ABSTRACT
Human Papilloma Virus infection is very frequent in humans and is mainly transmitted sexually. The majority of infections are transient and asymptomatic, however, if the infection persists, it can occur with a variety of injuries to skin and mucous membranes, depending on the type of HPV involved. Some types of HPV are classified as high oncogenic risk as associated with the onset of cancer. The tumors most commonly associated with HPV are cervical and oropharyngeal cancer, epigenetic mechanisms related to HPV infection include methylation changes to host and viral DNA and chromatin modification in host species. This review is focused about epigenethic mechanism, such as MiRNAs expression, related to cervix and oral cancer. Specifically it discuss about molecular markers associated to a more aggressive phenotype. In this way we will analyze genes involved in meiotic sinaptonemal complex, transcriptional factors, of orthokeratins, sinaptogirin, they are all expressed in cancer in a way not more dependent on cell differentiation but HPV-dependent.
Introduction
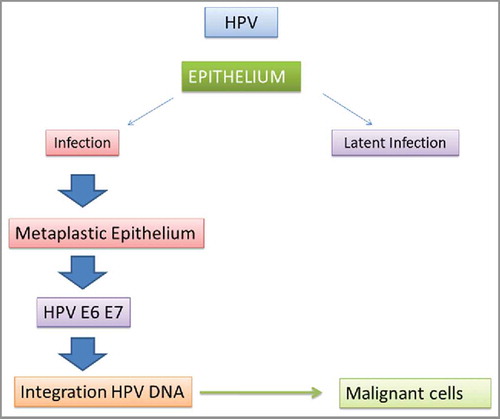
Papillomaviruses are circular, double-stranded DNA viruses with a size of about 8 Kb normally composed of 8 early genes (E1-E7) and 2 late genes (L1-L2). Genes E1 and E2 express 2 regulatory proteins and 3 oncoproteins are expressed by E5, E6 and E7 genes. Genes L1 and L2 encodes for capsid proteins. Human Papilloma Virus infection is very frequent in the humans and is mainly transmitted sexually. The majority of infections are transient and asymptomatic, however, if the infection persists, it can occur with a variety of injuries to skin and mucous membranes, depending on the type of HPV involved. Some types of HPV are classified as high oncogenic risk as associated with the onset of cancer. The tumor most commonly associated with HPV is the cervical cancer which is the first cancer to be recognized by the World Health Organization (WHO) entirely due to an infection. The known types of papilloma viruses are 160,Citation1 divided into 16 groups progressively designated with the letters A to P on the basis of their DNA sequence homologies. Papilloma viruses are also classified in skin and mucous according to their tissue specificity. Most of the viruses of this family do not cause serious diseases, such as skin warts. Some, however, may cause benign tumors such as genital wart and even malignant such as cancer of the cervix, the mouth, the anus, esophagus and the larynx. In particular, carcinomas of the uterine cervix are triggered by genotypes 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, where the types 16 and 18 are the most frequent, while the simple warts and warts are generated by genotypes 2, 7 (common warts), 1, 2, 4 (plantar warts) and 3, 10 (flat warts).
The HPV involvement in oral carcinogenesis was first proposed in 1983 by Syrjanen et al.Citation2 Nevertheless, the association between HPV and oral squamous cell carcinoma (OSCC) is until now not well defined, especially because of the wide range of HPV DNA detection in OSCC, varying from 0–100%.Citation3-Citation5 Basing on their association with cervical carcinogenesis, HPV types are classified as “low risk” (LR HPV), such as HPV-6 and −11, which are found in benign epithelial proliferations, and “high-risk” HPV (HR HPV), such as HPV-16 and 18, which are detected in up to 99% of high-grade cervical squamous intraepithelial lesions and invasive cervical carcinomas (CC). The expression of oncogenic HPV proteins (E5, E6, E7) should be able to down-regulate critical tumor suppressor (p53, pRB, p-16) in head and neck cancers comparing them to analog findings in cervical cancers (). The early virus proteins E6 and E7 act synergistically in maintaining the neoplastic cell phenotype preventing apoptosis. The combined actions of these oncoproteins, leads to p53 degradation, anti-apoptotic signaling and cell cycle promotion. Due to their binding properties and inactivating tumor suppressor genes such as p53 and Rb, proteins encoded by the viral genome promote intense cellular replication producing the formation of papillomas, warts, focal epithelial hyperplasia and cancers. The mechanisms that control the gene expression of HPV involve DNA methylation. The transcription of proteins E6 and E7 is directed by regulatory elements in the Long Control Region (LCR), which also contain the binding sites for the viral protein E2 that regulates the oncogene, expression. Since the majority of human neoplasms are characterized by the imbalance of the regulatory cell-cycle control processes, the expression study of proteins involved in the critical check-points of cell growth could elucidate the effects of the alteration of the cell death and growth ratio on the carcinogenetic process. Major efforts are currently made toward the chance to interfere with the 3 major anti- proliferative pathways influencing oncogenesis, namely, inhibition of cell growth, induction of differentiation and apoptosis. The proteins codified by the gene family of Inhibitor of Apoptosis (IAP) target a downstream step in apoptosis. Survivin is a recently characterized IAP protein, which is abundantly expressed in most solid and haematologic malignancies, but undetectable in normal adult tissues. Recent studies have also shown a survivin overexpression in oral SCC and a possible relation between HPV positivity and survivin expression.Citation3 A possible role of survivin in cervical carcinogenesis has been suggested.
Survivin expression tends to increase along with tumor progression and seems to be more closely related to the progression of cervical SCC compared with Fas-ligand, Fas and bcl−2. The first cause of death for oral SCC are nodal metastases; their incidence is significantly correlated with the loss of intercellular adherence of tumor cells. Intercellular adhesiveness is mediated by a glycoproteins family, named cadherins. In epithelial cells, homotypic cell-cell adhesiveness is mediated by epithelial-cadherin (E-cad). Down-regulation of E-cadherin was reported to be directly related to invasiveness and progression of many human epithelial tumors.Citation6 The importance of catenins in tumor progression has been suggested by the identification of tumor cell lines lacking β- or gamma-catenin.Citation7 Normally, catenins are membranous proteins and the free cytoplasmic pool of β-catenin is small. In fact, activated protein C (APC) forms a complex with β-catenin, axin and GSK-3β, which promotes the rapid degradation of β-catenin.Citation8 Elevation of the free pool of cytoplasmic β-catenin is the result of the inactivation of the APC-system or activation of the WNT-1 pathway. Normally, the WNT signal stabilizes free β-catenins, while mutation in APC or β-catenin mimic WNT signaling, stimulating cell proliferation or antagonizing apoptosis.Citation9 Some studies have investigated the possible role of catenins in oral SCC.Citation10,Citation11 A study supports the existence of an inverse relationship between the expression of β and gamma catenin and the degree of cellular differentiation,Citation12 as well the reduced expression of β and/or gamma catenin seems to be related with the progression of cervical SCC.Citation13 The transformation of human keratinocyte infected by HPV requires activation of the WNT pathway; this activation may serve as a screening tool in HPV-positive populations to detect malignant progression.Citation14 In the last decade the scientific research has shown a growing interest on several biologic markers (e.g. MIB-1, PCNA, cyclin D1) expressed during the cell cycle to use them as indexes of oral-cervical SCC progression and aggressiveness, also in relation to HPV-DNA detection. On the basis of these tendencies, the present review aims to examine the molecules involved in the control of cellular proliferation, like PCNA, Mib-1 and cyclin D1. In this regard an high expression of PCNA was found in oral and cervical SCC HPV-positive, due to HPV-related oncogenetic promotion.Citation15 Other studies, have been shown an aberrant expression of cyclin D1 in the majority of oral and cervical SCC: this situation resulted tightly related to their unfavorable prognosis.Citation16 Immunohistochemical detection of MIB1 (Ki-67) has been suggested to determine proliferation index in oral and cervical SCC.Citation17 Toll-like receptors (TLRs) are present on numerous cell types, also in human cells and have the ability to detect and bind distinct pathogen-associated ligands, thus signaling the presence of an invading microbe and consequently initiating an immune response against it.Citation18 In this review we will discuss about the expression of different type of TLRs in HPV+ and HPV- lesions to define the expression of receptor proteins associated with host immune response against virus infected cervix and oral cancerous cells.
Recent studies indicate that epigenetic regulation of gene expression has emerged as a fundamental pathway in the pathogenesis of numerous malignancies, including cancers of the oropharyngeal tract.Citation19,Citation20 Particular interest has been dedicated to the epigenetic silencing of SFRPs, WIF1 and DKK3 with the following chronic activation of WNT. Inactivation of several tumor-suppressor genes has been also attributed to aberrant hyper-methylation of their promoter regions (E-cadherin, p16, p15, hMLH1, hMSH2, MGMT, DAPK, RUNX3). We focus on small noncoding RNAs (MiRNAs) which play an essential role in the epigenetic regulation of gene expression. MiRNAs' function in the regulation of gene expression by binding the transcription product (mRNAs), and causing its degradation or blocking its translation.Citation21,Citation22
MicroRNAs are considered useful early diagnostic and prognostic markers of cancer, candidates for therapeutic intervention, and targets for basic biomedical research, also in cervix and oropharyngeal cancers. We will focus on the epigenetic regulation of multiple genes may be considered a common event in cancer.
The basis of HPV pathogenesis
It is by now assessed that the persistent infection of high-risk HPV is the main cause of cervical cancer. As reported in literature cervical cancer is the second most common cancer among women. Among 160 HPV genotypes 13 are classified as high-risk types regard the development of cervical cancer with the most common types 16 and 18. Since HPV transmission occurs mainly by skin-to-skin contact it is greatly influenced by sexual activity, especially among individuals having multiple partners. Furthermore, the virus is particularly resistant to heat and dehydration thus its propagation can occur by exposure to contaminated clothes.
The HPV infection proceeds through the binding of L1 capsid protein with the cellular membrane receptors of cervical epithelial cells, heparin sulfate proteoglycan molecules exposed on the cell surface are engaged in the binding. As consequence of binding to proteoglycan the capside undergoes to a conformational change triggers by cyclophilin B which enhance the infection, thus promoting initiation and progression of cancer. Indeed, multiple receptors interactions were found to be involved in the binding,Citation23 it has been hypothesized that the HPV infection involves several proteins among which HSPGs (HvSPG2 and SDC2), CyPB(PPIB), α 6 integrin (ITGA6), tetraspanins(TSPAN1), GFR(EGFR and FGFR2) and A2t. Furthermore, it has been found that receptor genes possess single nucleotide polymorphisms (SNP) expressing a certain variability which can affect the virus infection process and outcome of diseases. Thus the cervical lesions progression could be depended by the receptor gene other than virus variability. This finding increases the uncertainty in the prognosis of infection. Recently,Citation24 a paper concerning a case-control study of 1584 cases shows that TNFAIP8 a protein induced by tumor necrosis factor-α is associated with oral and cervical cancer risk. TNFAIP8 family expresses anti-apoptotic and pro-oncogenic activity, a single nucleotide polymorphisms of TNFAIP8 gene (TNFAIP8-rs11064 SNP) has found to be responsible of an increase of cancer risk. In fact, TNFAIP8 expression can be regulated by this SNP gene that functions by affecting the affinity of miR-22 binding to the 3-UTR of TNFAIP8.
HPV gene profile in oral and uterus cervix cancers
Epigenetic and Genetic profile may be useful in clinical practice, improving the current diagnostic tools and contributing indirectly, together with the classical histo-pathological parameters, to therapeutics as predictor of choice for the correct clinical management of patients. The epigenetic fingerprint of HPV related HN-cancer is very different from HPV unrelated cancers. Epigenetic fingerprint together with gene expression panel can differentiate cancers with opposite therapeutical strategies. In fact HPV related cancers are chemo- and radiotherapy sensitive whereas HPV unrelated cancer need the development of new therapy.
Despite being more advanced at diagnosis, HPV-positive head and neck SCC (HNSCC) is associated with better response to current radio-chemotherapy and longer survival than HPV-negative tumors. Thus, HPV-positive and- negative HNSCC appear to be clinically and biologically distinct.
Several studies, primarily on the high risk HPV16, have documented that the viral genome methylation status changes not only during the viral life cycle but also in the context of the progressive neoplastic disease that culminates in cancer. Most of these studies have focused on the high risk HPV most commonly associated with human cancer, HPV16, with a few studies looking at other high risk HPVs. Until recently, studies have focused on evaluating the HPV methylation status of the long control region (LCR; also referred to as the upstream regulatory region or URR) which contains the viral promoter driving expression of early genes including the viral oncogenes E6 and E7. These oncogenes are frequently found expressed in HPV-associated neoplastic disease including cancers, the viral transcriptional enhancer and the viral origin of DNA replication. Also commonly analyzed are regions neighboring the LCR on the circular double stranded DNA genome, including the 3′ region of L1, which is upstream of the LCR, and the E6 gene, which is downstream of the LCR. Studies have used the use of methylation-sensitive restriction endonucleases and/or bisulfite treatment to distinguish methylated from unmethylated cytosines. In the latter case, the standard approach consists to PCR amplification of bisulfite-treated DNA using regional specific primers followed by sequencing of the amplimer products directly or after cloning. The latter having the advantage that the methylation status of individual copies of the viral genome can be identified instead of the average methylation state of multiple genomes. More recently deep sequencing technologies have been used to interrogate the methylation status of bisulfite treated DNA, allowing one to look at the whole viral genome, as opposed to specific PCR-amplified regions. The general conclusions are that the DNA methylation state of the HPV genome is dynamic both in the context of the viral life cycle and associated neoplastic disease. The latter point has led to the suggestion that the methylation status of the HPV genome may be diagnostic if not prognostic of neoplastic progression as detailed below. The methylation dynamic status associated to neoplastic progression supports the hypothesis that epigenetic regulation of viral gene expression is an important factor in HPV-associated disease. Another observation is that the viral genome is likely subjected to de novo methylation by host DNA methyltransferases (DNMTs) implicated in innate responses to pathogens. Thus methylation of the viral genome may be in part a mechanism by which the host attempts to suppress viral gene expression and thereby HPV pathogenicity (dadoi:10.1016/j.virol.2013.07.016.).
Kim et al.Citation25 provided the first analysis of the methylation status of the HPV16 genome in the context of the viral life cycle. This study analyzed the methylation status of the LCR in a clonal cell line, W12E, derived from an HPV16-positive CIN,Citation26,Citation27 that harbors the viral genome as an extrachromosomal nuclear plasmid and that upon induction of differentiation supports the productive stage of the viral life cycle as evidenced by the production of virus particles.Citation28 Their analyses demonstrated that host DNA methyltransferases activity modified de novo the viral genome given the pattern of cytosine methylation at not only CpG dinucleotides but other CpX dinucleotides. They also observed, that upon induction of differentiation, the viral LCR becomes hypomethylated, demonstrating that the methylation status of the viral genome is dynamic. But they also observed an increase in methylation within the region of the LCR containing the early promoter. Interestingly among the sites hypermethylated in the promoter region were sites within the viral E2 transcription factor binding sites that are thought to mediate transcriptional repression of the early promoter.
Kalantari et al.,Citation29 who analyzed the LCR and adjoining regions of the HPV16 genome by sequencing of bisulfite treated DNA first observed that methylation within the 3- region of the L1 gene was associated with neoplastic progression: this region was heavily methylated in cervical cancers but poorly methylated in low grade CINs. These findings were validated by several follow-up studiesCitation30-Citation33 including 2 studies that evaluated the methylation status of the whole genomeCitation34 in which hypermethylation of not only the HPV16 L1 gene but also the L2 and E5 genes were observed in more severe oral and cervix neoplastic disease ().
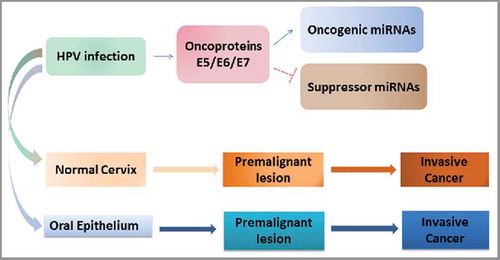
Figure 2. The Interplay of HPV-regulated miRNAs with malignant transformation in oral and cervical cancer.
The increased methylation found in E2, L2, and L1 genes was associated with increased risk of CIN3 in a serial sets of samples from women. Thus increases in methylation of the structural genes and E2 may not only be diagnostic of high grade CIN and cancer but also of potential prognostic value in predicting progression.
During neoplastic progression, the high risk HPV genomes often are found to become integrated into the host genome. Integration frequently arist fragile sites within the host genome, sites prone to undergoing genetic recombination.Citation35,Citation36 These recombination events commonly occur within the 3′ early region of the virus and often result in the deletion of 3′ part of the viral genome to the recombination break point, with continued if not enhanced expression of the 2 viral oncogenes E6 and E7.
Two types of integration are found in HPV-associated neoplasias.Citation26 Type 1 integration events occur when a single copy of the viral genome becomes integrated in the host chromosome. Type 2 integration takes place when a concatomer of viral genomes integrates into a site within the host chromosome. In both cases one can find situations in which the integrated viral genomes and neighboring host DNA are amplified. In the cases of type 2 integration events, it is often found that only the 3′ most copy of the viral genome (the one that is disrupted by the integration event) is transcriptionally active.
Unfortunately, sufficient attention has not been given to the state of the HPV genome in most methylation studies, and only one study has distinguished between type 1 vs type 2 integration events. In that study using clonal W12 lines derived from the HPV16-positive CIN,Citation31 differences in methylation status were observed, with type 2 integration events associated with greater hypermethylation of the LCR. This finding is consistent with the hypothesis that internal copies of the viral genome are epigenetically silenced.
Oropharyngeal cancer and MiRNAs
It is believed that cancer is a genetic and epigenetic disease, usually involving altered expression profiles of tumor-suppressor genes and of proto-oncogenes.Citation37 MicroRNAs (miRNAs) are endogenous small noncoding RNAs involved in tumorigenesis. MiRNAs have profound effects on epigenetic machinery. Besides, their control is also affected by epigenetic mechanisms. Indeed, a reduction in miRNAs accelerates oncogenic transformation thorough the deregulation of target oncogenes.Citation38
MiRNAs alterations correlate with cell proliferation, development, differentiation and metastasis in different tumor types. Several studies focused on miRNA profiling in HNC have been published in recent years.Citation39 They have identified specific miRNAs as candidate oncogenes and tumor-suppressor genes in head and neck squamous cell carcinoma.Citation40,Citation41 Interestingly, the investigation of a panel of 9 head and neck cancer cell lines and 261 mature miRNA gene identified specific subsets of miRNAs that provided candidate molecular signatures characteristic of the tumor-derived cell lines belonging to this cancer.Citation42 Results showed evidence of overexpression of 33 miRNAs, beside 22 miRNAs with low levels of expression. MiR-21 showed an overexpressed level, as for other kinds of malignancies, and was suggested to be a cancer-specific miRNA.Citation42 However, in contrast to many cancer cell lines (breast, lung, prostate, colorectal..), head and neck demonstrate overexpression of miR-205r that could be a specific marker of OSCC.Citation43 Functional changes of miRNAs could result from their genetic variants leading to different expressions of their target genes. Furthermore, a polymorphism in the complementary miRNA-binding site could also lead to miRNA disrupted expression. An SNP at the miR-885–5p or the let-7 binding sites have been associated with an increased risk of HNSCC and reduced survival.Citation44,Citation45
Although distinct miRNA profiles were shown in head and neck cancer only a few studies analyzing the miRNA profile in HNC with regard to HPV presence have been published to date. More than smoking and alcohol consumption, HPV infection is also recognized as another primary cause of HNC.Citation46 A recent study profiled HPV-positive and HPV-negative of HNC and identified the “HPV core” miRNAs. High-risk HPV infection leads to aberrant expression of cellular oncogenic and tumor suppressive miRNAs.Citation47
Many researchers have focused on the occurrence of epigenetic phenomena in OSCC developmentCitation48 Epigenetic changes represent an important mechanism for the silencing of tumor suppressor genes.Citation49 Further miRNA genes are subject to epigenetic changes. In fact, deregulation of DNA methylation alter the expression of some miRNAs. Hypermethylation causes downregulation of potentially tumor suppressive miRNAs such as miR-137 and −193a.Citation22,Citation50,Citation51 Whereas hypomethylation can cause overexpression of miRNA genes such as miR-663, which has been found to induce chemotherapy resistance in cancer.Citation52 Similarly the promoter regions associated with specific miRNAs can also be abnormally methylated leading to up or downregulation, as in the case of miR-137. This has shown some promise as a biomarker for Head Neck Squamous Carcinoma.Citation53
It is urgently needed to develop biomarkers for early clinical detection, diagnosis, prognosis, and novel effective therapies for oral carcinoma.Citation43 The knowledge of miRNA and its potential to play critical roles in a broad range of biologic activities, therefore creates new understanding of head and neck cancer development. Altered expression of miRNAs could be novel molecular biomarkers for the definite diagnosis of cancer, metastatic site, cancer stage, and its progression
Several attempts have been made by researches to identify a specific biomarker for a timely diagnosis and progression monitoring, at moment the more promising markers seem to be the specific virus DNA methylation or miRNA. However, final goal in the study of HPV infection will be to understand how the virus escapes the host immune system, the knowledge of this mechanism could open the door to a definitive therapy for this desease.
Cervical cancer and MiRNAs
Cervical cancer is one of the most common cancers in women worldwide that results of multistep process. Cervical cancer is usually associated with high-risk human papillomaviruses, which stratification of histological types from normal through to invasive carcinoma is well characterized and supported by molecular techniques based on HPV genotyping.Citation54 However, such infection is not sufficient to induce the malignant transformation. Hence, there is an urgent need for the identification of rapid and efficient molecular biomarkers of screening and diagnosis.
MicroRNAs are small non-coding RNAs with important functions in several biologic, and deregulation of these molecules was widely associated with human pathologies. MicroRNA expression is severely disrupted in carcinogenesis and specific dysregulated miRNAs may act as biomarkers of patients' outcome in different neoplasias.Citation55 Further, recent studies have shown that miRNA levels are significantly deregulated in a range of cancers including cervical cancer.Citation56 Indeed, a lot of miRNAs have been described to be closely associated with prognosis and susceptibility of cervical cancer. Furthermore, miRNAs acting as oncogenes (oncomiRs) or tumor suppressors, play important role in the regulation of numerous biologic processes such as cell proliferation, apoptosis, invasion and migration in cervical cancer.Citation57,Citation58
Several reports showed specific overexpression or underexpression of miRNAs in cervical cancer tissues or cell lines compared with normal cervical epithelial tissues that differ according to the particular tumor types.Citation59 Signature of miRNA expression in different histological samples, from normal to invasive cancer, is sometimes heterogeneous. Globally, many microarrays and TaqMan quantitative real-time polymerase chain reaction (PCR) analysis have demonstrated a highly preferential increased expression of recognized oncomirs such miR-20a,Citation60 miR-21,Citation61 miR-24,mir-146a, miR-155, miR-205 in cervical cancer. However, many others miRNAs are downregulated (e.g., miR-34a, miR-100, miR-let7b, miR-143, miR-145).Citation62,Citation63
Interestingly, the altered regulation of cellular oncogenic and tumor-suppressive miRNAs has been reported in association with HPV infection and cervical cancer. In high-risk HPV infections, several studies have been addressing on the impact of viral oncoproteins interactions with the aberrant expression of multiple miRNAs.Citation64,Citation65 Indeed, changes in the expressions of multiple miRNAs such as miR-34a,miR-218, miR-29a and miR-146a are attributed to interactions with viral oncoproteins E5, E6 and E7, which subsequently contribute to the initiation and progression of cervical cancer.Citation66 Yet, the detailed network of the events has not been fully elucidated. Expression level of miR-29 is upregulated in cervical cancer, and was suggested to be regulated by HPV oncoproteins E6/E7.Citation67 In contrast, miR196a expression is reduced in HPV16- positive cervical cancer cell lines. It has been suggested that HPV16 E5 specifically down-regulates miR196a upon infection of the human cervix facilitating the transformation of normal cervix cells to cervical carcinoma.Citation68,Citation69 Further, miR-34a showed also a reduced expression occurring in an early-onset event in the development of HPV associated cervical cancer. The oncoprotein E6 was suggested to reduce miR-34a expression in the p53-dependent pathway during precancerous lesions, although the exact mechanisms remain unclear.Citation70 miR-100 expression showed a reduced level in late phase of HPV associated cervical carcinogenesis. But, miR-100 inhibition would not be directly affected by HPV E6/E7 in cervical cells. Furthermore, HPV oncoproteins have reported to regulate crosstalk between miRNA levels and key cellular signaling pathways such STAT3.Citation71 It is well known that STAT3 play a crucial role that links chronic inflammation to cancer. Lee et al. strongly indicated a functional implication of altering levels of either miR-21, let-7a or STAT3 signaling on each other in cervical cancer cell lines and the loop was found controlled by HPV oncoprotein E6.Citation72 Besides, miRNAs, such as miR-125b and miR-203 seem to play an important role in the regulation of viral gene expression and DNA replication.
Conclusions
Collectively, several results support the involvement of miRNA in the pathogenesis and development of oral and cervical cancer. Further investigations are needed to bring more discoveries on miRNAs to develop new biomarkers associated to tumor progression, clinical outcome, or use them as therapeutic agents for these cancers.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Zou J, Cao Z, Zhang J, Chen T, Yang S, Huang Y, Hong D, Li Y, Chen X, Wang X, et al. Variants in human papillomavirus receptor and associated genes are associated with type-specific HPV infection and lesion progression of the cervix. Oncotarget 2016; 7:40135-47; PMID:27223085; https://doi.org/10.18632/oncotarget.9510
- Syrjänen K, Syrjänen S, Lamberg M, Pyrhönen S, Nuutinen J. Morphological and immunohistochemical evidence suggesting human papillomavirus (HPV) involvement in oral squamous cell carcinogenesis. Int J Oral Surg 1983; 12:418-24; PMID:6325356; https://doi.org/10.1016/S0300-9785(83)80033-7
- Al-Bakkal G, Ficarra G, McNeill K, Eversole LR, Sterrantino G, Birek C. Human papilloma virus type 16 E6 gene expression in oral exophytic epithelial lesions as detected by in situ rtPCR. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1999; 87:197-208; PMID:10052376; https://doi.org/10.1016/S1079-2104(99)70273-8
- Terai M, Burk RD. Characterization of a novel genital human papillomavirus by overlapping PCR: candHPV86 identified in cervicovaginal cells of a woman with cervical neoplasia. J Gen Virol 2001; 82:2035-40; PMID:11514712; https://doi.org/10.1099/0022-1317-82-9-2035
- Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev 2005; 14:467-75; PMID:15734974; https://doi.org/10.1158/1055-9965.EPI-04-0551
- Birchmeier W. E-cadherin as a tumor (invasion) suppressor gene. Bioessays 1995; 17:97-9; PMID:7748170; https://doi.org/10.1002/bies.950170203
- Sommers CL, Gelmann EP, Kemler R, Cowin P, Byers SW. Alterations in beta-catenin phosphorylation and plakoglobin expression in human breast cancer cells. Cancer Res 1994; 54:3544-52; PMID:8012979
- Yost C, Torres M, Miller JR, Huang E, Kimelman D, Moon RT. The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev 1996; 10:1443-54; PMID:8666229; https://doi.org/10.1101/gad.10.12.1443
- Peifer M. Beta-catenin as oncogene: the smoking gun. Science 1997; 275:1752-3; PMID:9122680; https://doi.org/10.1126/science.275.5307.1752
- Bagutti C, Speight PM, Watt FM. Comparison of integrin, cadherin, and catenin expression in squamous cell carcinomas of the oral cavity. J Pathol 1998; 186:8-16; PMID:9875134; https://doi.org/10.1002/(SICI)1096-9896(199809)186:1<8::AID-PATH156>3.0.CO;2-H
- Williams HK, Sanders DS, Jankowski JA, Landini G, Brown AM. Expression of cadherins and catenins in oral epithelial dysplasia and squamous cell carcinoma. J Oral Pathol Med 1998; 27:308-17; PMID:9725568; https://doi.org/10.1111/j.1600-0714.1998.tb01962.x
- Lo Muzio L, Staibano S, Pannone G, Grieco M, Mignogna MD, Cerrato A, Testa NF, De Rosa G. Beta- and gamma-catenin expression in oral squamous cell carcinomas. Anticancer Res 1999; 19:3817-26; PMID:10628318
- Fadare O, Reddy H, Wang J, Hileeto D, Schwartz PE, Zheng W. E-Cadherin and beta-Catenin expression in early stage cervical carcinoma: a tissue microarray study of 147 cases. World J Surg Oncol 2005; 3:38; PMID:15969753; https://doi.org/10.1186/1477-7819-3-38
- Uren A, Fallen S, Yuan H, Usubütün A, Küçükali T, Schlegel R, Toretsky JA. Activation of the canonical Wnt pathway during genital keratinocyte transformation: a model for cervical cancer progression. Cancer Res 2005; 65:6199-206; PMID:16024621; https://doi.org/10.1158/0008-5472.CAN-05-0455
- Soares CP, Benatti Neto C, Fregonezi PA, Teresa DB, Santos RT, Longatto Filho A, Maeda MY. Computer-assisted analysis of p53 and PCNA expression in oral lesions infected with human papillomavirus. Anal Quant Cytol Histol 2003; 25:19-24; PMID:12630078
- Zhao M, Kim YT, Yoon BS, Kim SW, Kang MH, Kim SH, Kim JH, Kim JW, Park YW. Expression profiling of cyclin B1 and D1 in cervical carcinoma. Exp Oncol 2006; 28:44-8; PMID:16614707
- Leung TW, Xue WC, Cheung AN, Khoo US, Ngan HY. Proliferation to apoptosis ratio as a prognostic marker in adenocarcinoma of uterine cervix. Gynecol Oncol 2004; 92:866-72; PMID:14984954; https://doi.org/10.1016/j.ygyno.2003.11.051
- Barton GM. Viral recognition by Toll-like receptors. Semin Immunol 2007; 19:33-40; PMID:17336545; https://doi.org/10.1016/j.smim.2007.01.003
- Sogabe Y, Suzuki H, Toyota M, Ogi K, Imai T, Nojima M, Sasaki Y, Hiratsuka H, Tokino T. Epigenetic inactivation of SFRP genes in oral squamous cell carcinoma. Int J Oncol 2008; 32:1253-61; PMID:18497987; https://doi.org/10.3892/ijo.32.6.1253
- Pannone G, Bufo P, Santoro A, Franco R, Aquino G, Longo F, Botti G, Serpico R, Cafarelli B, Abbruzzese A, et al. WNT pathway in oral cancer: epigenetic inactivation of WNT-inhibitors. Oncol Rep 2010; 24:1035-41; PMID:20811686; https://doi.org/10.3892/or_00000952
- Bentwich I, Avniel A, Karov Y, Aharonov R, Gilad S, Barad O, Barzilai A, Einat P, Einav U, Meiri E, et al. Identification of hundreds of conserved and nonconserved human microRNAs. Nat Genet 2005; 37:766-70; PMID:15965474; https://doi.org/10.1038/ng1590
- Berezikov E, Guryev V, van de Belt J, Wienholds E, Plasterk RH, Cuppen E. Phylogenetic shadowing and computational identification of human microRNA genes. Cell 2005; 120:21-4; PMID:15652478; https://doi.org/10.1016/j.cell.2004.12.031
- Kämper N, Day PM, Nowak T, Selinka HC, Florin L, Bolscher J, Hilbig L, Schiller JT, Sapp M. A membrane-destabilizing peptide in capsid protein L2 is required for egress of papillomavirus genomes from endosomes. J Virol 2006; 80:759-68; PMID:16378978; https://doi.org/10.1128/JVI.80.2.759-768.2006
- Shi TY, Cheng X, Yu KD, Sun MH, Shao ZM, Wang MY, Zhu ML, He J, Li QX, Chen XJ, et al. Functional variants in TNFAIP8 associated with cervical cancer susceptibility and clinical outcomes. Carcinogenesis 2013; 34:770-8; PMID:23299407; https://doi.org/10.1093/carcin/bgt001
- Kim K, Garner-Hamrick PA, Fisher C, Lee D, Lambert PF. Methylation patterns of papillomavirus DNA, its influence on E2 function, and implications in viral infection. J Virol 2003; 77:12450-9; PMID:14610169; https://doi.org/10.1128/JVI.77.23.12450-12459.2003
- Jeon S, Allen-Hoffmann BL, Lambert PF. Integration of human papillomavirus type 16 into the human genome correlates with a selective growth advantage of cells. J Virol 1995; 69:2989-97; PMID:7707525
- Jeon S, Lambert PF. Integration of human papillomavirus type 16 DNA into the human genome leads to increased stability of E6 and E7 mRNAs: implications for cervical carcinogenesis. Proc Natl Acad Sci U S A 1995; 92:1654-8; PMID:7878034; https://doi.org/10.1073/pnas.92.5.1654
- Flores ER, Lambert PF. Evidence for a switch in the mode of human papillomavirus type 16 DNA replication during the viral life cycle. J Virol 1997; 71:7167-79; PMID:9311789
- Kalantari M, Calleja-Macias IE, Tewari D, Hagmar B, Lie K, Barrera-Saldana HA, Wiley DJ, Bernard HU. Conserved methylation patterns of human papillomavirus type 16 DNA in asymptomatic infection and cervical neoplasia. J Virol 2004; 78:12762-72; PMID:15542628; https://doi.org/10.1128/JVI.78.23.12762-12772.2004
- Balderas-Loaeza A, Anaya-Saavedra G, Ramirez-Amador VA, Guido-Jimenez MC, Kalantari M, Calleja-Macias IE, Bernard HU, Garcia-Carranca A. Human papillomavirus-16 DNA methylation patterns support a causal association of the virus with oral squamous cell carcinomas. Int J Cancer 2007; 120:2165-9; PMID:17278110; https://doi.org/10.1002/ijc.22563
- Kalantari M, Villa LL, Calleja-Macias IE, Bernard HU. Human papillomavirus-16 and −18 in penile carcinomas: DNA methylation, chromosomal recombination and genomic variation. Int J Cancer 2008; 123:1832-40; PMID:18688866; https://doi.org/10.1002/ijc.23707
- Kalantari M, Chase DM, Tewari KS, Bernard HU. Recombination of human papillomavirus-16 and host DNA in exfoliated cervical cells: a pilot study of L1 gene methylation and chromosomal integration as biomarkers of carcinogenic progression. J Med Virol 2010; 82:311-20; PMID:20029805; https://doi.org/10.1002/jmv.21676
- Kalantari M, Garcia-Carranca A, Morales-Vazquez CD, Zuna R, Montiel DP, Calleja-Macias IE, Johansson B, Andersson S, Bernard HU. Laser capture microdissection of cervical human papillomavirus infections: copy number of the virus in cancerous and normal tissue and heterogeneous DNA methylation. Virology 2009; 390:261-7; PMID:19497607; https://doi.org/10.1016/j.virol.2009.05.006
- Brandsma JL, Sun Y, Lizardi PM, Tuck DP, Zelterman D, Haines GK, Martel M, Harigopal M, Schofield K, Neapolitano M. Distinct human papillomavirus type 16 methylomes in cervical cells at different stages of premalignancy. Virology 2009; 389:100-7; PMID:19443004; https://doi.org/10.1016/j.virol.2009.03.029
- Popescu NC, DiPaolo JA. Integration of human papillomavirus 16 DNA and genomic rearrangements in immortalized human keratinocyte lines. Cancer Res 1990; 50:1316-23; PMID:2153457
- Smith PP, Friedman CL, Bryant EM, McDougall JK. Viral integration and fragile sites in human papillomavirus-immortalized human keratinocyte cell lines. Genes Chromosomes Cancer 1992; 5:150-7; PMID:1381951; https://doi.org/10.1002/gcc.2870050209
- Garzon R, Marcucci G, Croce CM. Targeting microRNAs in cancer: rationale, strategies and challenges. Nat Rev Drug Discov 2010; 9:775-89; PMID:20885409; https://doi.org/10.1038/nrd3179
- Ji W, Sun B, Su C. Targeting MicroRNAs in Cancer Gene Therapy. Genes (Basel) 2017; 8:E21; PMID:28075356; https://doi.org/10.3390/genes8010021
- Zhang M, Zhao LJ, Liang WQ, Mao ZP. Identification of microRNAs as diagnostic biomarkers in screening of head and neck cancer: a meta-analysis. Genet Mol Res 2015; 14:16562-76; PMID:26681002; https://doi.org/10.4238/2015.December.11.3
- Irani S. miRNAs Signature in Head and neck squamous cell carcinoma metastasis: A literature review. J Dent (Shiraz) 2016; 17:71-83; PMID:27284551
- Koshizuka K, Hanazawa T, Fukumoto I, Kikkawa N, Okamoto Y, Seki N. The microRNA signatures: aberrantly expressed microRNAs in head and neck squamous cell carcinoma. J Hum Genet 2017; 62:3-13; PMID:27557665; https://doi.org/10.1038/jhg.2016.105
- Tran N, McLean T, Zhang X, Zhao CJ, Thomson JM, O'Brien C, Rose B. MicroRNA expression profiles in head and neck cancer cell lines. Biochem Biophys Res Commun 2007; 358:12-7; PMID:17475218; https://doi.org/10.1016/j.bbrc.2007.03.201
- Gomes CC, Gomez RS. MicroRNA and oral cancer: future perspectives. Oral Oncol 2008; 44:910-4; PMID:18620891; https://doi.org/10.1016/j.oraloncology.2008.01.002
- Guan X, Liu Z, Liu H, Yu H, Wang LE, Sturgis EM, Li G, Wei Q. A functional variant at the miR-885-5p binding site of CASP3 confers risk of both index and second primary malignancies in patients with head and neck cancer. FASEB J 2013; 27:1404-12; PMID:23271051; https://doi.org/10.1096/fj.12-223420
- Christensen BC, Moyer BJ, Avissar M, Ouellet LG, Plaza SL, McClean MD, Marsit CJ, Kelsey KT. A let-7 microRNA-binding site polymorphism in the KRAS 3′ UTR is associated with reduced survival in oral cancers. Carcinogenesis 2009; 30:1003-7; PMID:19380522; https://doi.org/10.1093/carcin/bgp099
- Chawla JP, Iyer N, Soodan KS, Sharma A, Khurana SK, Priyadarshni P. Role of miRNA in cancer diagnosis, prognosis, therapy and regulation of its expression by Epstein-Barr virus and human papillomaviruses: With special reference to oral cancer. Oral Oncol 2015; 51:731-7; PMID:26093389;https://doi.org/10.1016/j.oraloncology.2015.05.008
- Vojtechova Z, Sabol I, Salakova M, Smahelova J, Zavadil J, Turek L, Grega M, Klozar J, Prochazka B, Tachezy R. Comparison of the miRNA profiles in HPV-positive and HPV-negative tonsillar tumors and a model system of human keratinocyte clones. BMC Cancer 2016; 16:382; PMID:27377959; https://doi.org/10.1186/s12885-016-2430-y
- Gasche JA, Goel A. Epigenetic mechanisms in oral carcinogenesis. Future Oncol 2012; 8:1407-25; PMID:23148615; https://doi.org/10.2217/fon.12.138
- Mascolo M, Siano M, Ilardi G, Russo D, Merolla F, De Rosa G, Staibano S. Epigenetic disregulation in oral cancer. Int J Mol Sci 2012; 13:2331-53; PMID:22408457; https://doi.org/10.3390/ijms13022331
- Cuesta Gil M, Bucci T, Navarro Cuellar C, Duarte Ruiz B, Pannone G, Bufo P, Navarro Vila C. Intraosseous myoepithelioma of the maxilla: clinicopathologic features and therapeutic considerations. J Oral Maxillofac Surg 2008; 66:800-3; PMID:18355609; https://doi.org/10.1016/j.joms.2007.06.002
- Pannone G, Rodolico V, Santoro A, Lo Muzio L, Franco R, Botti G, Aquino G, Pedicillo MC, Cagiano S, Campisi G, et al. Evaluation of a combined triple method to detect causative HPV in oral and oropharyngeal squamous cell carcinomas: p16 Immunohistochemistry, Consensus PCR HPV-DNA, and In Situ Hybridization. Infect Agent Cancer 2012; 7:4; PMID:22376902; https://doi.org/10.1186/1750-9378-7-4
- Wilting SM, Miok V, Jaspers A, Boon D, Sørgård H, Lando M, Snoek BC, van Wieringen WN, Meijer CJ, Lyng H, et al. Aberrant methylation-mediated silencing of microRNAs contributes to HPV-induced anchorage independence. Oncotarget 2016; 7:43805-19; PMID:27270309; https://doi.org/10.18632/oncotarget.9698
- Langevin SM, Stone RA, Bunker CH, Grandis JR, Sobol RW, Taioli E. MicroRNA-137 promoter methylation in oral rinses from patients with squamous cell carcinoma of the head and neck is associated with gender and body mass index. Carcinogenesis 2010; 31:864-70; PMID:20197299; https://doi.org/10.1093/carcin/bgq051
- Roychowdhury A, Samadder S, Islam MS, Chaudhury K, Roy A, Banerjee D, Mandal R, Basu PS, Roychoudhury S, Panda CK. Identification of changes in the human papilloma virus 16 (HPV16) genome during early dissemination of cervical cancer cells may complement histological diagnosis of lymph node metastasis. Pathol Oncol Res 2017; PMID:28101801; https://doi.org/10.1007/s12253-017-0189-3
- Bahrami A, Aledavoud SA, Anvari K, Hassanian SM, Maftouh M, Yaghobzade A, Salarzaee O, ShahidSales S, Avan A. The Prognostic and Therapeutic Application of microRNAs in Breast Cancer: Tissue and Circulating microRNAs. J Cell Physiol 2017; PMID:28109133; https://doi.org/10.1002/jcp.25813
- Pereira PM, Marques JP, Soares AR, Carreto L, Santos MA. MicroRNA expression variability in human cervical tissues. PLoS One 2010; 5:e11780; PMID:20668671; https://doi.org/10.1371/annotation/2ae645ec-9413-4f7d-b51f-eb0678fa2f1b
- Cheung TH, Man KN, Yu MY, Yim SF, Siu NS, Lo KW, Doran G, Wong RR, Wang VW, Smith DI, et al. Dysregulated microRNAs in the pathogenesis and progression of cervical neoplasm. Cell Cycle 2012; 11:2876-84; PMID:22801550; https://doi.org/10.4161/cc.21278
- Lee JW, Choi CH, Choi JJ, Park YA, Kim SJ, Hwang SY, Kim WY, Kim TJ, Lee JH, Kim BG, et al. Altered MicroRNA expression in cervical carcinomas. Clin Cancer Res 2008; 14:2535-42; PMID:18451214; https://doi.org/10.1158/1078-0432.CCR-07-1231
- He Y, Lin J, Ding Y, Liu G, Luo Y, Huang M, Xu C, Kim TK, Etheridge A, Lin M, et al. A systematic study on dysregulated microRNAs in cervical cancer development. Int J Cancer 2016; 138:1312-27; PMID:26032913; https://doi.org/10.1002/ijc.29618
- Zhao S, Yao D, Chen J, Ding N, Ren F. MiR-20a promotes cervical cancer proliferation and metastasis in vitro and in vivo. PLoS One 2015; 10:e0120905; PMID:25803820; https://doi.org/10.1371/journal.pone.0120905
- Bumrungthai S, Ekalaksananan T, Evans MF, Chopjitt P, Tangsiriwatthana T, Patarapadungkit N, Kleebkaow P, Luanratanakorn S, Kongyingyoes B, Worawichawong S, et al. Up-Regulation of miR-21 Is Associated with Cervicitis and Human Papillomavirus Infection in Cervical Tissues. PLoS One 2015; 10:e0127109; PMID:26010154; https://doi.org/10.1371/journal.pone.0127109
- Banno K, Iida M, Yanokura M, Kisu I, Iwata T, Tominaga E, Tanaka K, Aoki D. MicroRNA in cervical cancer: OncomiRs and tumor suppressor miRs in diagnosis and treatment. ScientificWorldJournal 2014; 2014:178075; PMID:24516357; https://doi.org/10.1155/2014/178075
- Gocze K, Gombos K, Juhasz K, Kovacs K, Kajtar B, Benczik M, Gocze P, Patczai B, Arany I, Ember I. Unique microRNA expression profiles in cervical cancer. Anticancer Res 2013; 33:2561-7; PMID:23749909
- Harden ME, Prasad N, Griffiths A, Munger K. Modulation of microRNA-mRNA Target Pairs by Human Papillomavirus 16 Oncoproteins. MBio 2017; 8:e02170-16; PMID:28049151 https://doi.org/10.1128/mBio.02170-16
- Li J, Liu Q, Clark LH, Qiu H, Bae-Jump VL, Zhou C. Deregulated miRNAs in human cervical cancer: functional importance and potential clinical use. Future Oncol 2017; 13(8):743-53; PMID:27806630; https://doi.org/10.2217/fon-2016-0328
- Li B, Hu Y, Ye F, Li Y, Lv W, Xie X. Reduced miR-34a expression in normal cervical tissues and cervical lesions with high-risk human papillomavirus infection. Int J Gynecol Cancer 2010; 20:597-604; PMID:20442590; https://doi.org/10.1111/IGC.0b013e3181d63170
- Li Y, Wang F, Xu J, Ye F, Shen Y, Zhou J, Lu W, Wan X, Ma D, Xie X. Progressive miRNA expression profiles in cervical carcinogenesis and identification of HPV-related target genes for miR-29. J Pathol 2011; 224:484-95; PMID:21503900; https://doi.org/10.1002/path.2873
- Liu C, Lin J, Li L, Zhang Y, Chen W, Cao Z, Zuo H, Chen C, Kee K. HPV16 early gene E5 specifically reduces miRNA-196a in cervical cancer cells. Sci Rep 2015; 5:7653; PMID:25563170; https://doi.org/10.1038/srep07653
- Villegas-Ruiz V, Juárez-Méndez S, Pérez-González OA, Arreola H, Paniagua-García L, Parra-Melquiadez M, Peralta-Rodríguez R, López-Romero R, Monroy-García A, Mantilla-Morales A, et al. Heterogeneity of microRNAs expression in cervical cancer cells: over-expression of miR-196a. Int J Clin Exp Pathol 2014; 7:1389-401; PMID:24817935
- Ribeiro J, Sousa H. MicroRNAs as biomarkers of cervical cancer development: a literature review on miR-125b and miR-34a. Mol Biol Rep 2014; 41:1525-31; PMID:24402874; https://doi.org/10.1007/s11033-013-2998-0
- Shishodia G, Shukla S, Srivastava Y, Masaldan S, Mehta S, Bhambhani S, Sharma S, Mehrotra R, Das BC, Bharti AC. Alterations in microRNAs miR-21 and let-7a correlate with aberrant STAT3 signaling and downstream effects during cervical carcinogenesis. Mol Cancer 2015; 14:116; PMID:26051842; https://doi.org/10.1186/s12943-015-0385-2
- Shishodia G, Verma G, Srivastava Y, Mehrotra R, Das BC, Bharti AC. Deregulation of microRNAs Let-7a and miR-21 mediate aberrant STAT3 signaling during human papillomavirus-induced cervical carcinogenesis: role of E6 oncoprotein. BMC Cancer 2014; 14:996; PMID:25539644; https://doi.org/10.1186/1471-2407-14-996