ABSTRACT
MicroRNAs play important roles in tumorigenesis of various types of cancers. MiR-320a can inhibits cell proliferation of some cancers, but the biologic roles of miR-320a in lung cancer need to be further studied. Here, we investigated the roles of miR-320a in suppressing the proliferation of lung adenocarcinoma cells. MiR-320a treatment was found to effectively suppress LTEP-a-2 and A549 cell proliferation, and induce more apoptotic cells with irradiation treatment compared with control treatment. Our results also showed that miR-320a, as a novel miRNA, directly regulated signal transducer and activator of transcription 3 (STAT3) and its signals, such as Bcl−2, Bax, and Caspase 3. The siRNA-inhibited STAT3 levels further proved its roles in regulating STAT3 signals. Moreover, miR-320a treatment effectively suppressed cancer cell growth in mice xenografts compared with controls, and significantly inhibited cell migration in vitro and in vivo. Our findings collectively demonstrated that miR-320a, by directly regulating STAT3 signals, not only suppressed cell proliferation and metastasis, but also enhanced irradiation-induced apoptosis of adenocarcinomia cells.
Abbreviations
| GFP | = | green fluorescent protein |
| HE | = | hematoxylin–eosin staining |
| miRNAs | = | microRNAs |
| MTT | = | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NSCLC | = | Non-small cell lung cancer |
| STAT3 | = | signal transducer and activator of transcription 3 |
Introduction
Lung cancer still remains the leading cause of cancer-related mortality worldwide.Citation1 Non-small cell lung cancer (NSCLC) accounts for approximately 80% of all lung cancers and shows poor prognosis.Citation2 The 5-year survival rate for patients with NSCLC remains low at 15%. Radiotherapy is one of the most approaches for lung cancer treatment. Various of genes are activated during the progress of radiotherapy to alter the radiosensitivity of cancer cells, further promoting cancer cell proliferation.Citation3
MicroRNAs (miRNAs), approximately ∼22 nucleotides in length, regulate gene expression via translational repression or degradation of mRNA targets.Citation4 As oncogenes or tumor suppressors, miRNAs play important roles in various types of cancers, such as lung cancer, renal cell carcinoma, and gastric cancer.Citation5,6 Recent evidence has implicated miRNAs to participate in the radiosensitivity of various types of cancer cells. For instance, miR-622 reduces the radioresistance of colorectal cancer by downregulating retinoblastoma.Citation7 Qu et al. showed that miR-205 contributes to the radioresistance of nasopharyngeal carcinoma by directly targeting phosphatase and tensin homolog deleted on chromosome 10.Citation8 A recent report demonstrated that the radioresistance of lung adenocarcinoma is linked to dysregulated miR-451/c-Myc-survivin/rad-51 signaling. Furthermore, miR-451 can promote radioresistance of cancer cell apoptosis by directly targeting c-Myc.Citation9
MiR-320a is reported to be deregulated in many malignancy types. Recent studies indicated miR-320a inhibited gastric cancer cell proliferation by downregulating FoxM110 and suppressed cancer metastasis by directly targeting metadherin.Citation11 It can also inhibit cell proliferation of leukemia Citation12 and colon cancer.Citation13 Downregulation of its expression is associated with a poor prognosis of clinical patients with cancer.Citation14 However, the roles of miR-320a in lung cancer and irradiation-induced lung cancer cell apoptosis need to be further studied. Therefore, in this study, we investigated the tumor suppressive roles of miR-320a in the proliferation and irradiation-induced apoptosis of lung adenocarcinoma cells.
Results
MiR-320a suppresses the growth of lung adenocarcinoma cells
Though miR-320a is a suppressive miRNA to enhance the sensitivity of cancer cells to chemotherapy.Citation15,16 However, the roles of miR-320a in lung cancer remain unclear. To investigate the roles of miR-320a in proliferation of lung adenocarcinoma cells, we first detected miR-320a levels in lung cancer tissues. We found that miR-320a levels were considerably lower in lung carcinoma tissues (n = 18) than those in paracarcinoma tissues (2 cm from the paracarcinoma tissues, n = 18, ).
Figure 1. MiR-320a regulates LTEP-a-2 cell growth. (A) MiR-320a expression levels in lung adenocarcinoma tissues (n = 18) and paracarcinoma tissues (n = 18) as revealed by qRT-PCR. (B) MiR-320a levels were higher in cells transfected with miR-320a for 48 h compared with control treatment. *p < 0.01, vs. scrambled control. (C) MTT assay. LTEP-a-2 cells were transfected with miR-320a or scrambled for 24 h, subjected under 0 or 10 Gy X-ray, and then cultured for 24 h. MTT assay was conducted to detect cell growth of cells. *p < 0.05, vs. scrambled control or 0 Gy X-ray treatment. (D) Apoptotic cell detection. LTEP-a-2 cells were transfected with miR-320a for 24 h, subjected under 0 or 10 Gy X-ray, and further continued to culture for 24 h. miR-320a, cells treated with miR-320a oligos. Scrambled, treatment with scrambled oligo control RNA. Negative, vehicle-treated cells without oligos.
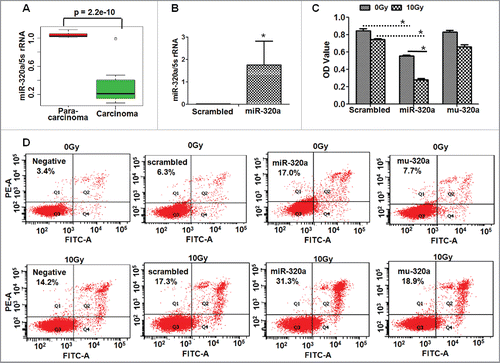
Then, we found that miR-320a overexpression () significantly suppressed cell proliferation in miR-320a-treated LTEP-a-2 cells compared with the mutated miR-320a (Mu-320a) or the scrambled control oligos treatment (). To test the effects of miR-320a on cell action to radiation, cells were irradiated after miRNA transfection. We found that miR-320a treatment further reduced the number of alive cells in 10 Gy X-ray-treated cultures compared with that in the Mu-320a- or scrambled-oligo-transfected cells (). Moreover, miR-320a treatment increased lung adenocarcinoma cell apoptosis in miR-320a-treated LTEP-a-2 cultures compared with the Mu-320a or scrambled control treatment, especially in 10 Gy X-ray-treated cultures (), which further supported that miR-320a is a suppressive miRNA and enhances the irradiation-induced apoptosis of lung cancer cells.
We cultured another lung adenocarcinoma cells (A549/34R) by exposing A549 cells to X-ray of up to 68 Gy for a prolonged period (2 Gy/day, 5 days/week). Similarly, miR-320a suppressed the proliferation and induced more cell apoptosis of A549/34R cells compared with control treatment (Fig. S1). MiR-320a treatment further reduced the number of alive cells and induced more apoptotic cells in 10 Gy X-ray -transfected cultures than those in scrambled-oligo-transfected cells (Fig. S1).
MiR-320a, as a novel miRNA, directly targets STAT3
We predicted that STAT3–3′UTR is the target site of miR-320a () by using microRNA informatics analysis software (http://www.microrna.org/microrna/getMirnaForm.do, or http://www.targetscan.org/ index.html). Next, a GFP reporter plasmid containing with STAT3–3′UTR was constructed to transfect cells with miR-320a. GFP intensity was considerably lower in miR-320a-transfected cells compared with control treatment, and the percentage of GFP positive cells decreased in miR-320a-treated cells. Mutation of the binding site of miR-320a (Mu-320a) could not reduce the the percentage of GFP positive cells compared with the wide type miR-320a ( and ), demonstrating that miR-320a directly regulated STAT3–3′UTR.
Figure 2. MiR-320a directly regulates STAT3 expression. (A) STAT3–3′UTR is targeted by miR-320a or Mu-320a (mutation of miR-320a) as shown by microRNA informatics. (B) GFP-positive cells were observed under a fluorescence microscope. Cells were treated with both pc3.1-GFP-STAT3–3′UTR and miR-320a or scrambled control. Bar = 100 μm. (C) Flow cytometry detected the percentage of GFP-positive cells after cells were co-transfected with pc3.1-GFP-STAT3–3′UTR and miR-320a (or scrambled control). (D) STAT3/p-STAT3 expression was detected through western bolt analysis. *p < 0.01, miR-320a treatment vs. scrambled control. (E) Immunofluorescence detection showed that p-STAT3 levels located in the nuclei were inhibited in miR-320a-treated cells compared with those treated with scrambled control treatment. p-STAT3 (green); DAPI-counterstained nuclei (blue). Bar = 50 μm. (F) q-PCR detected miR-320a levels in A549 or A549/R34 cells. *p < 0.01, miR-320a levels in A549/34R vs. those in A549.
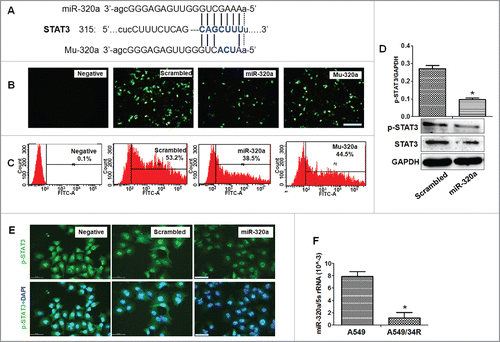
Western blot analysis further showed that miR-320a inhibited the expression of both STAT3 and phosphorylated p-STAT3 (p-STAT3) in miR-320a-treated cultures (), indicating that miR-320a directly regulated STAT3 expression. In addition, immunofluorescence detection also demonstrated that p-STAT3 level in the nucleus decreased sharply in miR-320a-treated cells compared with that in control treatment ().
MiR-320a regulates STAT3-related signals
The abovementioned experiments suggest that the inhibitory effect of miR-320a on proliferation of lung cancer cells is related to STAT3 signal regulation. To test this hypothesis, we first analyzed the levels of miR-320a, STAT3, p-STAT3, and their related signals in A549/34R cells treated by irradiation. qRT-PCR results showed that miR-320a levels in A549/34R cells with irradiation treatment was 5 times lower than those in A549 cells (). In contrast to the reduced miR-320a levels in A549/34R cells, western blot analysis showed that both the levels of STAT3 and p-STAT3 levels increased obviously in A549/34R cells compared with those in A549 controls (). Moreover, immunofluorescence assay showed that the amount of p-STAT3 increased in the nucleus of A549/R34 cells compared with that in A549 cells (). These results suggest that miR-320a and p-STAT3 are involved in the resistance of lung cancer cells to radiation, which was consistent with the previous findings, showing that nuclear translocation of STAT3 can be promoted by radiation.Citation17
Figure 3. MiR-320a inhibits STAT3-regulated signals. (A) STAT3 and p-STAT3 were detected through western blot analysis. *p < 0.01, protein levels in A549/34R vs. those in A549. (B) p-STAT3 levels in nuclei as detected by immunofluorescence staining. p-STAT3 (green); DAPI-counterstained nuclei (blue). Bar = 50 μm. (C) Bcl−2 and Bax expression in A549 and A549/R34 cells as shown by western blot analysis. *p < 0.05, A549/34R vs. A549. (D) Real-time PCR detection of Bcl−2 and Bax levels. *p < 0.01, miR-320a treatment vs. scrambled control. (E) Western blot analysis. Bcl−2 and Bax were expressed in A549/R34 cells transfected with miR-320a or scrambled control for 48 h. *p < 0.01, miR-320a treatment vs. scrambled control. (F) Levels of cleaved Caspase 9, 8, and 3 increased in miR-320a-treated A549/R34 cells.
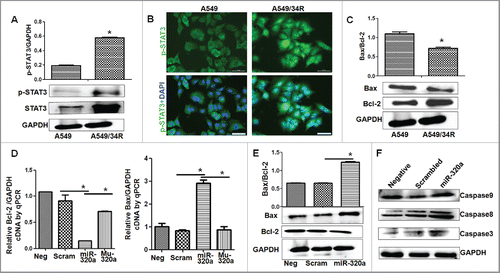
Nuclear translocation of p-STAT3 further activates its DNA binding genes, ultimately regulating its downstream effectors, including the anti-apoptotic protein Bcl−2.Citation18,19 Therefore, we detected Bcl−2 and its related BAX, Caspase-9 and Caspase-3 expression,Citation20 and found that Bcl−2 levels increased, whereas BAX levels decreased in irradiation-induced A549/34R cells compared with those in A549 controls (). Real-time PCR showed that miR-320a, treatment significantly reduced Bcl−2 and increased BAX levels compared with those in Mu-320a and scrambled controls (). Furthermore, western blot results proved the roles of miR-320a in reducing Bcl−2 and increasing BAX levels in A549/34R cells compared with those in controls (). MiR-320a intervention increased the levels of cleaved Caspase 9, Caspase 8, and Caspase 3 compared with those in scrambled control treatment (), demonstrating that miR-320a regulated STAT3-related signals.
Inhibition of STAT3 signals prevents cell growth
The results of abovementioned experiments suggest that the mechanisms of miR-320a-induced inhibition of proliferation of cancer cells involve in regulating STAT3 signals. To test this hypothesis, 2 siRNAs specific to STAT3 mRNA were synthesized to knockdown STAT3 expression. Not only that the STAT3 mRNA levels were knocked down (), but also the expression of STAT3 proteins decreased in siRNA-transfected cultures, especially in siRNA1-treated cells compared with those in siRNA-control treatment (si-Con, ). siRNA1 treatment effectively reduced p-STAT3 levels in the nucleus of A549/34R cells compared with control treatment (). The modified colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay further showed that siRNA1 effectively suppressed A549/34R cell proliferation compared with si-Con-treated cultures (). Cell apoptosis detection showed that siRNA1 induced more apoptotic cells in siRNA1-treated A549/34R and LTEP-a-2 cells compared with control treatment (, Fig. S2).
Figure 4. STAT3 signals are suppressed by siRNA. (A) STAT3 mRNA was reduced by siRNAs as shown by qRT-PCR analysis. *p < 0.01, siRNA1, or siRNA2 vs. si-Con. STAT3- siRNA1 or siRNA2 are the 2 kinds of siRNAs specific to STAT3 mRNA. (B) Western blot analysis showed that the levels of STAT3 and p-STAT3 decreased after treatment of A549/R34 cells with STAT3-siRNA1 and siRNA2, respectively, especially in siRNA1-treated culture. (C) Immunofluorescence detection showed that the p-STAT3 levels in nuclei were inhibited by siRNA1. p-STAT3 (green); DAPI-counterstained nuclei (blue). (D) MTT assay. Cells were transfected with STAT3-siRNA1 for 24 h, treated with 0 or 10 Gy X-ray, and then cultured for 24 h. *p < 0.05, vs. scrambled control or 0 Gy X-ray treatment. (E) Cell apoptosis detection. Cells were transfected with STAT3-siRNA1 for 24 h, treated with 0 or 10 Gy X-ray, and further cultured for 24 h. (F) Real-time PCR detection of Bcl−2 and Bax levels. *p < 0.01, miR-320a treatment vs. scrambled control. (G) Western blot analysis showed that Bcl−2 decreased and Bax increased in siRNA1-treated cells. *p < 0.01, vs. si-Con treatment. (H) The levels of cleaved Caspase 9, 8, and 3 increased in siRNA1-treated A549/R34 cells. siRNA1, cells treated with siRNA1. si-Con, treatment with siRNA scrambled control RNA. Negative, vehicle-treated cells without oligos.
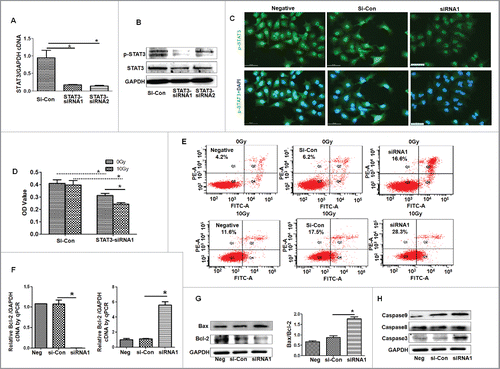
Moreover, 10 Gy X-ray treatment obviously decreased the number of the live cells in siRNA1-treated cells compared with that in siRNA-control-transfected cells (). SiRNA1 further induced apoptosis in X-ray-treated cultures by approximately, considerably higher than those in Control-transfected cells (, Fig. S2). In addition, Bcl−2 levels were reduced, whereas the levels of Bax, cleaved Caspase 9, cleaved Caspase 8 and cleaved Caspase 3 increased in siRNA1-treated cultures compared with those in si-Con treatment (). These experiments demonstrate that siRNA-regulated STAT3 levels inhibit A549/34R cell proliferation and enhance irradiation-induced apoptosis of A549/34R cells, further demonstrating that STAT3 signals are involved in cancer cell growth.
MiR-320a inhibits cancer cell growth in mouse xenografts
To verify the suppressive roles of miR-320a in cancer cell growth in vivo, we hypodermically injected A549/34R cells transfected with miR-320a or STAT3-siRNA or their control RNA into BALB/c-nu/nu mice to produce xenografts. One month after injection, the volumes of xenografts (n = 6) were considerably smaller in miR-320a-treated mice than in scrambled control (n = 6, ). STAT3-specific siRNA1 treatment also inhibited A549/34R cell growth (n = 6) and reduced the volumes of xenografts (n = 6, ). Moreover, the weights of A549/34R cell xenografts were considerably lower in miR-320a-treated mice (n = 6) or in siRNA1-treated mice (n = 6) compared with those in scrambled control (n = 6, ). On the basis of dynamic changes in volumes, we found that the growth rate of A549/34R cell xenografts (n = 6) of was inhibited effectively in vivo by miR-320a or siRNA1 compared with scrambled control treatment (n = 6, ).
Figure 5. MiR-320a inhibits cell growth in mouse xenografts. (A) Images showing the changes in size of xenografts 30 d after injection. The cells were treated with control oligo, miR-320a, or STAT3-siRNA1 and then injected subcutaneously in nude mice. One week after cell injection, partial treatment with 0 or 10 Gy X-ray was performed once a week. (B) The xenografts were weighted 30 d after injection. (C) Dynamic changes in tumor size were measured every 3 d. (D, E) The expression of STAT3, p-STAT3, and cleaved Caspase 3 in the xenografts treated with 0 or 10 Gy X-ray.
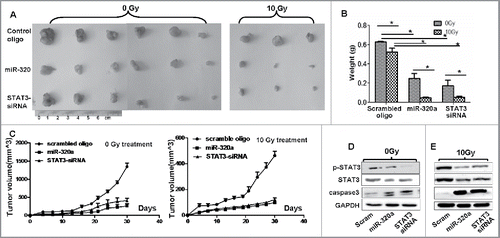
One week after injection, we locally treated the xenografts in the mice every week with 10 Gy X-rays for a total of 3 treatments. Our results showed that 10 Gy X-ray treatment obviously further reduced the volumes of xenografts (n = 3) in the miR-320a- or siRNA1-treated mice compared with those in scrambled control (n = 3, ). The weights of xenografts in miR-320a- (n = 3) or siRNA1-treated mice (n = 3) were decreased by more than 3-fold after 10 Gy X-ray treatment compared with those in scrambled control treatment, in which the xenograft weights decreased only slightly after 10 Gy X-ray treatment (n = 3, ). The growth rate of A549/34R cell xenografts (n = 3) was further inhibited in vivo by miR-320a or siRNA1 after treatment with 10 Gy X-ray compared with that in scrambled control treatment (n = 3, ).
These experiments further demonstrated that miR-320a effectively suppressed cell growth and increased the sensitivity of A549/34R cells to irradiation in vivo. We then tested the expression of STAT3 and its signals in the xenografts. The results of treatment with or without X-ray showed that the expression of STAT3 and p-STAT3 decreased sharply, whereas that of cleaved Caspase 3 increased in the miR-320a- or siRNA1-treated xenografts (n = 3) compared with that in scrambled control (n = 3, ), supporting the roles of miR-320a or siRNA in regulating STAT3 signals in vivo.
MiR-320a inhibits cancer cell metastasis
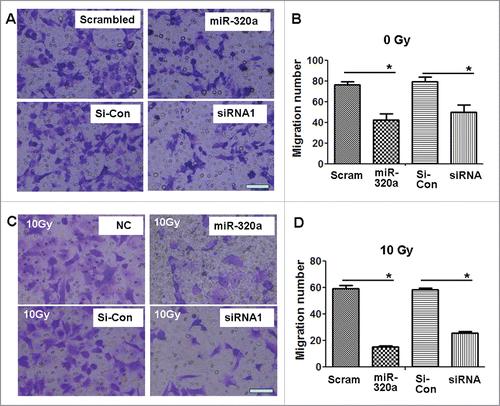
We subsequently investigated the effect of miR-320a on the migration of lung adenocarcinoma cells. Transwell chamber experiments showed that miR-320a treatment obviously reduced the number of migrating A549/34R cells in vitro compared with scrambled control treatment (). SiRNA1 treatment specific to STAT3 also obviously reduced the number of migrating A549/34R cells (). Moreover, we found that 10 Gy X-ray treatment further reduced the number of migrating A549/34R cells by approximately 2- to 3-fold in miR-320a- or siRNA1-treated cells compared with scrambled control treatment (). These data suggest that miR-320a or siRNA inhibits A549/34R cell migration in vitro.
Figure 6. MiR-320a suppresses cell migration in vitro. (A) The migration assay showed that the number of migrating A549/34R cells decreased in the miR-320a- or siRNA1-treated cultures. (B) Determination of the number of migratory cells. *p < 0.01, miR-320a vs. scrambled, or siRNA1 vs. si-con, respectively. (C) X-ray treatment (10 Gy) further reduced the numbers of migratory A549/34R cells in the miR-320a- or siRNA1-treated cultures. (D) Determination of the number of migratory cells. *p < 0.01, miR-320a vs. scrambled, or siRNA1 vs. si-con. miR-320a, cells treated with miR-320a oligos. Scrambled, treated with scrambled oligo control RNA. siRNA1, cells treated with siRNA1. si-Con, cells treated with siRNA scrambled control RNA. Bar = 100 μm.
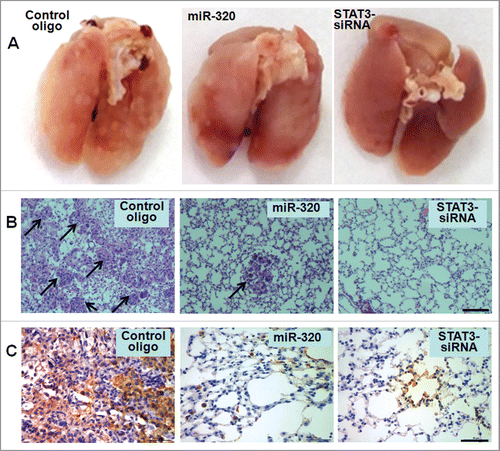
To understand the effects of miR-320a on metastasis of A549/34R cells in vivo, we injected 2 × 106 cells transfected with miR-320a or siRNA or controls into male nude mice through their tail vein. Seven weeks after injection, we observed that miR-320a or siRNA1 reduced the number of metastatic nodules on the surface of lungs compared with the scrambled control treatment (). In addition, HE staining of lung sections showed the significantly reduced migration of miR-320a- or siRNA-treated tumors (). CD44, a cell adhesion molecule, increases the migratory and invasive capacity of various cancers.Citation21 We further detected human CD44 expression in metastatic A549/34R cell nodules, and found that significantly lower levels of CD44 in miR-320a- or siRNA-treated tumors than that in scrambled control treatment (). The in vivo results further confirmed the functional effects of miR-320a on tumor migration.
Figure 7. MiR-320a suppresses lung adenocarcinoma cell metastasis in vivo. (A) An experimental metastasis mouse model was injected with control miR-320a, STAT3-siRNA1-transfected A549/34R cells, or control oligos. (B) Visualization of the HE-stained lung section. Bar = 100 μm. (C) Immunohistochemistry was conducted to detect CD44 expression. Bar = 50 μm.
Lung adenocarcinoma samples show high STAT3 levels
Finally, we measured STAT3 mRNA levels of STAT3 in lung adenocarcinoma samples. In contrast to the lower levels of miR-320a in adenocarcinoma samples, qRT-PCR results showed that the mRNA levels of STAT3 were considerably higher in adenocarcinoma samples than that in para-carcinomas (n = 18, , p = 2.204 × 10−10), and
Figure 8. STAT3 levers in samples correlated with overall survival E. STAT3 mRNA levels were detected in lung adenocarcinoma tissues and para-carcinoma tissues (n = 18) by qRT-PCR. F. Overall survival was determined through Kaplan–Meier survival analysis.
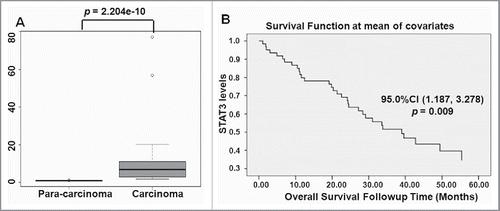
Data on STAT3 expression data were further downloaded from data link Data Link(s): http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc = GSE3141. Overall survival was determined using Kaplan–Meier survival analysis. Data showed that the poor survival time of patients is not significantly correlated with the age (p = 0.756), or sex (p = 0.474). Interestingly, patients showing high STAT3 levels displayed short survival time (n = 59, p = 0.009, ), suggesting that higher STAT3 levels increase the risks of lung adenocarcinoma.
Discussion
MiRNAs take part in the tumorigenesis of various types of cancer cells.Citation8,9 The present study investigated the roles of miR-320a in the proliferation of lung cancer cells, and found that overexpression of miR-320a enhanced the apoptosis of lung adenocarcinoma cells induced by irradiation in vitro and in vivo. Moreover, miR-320a significantly inhibited lung adenocarcinoma cell proliferation and migration by directly regulating STAT3 signals, which was supported by zhao et al., who found that miR-320a plays a critical role in invasion and metastasis of colorectal cancer by regulating Rac1 gene.Citation22
STAT3, an important member of the JAK/STAT3 signaling pathway, is one of the key players in many physiologic and pathological processes.Citation17 STAT3 is involved in essential processes, such as cell survival, growth, and proliferation in many types of tumors, as well as in immune diseases.Citation23 We found that the mRNA levels of STAT3 were considerably higher in adenocarcinoma tissues than in paracarcinomas, and that patients showing higher STAT3 levels displayed short poor survival time, indicating that STAT3 plays important roles in tumorigenesis of lung cancer.
Suppression of STAT3 signals would reduce cell proliferation and increase apoptosis. STAT3 inhibitor galiellalactone treatment obviously reduced the tumor growth and metastatic spread in orthotopic xenografts in mice.Citation24 Recently, scientists aimed to explore the roles of miRNAs in suppressing STAT3. MiR-506 can markedly suppress glioma cell proliferation by targeting STAT3.Citation25 MiR-203 potentially controls DNA damage repair via the JAK/STAT3 pathways, which contribute to the modulation of radiation sensitivity of human malignant glioma cells.Citation26 In the present study, we identified miR-320a, as a novel miRNA, directly suppressed STAT3 expression and its signals by targeting STAT3–3′UTR, further effectively inhibiting cell growth and inducing cell apoptosis.
P-STAT3 translocates into the nucleus to regulate gene expression by binding to the elements within their promoters, resulting in the expression of the downstream factors, Bcl−2, Bcl−XL, and survivin.Citation27 These genes are associated with cell proliferation and migration. Here, we found that miR-320a significantly suppressed p-STAT3 levels in the nucleus of miR-320a-treated cancer cells. Being downstream factors of STAT3, Bcl−2 decreased, whereas Bax increased in miR-320a-treated A549 cells. These phenomena are possibly related to the inhibitory effect of miR-320a on A549/R34 cell proliferation. The regulation of Bcl−2 by STAT3 induced the mitochondria to release cytochrome C, which further induced cell apoptosis through Caspase signals. Our results proved that miR-320a induced lung cancer cell apoptosis by reducing Bcl−2 and by upregulating cleaved Caspase 9, 8, and 3.
STAT3 also played a critical role in radioresistance of cancer. By inhibiting IL-8/STAT3 signals, miR-23a can reduce the radioresistance of nasopharyngeal carcinoma cells.Citation28 Suppression of the ERp57-STAT3 pathway is necessary to enhance the efficacy of radiotherapy in human laryngeal cancer.Citation29 In lung adenocarcinoma study, we found that the levels of STAT3/p-STAT3 were higher in irradiation-induced A549/R34 cells than in A549 cells. Knockdown of STAT3 by miR-320a significantly increased the apoptosis of A549/R34 cells induced by irradiation in vitro and in vivo.
In summary, our study demonstrates that miR-320a, as a novel miRNA, directly regulates STAT3 signals, which further significantly inhibits cell proliferation and enhances irradiation-induced apoptosis of lung adenocarcinoma cells in vitro and in vivo. Our findings highlight the potential of miR-320a as novel therapeutic target for patients with lung adenocarcinomas.
Materials and methods
Lung adenocarcinoma tissues
This study was conducted between August 1, 2014 and July 31, 2015 at the Inpatient Department of Medical Oncology, Yantai Shan Hospital, the Teaching Hospital of Binzhou Medical University (Yantai, China). The experiments were performed in accordance to the relevant guidelines of the Code of Ethics of the World Medical Association for experiments involving humans and the Medical Ethics Committee of Binzhou Medical University.
Eighteen patients (9 male and 9 female, aged 40–59 years) who were pathologically diagnosed with lung adenocarcinoma for the first time and have not yet received chemotherapy, were included in the present study. Fresh lung adenocarcinoma and controls were obstained from the patients who underwent surgery.
Real-time qRT-PCR
Real-time qRT-PCR was performed as previous study.Citation30 Primers used to amplify miR-320a were as follows: forward: 5′- AAAAGCTGGGTTGAGAGG -3′; reverse: 5′-AACATGTACAGTCCATGGATG-3′. Human 5S rRNA served as positive control. The primers used to amplify STAT3 were as follows: forward: 5′- TGCGGAGAAGCATCGTGAGT-3′; reverse: 5′- CCTCCAATGCAGGCAATCTGT-3′.
Bcl−2 forward: 5′-GTTCGGTGGGGTCATGTGTGTGGAGAGCG-3′; reverse: 5′-TAGCTGATTCGACGTTTTGCCTGA-3′. Bax forward: 5′-CGCCCTTTTCTACTTTGCCAGCA-3′; reverse: 5′-AGGAGTCTCACCCAACCACCCTG-3′. qRT-PCR was performed on a RG3000 system (Qiagen) under the following reaction condition: initial denaturation at 95°C for 30 s, followed by 40 cycles at 95°C for 5 s, annealing at 60 °for 20 s, and extension at 72°C for 30 s. GAPDH cDNAs served as positive control.
Cell culture and transfection
Lung adenocarcinoma (LTEP-a-2 and A549) cells were obtained from Shanghai Institute of Cell Biology, China. A549/R34 cells were induced by exposing A549 cells to X-ray irradiation of up to 68 Gy using X-RAD 320 (PXi, Connecticut, USA) as previous described.Citation31 The cells were cultured in RPMI-1640 medium (Gibco, Grand Island, New York, USA) supplemented with 10% fetal bovine serum (Gibco) at 37°C with 5% CO2. Cells (2 × 105) were seeded into 6-well plates. Transfection was performed in triplicate at approximately 60% confluence by using Lipofectamine™ 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. MTT assay was performed as previous studies.Citation32
Apoptosis assays
Apoptosis assay was performed 48 h after transfection by using Annexin V-FITC/PI31 (KeyGEN Biotech. Co. Ltd., Nanjing, China) according to the manufacturer's protocols.
Western blot analysis
Western blot analysis was performed as described previously.31The antibodies used were as follows: rabbit anti-humanSTAT3 (1:800; Bioworld Technology, co. Ltd), rabbit anti-human p-STAT3 (1:800; Bioworld Technology, co. Ltd), rabbit anti-human Bax (1:500; Bioworld Technology, co. Ltd), Bcl2 (1:500; Bioworld Technology, co. Ltd), rabbit anti-human Caspase 3 (1:500; Bioworld Technology, co. Ltd), rabbit anti-human Caspase 8(1:500; Bioworld Technology, co. Ltd), rabbit anti-human Caspase 9 (1:500; Bioworld Technology, co. Ltd), and rabbit anti-human GAPDH (1:4000; Bioworld Technology, co. Ltd).
Xenografts in mice
Tumor cells (5 × 106) in 0.1 mL of PBS, transfected with miR-320a or STAT3-small interfering RNAs (siRNAs) or controls, were transplanted subcutaneously into the right or left flanks of 5–6-week-old male BALB/c-nu/nu mice (Charles River. Beijing, China). The maximum and minimum tumor diameters were measured every 3 d. The nude mice were raised in SPF environment. Tumor volumes (mm3) = A× B2/2, where A and B are the maximum and minimum tumor diameters, respectively. After 4 weeks, the tumors were anatomized and weighed. All animal experiments were approved by the Committee on the Ethics of Animal Experiments of Binzhou Medical University and performed in accordance with the National Institutes of Health guide for the care and use of Laboratory animals.
Injection of cells into the nude mice via tail vein
Cells transfected with miR-320a, STAT3-siRNA, or controls were harvested from Petri dish, in 100 uL normal saline at 1.5 × 106 cells. Subsequently, the collected cells were injected into the tail veins of nude mice (5 weeks old). After 6–7 weeks, the lungs were dissected, and hematoxylin–eosin staining (HE) was performed as described previously.Citation33
Statistics
SPSS Statistics Client 22 (IBM) software was performed to analyze the significance of all results. ANOVA was used to compare different groups with respect to continuous variables. Group means comparisons were compared using an unpaired, 2-sided, Student's t-test. Correlations were calculated with a Spearman rank test. Array data of STAT3 were downloaded from data link Data Link(s): http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc = GSE3141. Overall survival was determined using Kaplan–Meier survival analysis. P values < 0.05 indicates statistically significant differences.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplementary_Data.pdf
Download PDF (323.8 KB)Acknowledgments
The authors thank Zheng-Ping Hu (Medical Center, Binzhou Medical University) for kindly help for treating cells or mice with X-ray irradiation by using X-RAD 320.
Funding
This study was supported by the National Natural Science Foundation of China (Nos. 31371321, 31440061, 81502536), and the Shandong Science and Technology Committee (Nos.2015GSF118073, ZR2016CL09, 2014HP004).
References
- Guo W, Zhang Y, Zhang Y, Shi Y, Xi J, Fan H, Xu S. Decreased expression of miR-204 in plasma is associated with a poor prognosis in patients with non-small cell lung cancer. Int J Mol Med 2015; 36(6):1720-6; PMID:26497897; http://dx.doi.org/10.3892/ijmm.2015.2388
- Ye L, Wang H, Liu B. miR-211 promotes non-small cell lung cancer proliferation by targeting SRCIN1. Tumour Biol 2016; 37(1):1151-7; PMID:26277787; http://dx.doi.org/10.1007/s13277-015-3835-y
- Toulany M, Rodemann HP. Phosphatidylinositol 3-kinase/Akt signaling as a key mediator of tumor cell responsiveness to radiation. Semin Cancer Biol 2015; 35:180-90; PMID:26192967; http://dx.doi.org/10.1016/j.semcancer.2015.07.003
- Li Z, Yu X, Shen J, Jiang Y. MicroRNA dysregulation in uveal melanoma: a new player enters the game. Oncotarget 2015; 6:4562-8; PMID:25682876; http://dx.doi.org/10.18632/oncotarget.2923
- Gorur A, Balci Fidanci S, Dogruer Unal N, Ayaz L, Akbayir S, Yildirim Yaroglu H, Dirlik M, Serin MS, Tamer L. Determination of plasma microRNA for early detection of gastric cancer. Mol Biol Rep 2013; 40(3):2091-6; PMID:23212612; http://dx.doi.org/10.1007/s11033-012-2267-7
- Li JH, Luo N, Zhong MZ, Xiao ZQ, Wang JX, Yao XY, Peng Y, Cao J. Inhibition of microRNA-196a might reverse cisplatin resistance of A549/DDP non-small-cell lung cancer cell line. Tumour Biol 2016; 37(2):2387-94; PMID:26376998; http://dx.doi.org/10.1007/s13277-015-4017-7
- Ma W, Yu J, Qi X, Liang L, Zhang Y, Ding Y, Lin X, Li G, Ding Y. Radiation-induced microRNA-622 causes radioresistance in colorectal cancer cells by down-regulating Rb. Oncotarget 2015; 6:15984-94; PMID:25961730; http://dx.doi.org/10.18632/oncotarget.3762
- Li G, Liu Y, Su Z, Ren S, Zhu G, Tian Y, Qiu Y. MicroRNA-324-3p regulates nasopharyngeal carcinoma radioresistance by directly targeting WNT2B. Eur J Cancer 2013; 49(11):2596-607; PMID:23583221; http://dx.doi.org/10.1016/j.ejca.2013.03.001
- Wang R, Chen DQ, Huang JY, Zhang K, Feng B, Pan BZ, Chen J, De W, Chen LB. Acquisition of radioresistance in docetaxel-resistant human lung adenocarcinoma cells is linked with dysregulation of miR-451/c-Myc-survivin/rad-51 signaling. Oncotarget 2014; 5:6113-29; PMID:25026294; http://dx.doi.org/10.18632/oncotarget.2176
- Wang Y, Zeng J, Pan J, Geng X, Li L, Wu J, Song P, Wang Y, Liu J, Wang L. MiR-320a inhibits gastric carcinoma by targeting activity in the FoxM1-P27KIP1 axis. Oncotarget 2016; 7(20):29275-86; PMID:27086911; http://dx.doi.org/10.18632/oncotarget.8676
- Yu J, Wang JG, Zhang L, Yang HP, Wang L, Ding D, Chen Q, Yang WL, Ren KH, Zhou DM, et al. MicroRNA-320a inhibits breast cancer metastasis by targeting metadherin. Oncotarget 2016; 7(25):38612-38625; PMID:27229534; http://dx.doi.org/10.18632/oncotarget.9572
- Schaar DG, Medina DJ, Moore DF, Strair RK, Ting Y. miR-320 targets transferrin receptor 1 (CD71) and inhibits cell proliferation. Exp Hematol 2009; 37(2):245-55; PMID:19135902; http://dx.doi.org/10.1016/j.exphem.2008.10.002
- Zhang Y, He X, Liu Y, Ye Y, Zhang H, He P, Zhang Q, Dong L, Liu Y, Dong J. microRNA-320a inhibits tumor invasion by targeting neuropilin 1 and is associated with liver metastasis in colorectal cancer. Oncol Rep 2012; 27(3):685-94; PMID:22134529; http://dx.doi.org/10.3892/or.2011.1561
- Sun JY, Huang Y, Li JP, Zhang X, Wang L, Meng YL, Yan B, Bian YQ, Zhao J, Wang WZ, et al. MicroRNA-320a suppresses human colon cancer cell proliferation by directly targeting beta-catenin. Biochem Biophys Res Commun 2012; 420(4):787-92; PMID:22459450; http://dx.doi.org/10.1016/j.bbrc.2012.03.075
- Vishnubalaji R, Hamam R, Yue S, Al-Obeed O, Kassem M, Liu FF, Aldahmash A, Alajez NM. MicroRNA-320 suppresses colorectal cancer by targeting SOX4, FOXM1, and FOXQ1. Oncotarget 2016; 7(24):35789-35802; PMID:27119506; http://dx.doi.org/10.18632/oncotarget.8937
- Wan LY, Deng J, Xiang XJ, Zhang L, Yu F, Chen J, Sun Z, Feng M, Xiong JP. miR-320 enhances the sensitivity of human colon cancer cells to chemoradiotherapy in vitro by targeting FOXM1. Biochem Biophys Res Commun 2015; 457(2):125-32; PMID:25446103; http://dx.doi.org/10.1016/j.bbrc.2014.11.039
- Gao L, Li FS, Chen XH, Liu QW, Feng JB, Liu QJ, Su X. Radiation induces phosphorylation of STAT3 in a dose- and time-dependent manner. Asian Pac J Cancer Prev 2014; 15(15):6161-4; PMID:25124591; http://dx.doi.org/10.7314/APJCP.2014.15.15.6161
- Zhu WQ, Wang J, Guo XF, Liu Z, Dong WG. Thymoquinone inhibits proliferation in gastric cancer via the STAT3 pathway in vivo and in vitro. World J Gastroenterol 2016; 22(16):4149-59; PMID:27122665; http://dx.doi.org/10.3748/wjg.v22.i16.4149
- Li T, Liu X, Shen Q, Yang W, Huo Z, Liu Q, Jiao H, Chen J. Salinomycin exerts anti-angiogenic and anti-tumorigenic activities by inhibiting vascular endothelial growth factor receptor 2-mediated angiogenesis. Oncotarget 2016; 7(18):26580-92; PMID:27058891; http://dx.doi.org/10.18632/oncotarget.8555
- Guo X, Fan W, Bian X, Ma D. Upregulation of the Kank1 gene-induced brain glioma apoptosis and blockade of the cell cycle in G0/G1 phase. Int J Oncol 2014; 44(3):797-804; PMID:24399197; http://dx.doi.org/10.3892/ijo.2014.2247
- Yokoyama T, Nakamura T. Tribbles in disease: Signaling pathways important for cellular function and neoplastic transformation. Cancer Sci 2011; 102(6):1115-22; PMID:21338441; http://dx.doi.org/10.1111/j.1349-7006.2011.01914.x
- Zhao H, Dong T, Zhou H, Wang L, Huang A, Feng B, Quan Y, Jin R, Zhang W, Sun J, et al. miR-320a suppresses colorectal cancer progression by targeting Rac1. Carcinogenesis 2014; 35(4):886-95; PMID:24265291; http://dx.doi.org/10.1093/carcin/bgt378
- Li CJ, Li YC, Zhang DR, Pan JH. Signal transducers and activators of transcription 3 function in lung cancer. J Cancer Res Ther 2013; 9(Suppl 2):S67-73; PMID:24135245; http://dx.doi.org/10.4103/0973-1482.119100
- Canesin G, Evans-Axelsson S, Hellsten R, Sterner O, Krzyzanowska A, Andersson T, Bjartell A. The STAT3 inhibitor Galiellalactone effectively reduces tumor growth and metastatic spread in an Orthotopic Xenograft mouse model of prostate cancer. Eur Urol 2016; 69(3):400-4; PMID:26144873; http://dx.doi.org/10.1016/j.eururo.2015.06.016
- Peng T, Zhou L, Zuo L, Luan Y. MiR-506 functions as a tumor suppressor in glioma by targeting STAT3. Oncol Rep 2016; 35(2):1057-64; PMID:26554866; http://dx.doi.org/10.3892/or.2015.4406
- Chang JH, Hwang YH, Lee DJ, Kim DH, Park JM, Wu HG, Kim IA. MicroRNA-203 modulates the radiation sensitivity of human malignant glioma cells. Int J Radiat Oncol Biol Phys 2016; 94(2):412-20; PMID:26678661; http://dx.doi.org/10.1016/j.ijrobp.2015.10.001
- Mukthavaram R, Ouyang X, Saklecha R, Jiang P, Nomura N, Pingle SC, Guo F, Makale M, Kesari S. Effect of the JAK2/STAT3 inhibitor SAR317461 on human glioblastoma tumorspheres. J Transl Med 2015; 13:269; PMID:26283544; http://dx.doi.org/10.1186/s12967-015-0627-5
- Qu JQ, Yi HM, Ye X, Li LN, Zhu JF, Xiao T, Yuan L, Li JY, Wang YY, Feng J, et al. MiR-23a sensitizes nasopharyngeal carcinoma to irradiation by targeting IL-8/Stat3 pathway. Oncotarget 2015; 6(29):28341-56; PMID:26314966; http://dx.doi.org/10.18632/oncotarget.5117
- Choe MH, Min JW, Jeon HB, Cho DH, Oh JS, Lee HG, Hwang SG, An S, Han YH, Kim JS. ERp57 modulates STAT3 activity in radioresistant laryngeal cancer cells and serves as a prognostic marker for laryngeal cancer. Oncotarget 2015; 6:2654-66; PMID:25605256; http://dx.doi.org/10.18632/oncotarget.3042
- Wang PY, Sun YX, Zhang S, Pang M, Zhang HH, Gao SY, Zhang C, Lv CJ, Xie SY. Let-7c inhibits A549 cell proliferation through oncogenic TRIB2 related factors. FEBS Lett 2013; 587(16):2675-81; PMID:23850892; http://dx.doi.org/10.1016/j.febslet.2013.07.004
- Zhang HH, Pang M, Dong W, Xin JX, Li YJ, Zhang ZC, Yu L, Wang PY, Li BS, Xie SY. miR-511 induces the apoptosis of radioresistant lung adenocarcinoma cells by triggering BAX. Oncol Rep 2014; 31(3):1473-9; PMID:24402374; http://dx.doi.org/10.3892/or.2014.2973
- Zhang C, Chi YL, Wang PY, Wang YQ, Zhang YX, Deng J, Lv CJ, Xie SY. miR-511 and miR-1297 inhibit human lung adenocarcinoma cell proliferation by targeting oncogene TRIB2. PLoS One 2012; 7(10):e46090; PMID:23071539; http://dx.doi.org/10.1371/journal.pone.0046090
- Zhang YX, Yan YF, Liu YM, Li YJ, Zhang HH, Pang M, Hu JX, Zhao W, Xie N, Zhou L, et al. Smad3-related miRNAs regulated oncogenic TRIB2 promoter activity to effectively suppress lung adenocarcinoma growth. Cell Death Dis 2016; 7(12):e2528; PMID:28005074; http://dx.doi.org/10.1038/cddis.2016.432
