ABSTRACT
Objective: This study was designed to compare the clinical efficacy and safety of transcatheter arterial chemoembolization (TACE) combined with apatinib and TACE alone in the treatment of intermediate and advanced hepatocellular carcinoma (HCC). Methods: From March 2015 to August 2015, a total of 44 patients with moderate and advanced HCC, who were admitted in the Navy General Hospital of China, were included into this study. These patients were randomly divided into 2 groups: group A and group B. Patients in group A underwent TACE alone, while patients in group B underwent the combined treatment of TACE with apatinib. Differences in preoperative general data between these 2 groups were not statistically significant (P > 0.05). All patients were followed up for 12–18 months. Changes in α-fetal protein (AFP) at 3 months after treatment and the objective response rate (ORR) at 3, 6, 9 and 12 months after treatment were compared between these 2 groups. Furthermore, progression-free survival (PFS) and the incidence of adverse reactions were also compared between these 2 groups. Results: AFP levels in groups A and B significantly decreased after 3 months of treatment, compared with the levels before treatment, and the differences were statistically significant (P < 0.05). However, at 3 months after treatment, the difference between these 2 groups was not statistically significant (P > 0.05). ORR at 3, 6, 9 and 12 months after treatment was 36.36%, 27.27%, 13.64% and 9.09%, respectively, in group A; and 60%, 50%, 45% and 35%, respectively, in group B. At 3 and 6 months after treatment, the differences between these 2 groups were not statistically significant (P > 0.05); while at 9 and 12 months after treatment, the differences between these 2 groups were statistically significant (P < 0.05). The median PFS was 6.0 months in group A and 12.5 months in group B, and the difference was statistically significant (P < 0.05). The incidences of complications were related to oral apatinib, such as hypertension, hand-foot syndrome and proteinuria, were higher in group B than in group A, and the differences were statistically significant (P < 0.05). These symptoms all alleviated after symptomatic treatments. Conclusions: For intermediate and advanced HCC, the long-term curative effect of TACE combined with apatinib is better than that of TACE alone. The former can obviously prolong the PFS of patients and has a confirmed safety.
Introduction
Primary hepatocellular carcinoma (HCC) is one of the most common malignant tumors in the world, and is the second cause of cancer-related deaths.Citation1 According to the Barcelona Clinic Liver Cancer (BCLC) clinical staging system, the standard treatment of HCCs in BCLC stages B and C are transcatheter arterial chemoembolization (TACE) and oral sorafenib.Citation2 Sorafenib is the first molecular targeted drug approved for the treatment of liver cancer. However, its therapeutic effect is limited; and no other alternative therapy can work once drug resistance occurs. Vascular endothelial growth factor receptor (VEGFR)-2 is closely associated to the occurrence of liver cancer. Apatinib mesylate is a novel VEGFR-2 inhibitor that has the highest selectivity, it has 10 times binding affinity of VEGFR-2 tyrosine kinase than that of sorafenib. This drug can block the migration and proliferation of vascular endothelial cells, reduce tumor microvessel density, and inhibit tumor growth.Citation3 In addition, apatinib can also inhibit multiple ATP binding sites, reverse multidrug resistance and improve the efficacy of traditional chemotherapy drugs.Citation4,5 Studies have revealed that the number of vascular endothelial growth factor (VEGF)-positive cells in residual liver cancer tissues significantly increased after TACE operation, and VEGF-A level significantly increased in patients with rapid progression after TACE, promoting neovascularization.Citation6,7 Therefore, due to the characteristics of the selective inhibition on VEGFR-2, apatinib may inhibit tumor neovascularization after TACE, and thereby play an anticancer role. When concurrently used, it may increase anti-tumor treatment efficacy.
At present, the phase II clinical trial of apatinib for patients with BCLC stage B or C of HCCs has been completed. The trail reveals that apatinib is effective in treating moderate and advanced HCCs. The objective response rate (ORR, complete remission [CR]+ partial remission [PR]) was 5.00%, and the disease control rate (DCR, CR+PR+ stable disease [SD]) was 43.80% in 121 HCCs as evaluated at the end of 3 treatment cycles. Serum AFP level was significantly decreased after the treatment. The therapeutic effects of apatinib for liver cancer has been recognized to a certain extent.Citation8 The phase III clinical trial is being performed. In this single-center randomized study, efficacy and adverse reactions between TACE combined with apatinib and TACE alone in treatment of moderate and advanced HCCs are compared with provide more clinical bases for the combined treatment of TACE and apatinib for moderate and advanced HCC. At present, similar studies have not been reported.
Data and methods
Inclusion and exclusion criteria
Inclusion criteria: (1) patients with HCC diagnosed by biopsy pathology; (2) patients who were in stage B or C according to the BCLC staging system, and could not tolerate surgical resection or refused surgical resection; (3) patients with Child-Pugh A or B liver function; (4) patients without heart, lung oror kidney dysfunction; (5) patients with an Eastern Cooperative Group Performance Status (ECOG) score of ≤ 2 points; (4) patients who did not receive other treatments before the operation.
Exclusion criteria: (1) patients with severe dysfunction of the heart, lung, kidney and other important organs; (2) patients with chemotherapy contraindications: white blood cell count <3× 109/L, platelet count <40× 109/L, or hemoglobin <60 g/L; (3) patients with severe coagulation disorders (prothrombin time >18 seconds or hemorrhagic tendency); (4) patients with hepatic artery-portal vein fistula or tumor thrombi in the middle trunk or in the left and right trunks of the hepatic vein; (5) patients with large amounts of ascites or refractory ascites; (6) patients with metastasis in other distant organs; (7) patients who discontinued treatment due to severe adverse reactions of TACE (such as Child-Pugh score C, liver abscesses, hepato-renal syndrome,etc.) or stopped taking apatinib for more than one month due to various reasons.
General clinical data
From March 2015 to August 2015, a total of 58 patients with moderate and advanced HCCs, who were admitted in the Navy General Hospital of China, were enrolled into this study. All patients were confirmed to have HCCs by biopsy and were in line with the diagnostic criteria of primary liver cancer. Among these patients, 14 patients were excluded based on exclusion criteria, including 6 patients with distant metastasis, 2 patients with portal vein trunk tumor thrombosis, one patient with refractory ascites, one patient with renal dysfunction and 4 patients who have received other strategies before. All patients were fully informed of the study and provided a signed informed consent that stated their willingness to participate in the randomized controlled trial. According to the random number table method, the 44 included HCC patients were randomly divided into 2 groups: group A and group B (n = 22, each group). General clinical data such as age, gender, the etiology of liver cancer (hepatitis B, hepatitis C, alcoholic liver and others), the primary tumor diameter and Child-Pugh grade are shown in . Differences in preoperative general data between these 2 groups were not statistically significant (P > 0.05, ).
Table 1. Comparison of 2 groups of patients with general data.
Therapeutic methods
Patients in group A underwent TACE alone, while patients in group B underwent the combined treatment of TACE and oral apatinib.
TACE method The Seldinger method was adopted: Conventional femoral artery puncture and intubation were performed, and angiography of proper hepatic artery, superior mesenteric artery, arteria phrenica, left gastric artery and adrenal gland was performed to determine the source of the blood supply of the tumors. General situations of the portal vein were explored by indirect portal vein angiography through the superior mesenteric artery or splenic artery. Next, conventional superselective catheterization of the hepatic artery and other feeding arteries were performed, in which the superselective catheters were inserted to each feeding artery of the tumor lesions using a microcatheter. Subsequently, 30 mg of epirubicin powder, 50 mg of oxaliplatin powder, 10–20 ml of lipiodol and 10–20 ml of iodine contrast agent were prepared into the emulsifier and was slowly infused via the transcatheter; and the feeding arteries were embolized using Embosphere particles (300–500 μm; Merit Medical, USA). The end-point of the embolization was the stagnation of blood flow in the feeding arteries. Routine antiemetic, stomach protecting treatments and other symptomatic treatments, as well as liver protection therapy, were performed after the operation. Abdominal CT, blood biochemistry, and blood routine index were rechecked at 45 d after the operation. TACE would be performed again when the following criteria were met: (1) lipiodol was not found in the lesions, and enhanced CT revealed residual or recurrent lesions; (2) patients had Child-Pugh A or B liver function; (3) patients did not have contraindications of TACE treatment such as serious hepatic and renal damage, uncorrectable coagulation dysfunction, or dyscrasia. When lesions were completely necrotized and AFP was reduced to normal levels, TACE treatment could be stopped. Patients in group A were observed until the lesions progressed, while patients in group B continued to take apatinib orally. Patients who encountered disease progression withdrew from this study and turned to other treatments.
Administration method of apatinib Apatinib was orally taken for the first time 4 d after TACE, and the initial dose was 500 mg/day. The dose was adjusted after 1–2 weeks, according to the tolerance of patients to the drug. The original dose was maintained when the patient had good tolerance or mild adverse reactions. If obvious adverse reactions appeared, the dose was reduced to 250 mg/day; and adverse reactions were observed for a period of time. If patients could still not tolerate the treatment or serious adverse reactions appeared, the discontinuation of the administration of the drug was considered; and the situation of the patient was observed for 1–2 weeks. When adverse reactions degraded or disappeared, the dose was recovered gradually to the original dose. Apatinib was stopped 4 d before the next course of TACE, and was resumed 4 d after TACE. If stopped taking the drug for more than one month, the patient was considered for exclusion from the study.
Evaluation method of therapeutic effect
Enhanced CT or MRI, as well as the examination of serum AFP value, were performed at 3, 6, 9 and 12 months after treatment. The curative effects were evaluated according to the modified response evaluation criteria in solid tumor (mRECIST), which has 4 levels: CR, all enhanced imaging of the target lesions in the arterial phase disappeared; PR, the total reduction of the diameter of the target lesions (enhanced arterial phase) was ≥ 30%; SD, the diameter of the target lesion did not reduce to that in PR and did not increase to that in disease progression (PD); PD, the diameter of the target lesion (enhanced imaging in the arterial phase) increased by at least 20% compared with the baseline value, or new lesions appeared.Citation9 ORR = (CR+PR) / total number of cases × 100%. Secondary endpoints of the study also included progression-free survival (PFS), which was defined as the interval from the beginning of intervention treatment to the time of PD or death. The starting point was the beginning of the first treatment, and deadline was the time of PD or the last follow-up. Other treatments such as radiotherapy or radiofrequency ablation were performed based on the patient's condition when the patient's disease progressed.
Statistics analysis
Statistical analysis was conducted using statistical software SPSS 21.0. Measurement data were expressed as mean ± standard deviation (x¯ ± SD). Intra-group comparison was conducted using paired t-test. Intergroup comparison was conducted using independent samples t-test. Count data were expressed as percentage and counts, and were compared using Chi-square test. Data with non-normal distribution were analyzed using Wilcoxon rank-sum test. The statistics of median PFS was conducted using the Kaplan-Meier method, and the survival rate curve was drawn. Intergroup difference in survival was analyzed using the log-rank test. Inspection level was set as α = 0.05.
Results
In group A, 22 patients were treated with 95 times of TACE, while in group B, 22 patients were treated with 75 times of TACE. There were no serious complications in these 2 groups of patients after TACE. In group B, 2 patients withdrew from the group. Among them, one patient developedmassive upper gastrointestinal tract hemorrhage on the second day after oral administration of apatinib and one course of TACE. The treatment was stopped and the patient withdrew from the group. The other patient developed side effects such as severe hypertension, oral and tongue ulcers. This patient decided to stop the administration of the drug for more than a month, and thereby withdrew from the group. Practically, the curative effects in a total of 42 patients were evaluated (22 patients in group A and 20 patients in group B). There were no significant difference in the basic situations of these 2 groups such as age, gender, Child-Pugh classification, ECOG score, etiology, BCLC staging, primary tumor diameter, AFP level and TACE number between these 2 groups of patients (). All patients were followed-up for 12–18 months. All enrolled patient must be followed up for at least 12 months. The cut-off date for follow-ups was August 15, 2016, namely the date when the last enrolled patient had been followed up for 12 months. The median duration of follow-up for all the patients was 9.65 months.
Comparison of the clinical curative effect between these 2 groups
At 3 months after treatment, AFP levels in groups A and B both significantly decreased. These levels decreased to 67.20 (3.5–265.8) in group A and 43.80 (2.5–209.6) in group B. Furthermore, compared with levels before treatment, the differences were statistically significant (Z: -2.289 and -2.953, respectively; P < 0.05, both). There was no significant difference between the 2 groups (Z = -0.126, P = 0.9 > 0.05). Moreover, AFP levels decreased to normal levels in 5/19 patients in group A, who previously had elevated AFP; while AFP levels decreased to normal levels in 6/16 patients in group B, who previously had elevated AFP.
As shown in , the ORR at 3 and 6 months after treatment were 36.36% (8/22) and 27.27% (6/22), respectively, in group A; while ORR was 60% (12/20) and 50% (10/20), respectively, in group B. Even though there were differences between these 2 groups, these differences were not statistically significant (both, P > 0.05). The ORR at 9 and 12 months after treatment were 13.64% (3/22) and 9.09% (2/22), respectively, in group A; while ORR was 45% (9/20) and 35% (7/20), respectively, in group B. Differences between these 2 groups were statistically significant (P < 0.05, for both). The oral use of apatinib after TACE was advantageous in controlling and reducing the lesions, thereby improving ORR ().
Table 2. In the 2 groups were followed up for 3, 6, 9 and 12 months ORR more.
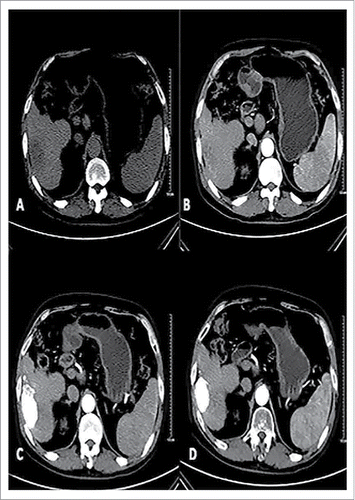
Figure 1. Male patients, 57, primary liver cancer. Patients with preoperative CT scan of the right liver lobe low-density lesions, boundary is not clear, Size of 7.0 * 3.8 cm (A). Enhanced CT of the right liver lobe lesions early uneven arterial enhancement (B).With conventional therapy and oral path for 3 months after, the CT in tumor iodine oil deposits are good in the oven, enhanced scan did not see lesions.Curative effect evaluation for CR (C); Follow-up of 12 months after treatment tumors had the previous narrow (5.0× 2.7 cm), enhanced scan within tumors had no reinforcement (D).
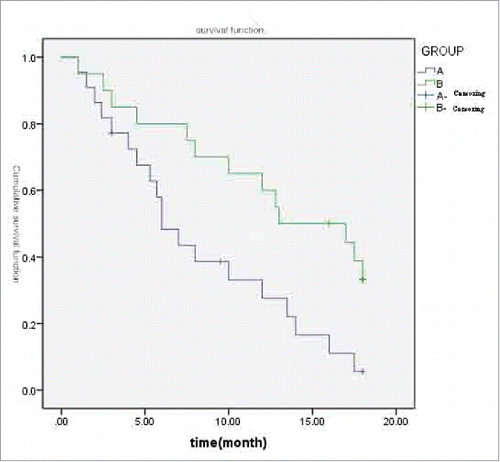
Comparison of PFS between the 2 groups
All patients were followed-up for 12–18 months. The median PFS was 6.0 months in group A and 12.5 months in group B, and the difference between these 2 groups was statistically significant (X2 = 6.576, P = 0.01 < 0.05; ).
Comparison of adverse reactions between these 2 groups
Different extents of postembolization syndrome (PSE) appeared in groups A and B after TACE, including fever, abdominal pain, nausea, vomiting, etc. The incidences in these 2 groups are listed in . Differences between these 2 groups were not statistically significant. There was no significant difference in the incidence of bone marrow suppression induced by chemotherapy drugs (P > 0.05).
Table 3. Compared 2 groups of adverse reactions.
In group B, apatinib-related adverse reactions included the following: hypertension (16 patients, 80%), hand-foot syndrome (11 patients, 55%), proteinuria (9 patients, 45%), diarrhea (4 patients, 20.0%), and oral ulcers (2 patients, 10%). Among those patients, grade 3 or 4 adverse effects occurred in 3 patients. Severe hand-foot syndrome(grade 3) occurred in one patient, severe diarrhea(grade 3) occurred in one patient and massive upper gastrointestinal hemorrhage(grade 4) occurred in one patient. The first patient with severe hand-foot syndrome stopped the treatment of 2 weeks. After symptomatic treatment, hand-foot skin reaction degraded, and the treatment was resumed. The latter 2 patients with severe hand-foot syndrome were excluded due to severe adverse effects. In the rest of patients, their syndromes were alleviated after drug reduction and symptomatic treatments. The incidences of complications related to oral apatinib such as hypertension, hand-foot syndrome and proteinuria were higher in group B than in group A, and the differences were statistically significant (P < 0.05, ). No severe complications such as hypertensive crisis, heart failure, or liver/kidney failure were observed in these 2 groups.
Discussion
China is one of the countries that have a high incidence of HCC in the world. When seeing a doctor after onset, most patients are already in the late stage of the disease. Hence, they cannot tolerate radical surgery. TACE can effectively prolong the survival time of patients with HCC, and improve the 2-years survival rate of patients. Therefore, TACE has become the first-choice non-surgical treatment of HCC.Citation10 The occurrence and metastasis of HCC depend on neovascularization. However, chemoembolization usually cannot completely destroy these tumors, and the local hypoxia environment induced by embolization also stimulates neovascularization in tumors.Citation11 At present, it has been confirmed that there are a variety of angiogenic factors in patients with HCC. Among these factors, vascular endothelial growth factor (VEGF) is the strongest angiogenic factors in the body.Citation12 After TACE, the expression of VEGF in remnant peripheral tumor tissues becomes higher, more invasive and metastatically active.Citation13 These factors increase the invasion and metastasis ability of tumors, and become the basis of disease progression or the production of new lesions. Therefore, inhibiting high expression levels of VEGF in tumor cells induced by TACE may become an important link to improve the long-term efficacy of TACE.
Sorafenib is currently the only oral targeted drug approved to treat advanced HCC. It is a multi-kinase inhibitor that plays an anti-angiogenesis role by exerting on RAF kinase, VEGFR-2, VEGFR-3 and PDGFR-β tyrosine kinase.Citation14 Llovet et al. reported the clinical trial of SHARP. Results revealed that sorafenib could prolong the PFS of patients with advanced HCC by 3 months.Citation15 However, its response rate is very low in HCC ,Citation16 and its curative effect has some limitations when concurrently used with TACE. A multi-center study revealed that TACE combined with sorafenib could prolong mTTP by nearly 2 months, but the difference was not statistically significant.Citation17 A study in China also revealed that TACE combined with sorafenib in the treatment of HCC in BCLC stage B did not show significant advantages, compared with TACE alone.
As a highly selective VEGFR-2 blocker, apatinib can block the migration and proliferation of vascular endothelial cells, decrease tumor microvessel density, and inhibit tumor growth. Its affinity is 10 times of that of sorafenib.Citation3 In a randomized, open-labeled, multi-center Phase II clinical study, the effects of 2 different doses of apatinib on the mOS were compared. The mOS of patients who received 850 mg/d vs. 750 mg/g of apatinib were 9.71 vs. 9.82 months respectively. The time to progression (TTP) were 4.21 vs. 3.32 months in patients with BCLC stage B and C in the phase II clinical trial.Citation8 These were longer compared with those in the study of heart and kidney protection (SHARP).Citation15 Therefore, apatinib combined with TACE can inhibit neovascularization in tumor tissues after TACE, and thereby plays an anti-cancer role. Furthermore, the concurrent administration of apatinib may increase anti-tumor efficacy, providing more choices for patients with advanced HCC
This study revealed that for patients with intermediate and advanced HCC, TACE combined with apatinib treatment could effectively prolong PFS compared with TACE alone; and bring a higher long-term ORR. However, the difference in short-term ORR was not statistically significant. The cause may be as follows: (1) the number of cases in the group was too small to reveal a statistically significant difference; (2) VEGF level increased after TACE and was inhibited by apatinib again, and an amount of time was required to achieve efficacy, thereby allowing this drug to primarily improved the long-term efficacy instead of short-term efficacy in patients. In addition, these 2 treatment options could effectively decrease AFP levels and inhibit tumor growth. This need to be further confirmed by large-sample multi-center randomized controlled studies.
One patient developed severe diarrhea in the TACE combined with apatinib group, who stopped the treatment of more than one month and withdrew from the study. The symptoms gradually relieved after stopping treatment and symptomatic treatment. The remaining patients could all tolerate the adverse reactions, and all symptoms alleviated after symptomatic treatments. There was no significant difference in the incidences of PES after embolization in the 2 groups. The adverse reaction rate was higher in the TACE combined with apatinib group than in the TACE alone group, which mainly manifested as hypertension, hand-foot syndrome, proteinuria and oral ulcer. Among these patients, one patient developed grade 3 hand-foot syndrome, and symptoms improved 2 weeks after stopping treatment and symptomatic treatment. Afterwards, the administration of apatinib was recovered. Overall adverse reactions in patients were acceptable and serious adverse reactions did not occur. Furthermore, its safety was confirmed.
We conducted the first randomized control study to investigate the effects of TACE combined with apatinib in the treatment of HCC. The curative effect of TACE combined with apatinib on intermediate and advanced HCC was better than TACE alone, and the former significantly prolonged the PFS of patients. After symptomatic treatments, most of the adverse reactions did not affect the treatment. Furthermore, its safety was confirmed, which is worth of popularization and application in clinic. However, The shortcomings of our study is small number of enrolled patients and a short time of follow-up. OS is not discussed in this paper. Therefore, perspective randomized controlled study in multi-center need to be conducted to demonstrate the effects and safety of TACE combined apatinib in treatment of HCCs.
Disclosure of potential conflicts of interest
We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
References
- Mittal S, Hashem B, El-Serag . Epidemiology of HCC: Consider the Population[J]. J ClinGastroenterol 2013; 47 Suppl:S2-6; PMID:23632345; https://doi.org/10.1097/MCG.0b013e3182872f29
- Llovet JM, Ducreux M, Lencioni R, Galle PR, Dufour JF, Greten TF, Raymond E, Roskams T, Ducreux M, Bernardi M, et al. Clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012; 56:908-43; PMID:22424438; https://doi.org/10.1016/S0168-8278(12)61409-3
- Peng S, Zhang Y, Peng H, Ke Z, Xu L, Su T, Tsung A, Tohme S, Huang H, Zhang Q, et al. Intracellular autocrine VEGF signaling promotes EBDC cell proliferation, which can be inhibited by Apatinib. Cancer Lett 2016; 373:193-202; PMID:26805764; https://doi.org/10.1016/j.canlet.2016.01.015
- Mi YJ, Liang YJ, Huang HB, Zhao HY, Wu CP, Wang F, Tao LY, Zhang CZ, Dai CL, Tiwari AK, et al. Apatinib (YN968D1) reverses multidrug resistance by inhibiting the efflux function of multiple ATP-binding cassette transporters. Cancer Res 2010; 70:7981-91; PMID:20876799; https://doi.org/10.1158/0008-5472.CAN-10-0111
- Tong XZ, Wang F, Liang S, Zhang X, He JH, Chen XG, Liang YJ, Mi YJ, To KK, Fu LW. Apatinib (YN968D1) enhances the efficacy of conventional chemotherapeutical drugs in side population cells and ABCB1-overexpressing leukemia cells. BiochemPharmacol 2012; 83:586-97; PMID:18568538; https://doi.org/10.1080/02841850801958890
- Wang B, Xu H, Gao ZQ, Ning HF, Sun YQ, Cao GW. Increased expression of vascular endothelial growth factor in hepatocellular carcinoma after transcatheter arterial chemoembolization. ActaRadiol 2008; 49:523-9; PMID:18568538; https://doi.org/10.1080/02841850801958890
- Hsieh MY, Lin ZY, Chuang WL. Serial serum VEGFR-A, angiopoietin-2, and endostatin measurements in cirrhotic patients with hepatocellular carcinoma treated by transcatheter arterial chemoembolization. Kaohsiung J Med Sci 2011; 27:314-22; PMID:21802642; https://doi.org/10.1016/j.kjms.2011.03.008
- Qin SK, Ouyang XN, Bai YX, Cheng Y, Xu JM, Wu W, Li Q, Liu W, Wang D, Liang J, et al. Multicenter phase II study of apatinib, a novel inhibitor of VEGFR, in patients with advanced hepatocellular carcinoma. ASCO annual meeting 2014. Abstract 4019
- Lencioni R, Llovet JM. Modified RECIST(mRECIST)assessment for hepatocellular carcinoma. Semin Liver Dis 2010; 30:52-60; PMID:20175033; https://doi.org/10.1055/s-0030-1247132
- Llovet JM, Bruix J. Systematic review of randomized trials forunresectable hepatocellular carcinoma: Chemoembolizationimproves survival[J]. Hepatology 2003; 37:429-42; PMID:12540794; https://doi.org/10.1053/jhep.2003.50047
- Iwashita YK, Ohta M, Kitano S, Mori M. Preoperative transcatheter arterial chemoembolization reduces long-term survival rate after hepatic resection for resectable hepatocellular carcinoma[J]. Eur J SurgOncol 2006; 32:773-9; PMID:16797156; https://dx.doi.org/10.1016/j.ejso.2006.04.002
- Chen J, Hou EC. Progress of VEGF and its receptors in HCC angiogenesis and antiangiogenesis therapy. Xian Dai Zhong Liu Yi Xue, 2016, 24( 03):498-502
- Kim YB, Berek JS, Maza OM, Satyaswaroop PG. Vascular endothelial growth factor expression is not regulated by estradiol or medroxyprogesterone acetate in endometrial carcinoma[J]. GynecolOncol 1996; 61:97-100; PMID:8626126; https://doi.org/10.1006/gyno.1996.0104
- Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, Chen C, Zhang X, Vincent P, Cao Y, et al. BAY 43–9006 exhibitsbroad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved intumor progression and angiogenesis[J]. Cancer Res 2004; 64:7099-109; PMID:15466206; https://doi.org/10.1158/0008-5472.CAN-04-1443
- Carter C, Tang L, Wilkie D, Rong H, Chen C, Zhang X, Vincent P, Cao Y, Shujath J, Gawlak S, et al. Sorafenib in advancedhepatocellular carcinoma[J]. N Engl J Med 2008; 359:378-90; PMID:18650514; https://doi.org/10.1056/NEJMoa0708857
- Pallota MG, Zarba JJ, Boyer M, Riordan S, Strickland A, Tebbutt N, Thomson B, Borbath I, Barrios C, Takov D, et al. Activation of phosphatidylinositol3-kinase/ Akt signaling pathway mediates acquiredresistance to sorafenib in hepatocellular carcinoma. J PharmacolExpTher 2011; 337:155-61; PMID:21205925; https://doi.org/10.1124/jpet.110.175786
- Kudo M, Imanaka K, Chida N, Nakachi K, Tak WY, Takayama T, Yoon JH, Hori T, Kumada H, Hayashi N, et al. Phase III study ofsorafenib after transarterialchemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma [J]. Eur J Cancer 2011; 47:2117-27; PMID:21664811; https://doi.org/10.1016/j.ejca.2011.05.007