Gynecol Oncol 2004; 93(1):242-7; PMID:15047244; http://dx.doi.org/10.1016/j.ygyno.2003.12.036
- Bhalla R, Evans H, Berger L, Crow J, Deheragoda M, Taper Y. A uterus didelphys bicollis, with endometrial cancer in both uteruses. J Obstet Gynaecol 2005; 25(8):823-5; PMID:16368601; http://dx.doi.org/10.1080/01443610500338214
- Fanfani F, Fagotti A, Restaino G, Guerriero M, Scambia G. Endometrial cancer arising in both horns of didelphys uterus in a Down's syndrome woman. Gynecol Oncol 2006; 101(3):537-9; PMID:16487578; http://dx.doi.org/10.1016/j.ygyno.2006.01.002
- Chen CY, Yen MS, Yang MJ, Wu YC. Uterus didelphys with adenocarcinoma in the right cavity diagnosed by 2-dimensional sonography and magnetic resonance imaging. J Ultrasound Med 2008; 27(12):1802-3; PMID:19023011; http://dx.doi.org/10.7863/jum.2008.27.12.1711
- Dane C, Tatar Z, Dane B, Erqinbas M, Cetin A. A single horn endometrial carcinoma of a uterus bicornis unicollis. J Gynecol Oncol 2009; 20(3):195-7; PMID:19809556; http://dx.doi.org/10.3802/jgo.2009.20.3.195
- Shirley S, Devi VS, Krishnamurthy R, Nabhi MV, Majhi U, Selvaluxmy G. Endometrial adenocarcinoma involving both horns of a bicornuate uterus. J Cancer Res Ther 2010; 6(3):304-6; PMID:21119258; http://dx.doi.org/10.4103/0973-1482.73322
- Suprasert P, Khunamornpong S. Carcinosarcoma arising in uterine didelphys after tamoxifen therapy for breast cancer: a case report. J Med Assoc Thai 2010; 93(5):608-12; PMID:20524448
- Kunos C, Woods C, Colussi VC, Abdul-Karim FW, Waggoner S. Low-dose-rate brachytherapy for treatment of uterine didelphys malignancy. J Clin Oncol 2011; 29(5):e104-6; PMID:21098325; http://dx.doi.org/10.1200/JCO.2010.31.7545
- Iavazzo C, Kokka F, Sahdev A, Singh N, Reynolds K. Uterine carcinosarcoma in a patient with didelphys uterus. Case Rep Obstet Gynecol 2013; 2013:401962; PMID:23533863; http://dx.doi.org/10.1155/2013/401962
- Vazquez VD, Di Fiore HA, Garcia-Foncillas J, Plaza AJ. Endometrial adenocarcinoma in one horn of a didelphic uterus with vaginal duplication. BMJ Case Rep 2014; 2014.; PMID:24842355; http://dx.doi.org/10.1136/bcr-2013-203280
- Munkhdelger J, Mia-Jan K, Cha DS, Eom M. Endometrial carcinoma arising in a bicornuate uterus. Obstet Gynecol Sci 2014; 57(5):401-4; PMID:25264532; http://dx.doi.org/10.5468/ogs.2014.57.5.401
- The American Fertility Society classifications of adnexal adhesions, distal tubal occlusion, tubal occlusion secondary to tubal ligation, tubal pregnancies, mullerian anomalies and intrauterine adhesions. Fertil Steril 1988; 49(6):944-55; PMID:3371491; http://dx.doi.org/10.1016/S0015-0282(16)59942-7
- Acien P, Acien M, Sanchez-Ferrer M. Complex malformations of the female genital tract. New types and revision of classification. Hum Reprod 2004; 19(10):2377-84; PMID:15333604; http://dx.doi.org/10.1093/humrep/deh423
- Oppelt P, Renner SP, Brucker S, Strissel PL, Strick R, Oppelt PG, Doerr HG, Schott GE, Hucke J, Wallwiener D, Beckmann MW. The VCUAM (Vagina Cervix Uterus Adnex-associated Malformation) classification: a new classification for genital malformations. Fertil Steril 2005; 84(5):1493-7; PMID:16275249; http://dx.doi.org/10.1016/j.fertnstert.2005.05.036
- Acien P, Acien MI. The history of female genital tract malformation classifications and proposal of an updated system. Hum Reprod Update 2011; 17(5):693-705; PMID:21727142; http://dx.doi.org/10.1093/humupd/dmr021
- Grimbizis GF, Gordts S, Di Spiezio SA, Brucker S, De Angelis C, Gergolet M, Li TC, Tanos V, Brolmann H, Gianaroli L, Campo R. The ESHRE/ESGE consensus on the classification of female genital tract congenital anomalies. Hum Reprod 2013; 28(8):2032-44; PMID:23771171; http://dx.doi.org/10.1093/humrep/det098
- Breech LL, Laufer MR. Mullerian anomalies. Obstet Gynecol Clin North Am 2009; 36(1):47-68; PMID:19344847; http://dx.doi.org/10.1016/j.ogc.2009.02.002
- Hall-Craggs MA, Kirkham A, Creighton SM. Renal and urological abnormalities occurring with Mullerian anomalies. J Pediatr Urol 2013; 9(1):27-32; PMID:22129802; http://dx.doi.org/10.1016/j.jpurol.2011.11.003
- Sugiura-Ogasawara M, Ozaki Y, Suzumori N. Mullerian anomalies and recurrent miscarriage. Curr Opin Obstet Gynecol 2013; 25(4):293-8; PMID:23812381; http://dx.doi.org/10.1097/GCO.0b013e3283632849
- Colombo N, Preti E, Landoni F, Carinelli S, Colombo A, Marini C, Sessa C. Endometrial cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013; 24(Suppl 6):i33-8; PMID:24078661;http://dx.doi.org/10.1093/annonc/mdt353
- Lee DO, Jung MH, Kim HY. Prospective comparison of biopsy results from curettage and hysteroscopy in postmenopausal uterine bleeding. J Obstet Gynaecol Res 2011; 37(10):1423-6; PMID:21651668; http://dx.doi.org/10.1111/j.1447-0756.2011.01558.x
- Epstein E, Ramirez A, Skoog L, Valentin L. Dilatation and curettage fails to detect most focal lesions in the uterine cavity in women with postmenopausal bleeding. Acta Obstet Gynecol Scand 2001; 80(12):1131-6; PMID:11846711; http://dx.doi.org/10.1034/j.1600-0412.2001.801210.x
- Mueller GC, Hussain HK, Smith YR, Quint EH, Carlos RC, Johnson TD, DeLancey JO. Mullerian duct anomalies: comparison of MRI diagnosis and clinical diagnosis. AJR Am J Roentgenol 2007; 189(6):1294-302; PMID:18029861; http://dx.doi.org/10.2214/AJR.07.2494
- Teng F, Zhang YF, Wang YM, Yu J, Lang X, Tian WY, Jiang CX, Xue FX. Contrast-enhanced MRI in preoperative assessment of myometrial and cervical invasion, and lymph node metastasis: diagnostic value and error analysis in endometrial carcinoma. Acta Obstet Gynecol Scand 2015; 94(3):266-73; PMID:25545203; http://dx.doi.org/10.1111/aogs.12570
- Dijkhuizen FP, Mol BW, Brolmann HA, Heintz AP. The accuracy of endometrial sampling in the diagnosis of patients with endometrial carcinoma and hyperplasia: a meta-analysis. Cancer-Am Cancer Soc 2000; 89(8):1765-72.; PMID:11042572; http://dx.doi.org/10.1002/1097-0142(20001015)89:8<1765
- Clark TJ, Mann CH, Shah N, Khan KS, Song F, Gupta JK. Accuracy of outpatient endometrial biopsy in the diagnosis of endometrial cancer: a systematic quantitative review. BJOG 2002; 109(3):313-21; PMID:11950187; http://dx.doi.org/10.1111/j.1471-0528.2002.01088.x
- Gkrozou F, Dimakopoulos G, Vrekoussis T, Lavasidis L, Koutlas A, Navrozoglou I, Stefos T, Paschopoulos M. Hysteroscopy in women with abnormal uterine bleeding: a meta-analysis on four major endometrial pathologies. Arch Gynecol Obstet 2015; 291(6):1347-54; PMID:25524536; http://dx.doi.org/10.1007/s00404-014-3585-x
- Li LT, Jiang G, Chen Q, Zheng JN. Ki67 is a promising molecular target in the diagnosis of cancer (review). Mol Med Rep 2015; 11(3):1566-72; PMID:25384676; http://dx.doi.org/10.3892/mmr.2014.2914
- Gadducci A, Cosio S, Genazzani AR. Tissue and serum biomarkers as prognostic variables in endometrioid-type endometrial cancer. Crit Rev Oncol Hematol 2011; 80(2):181-92; PMID:21134766; http://dx.doi.org/10.1016/j.critrevonc.2010.11.005
