ABSTRACT
Zinc oxide nanoparticles (ZnO-NPs) are being used in many cosmetic products and have been shown to induce tumor-selective cell death in human head and neck squamous cell carcinoma (HNSCC) in vitro. Cetuximab is a monoclonal antibody directed against the epidermal growth factor receptor (EGFR), whose effectiveness for HNSCC, alone or in combination with cytostatic drugs, has been demonstrated intensively in the last decades. Nanoparticles are known to interact with protein structures and thus may influence their functionality. The aim of the current study was to evaluate the effect of ZnO-NPs on the antitumor properties of Cetuximab in HNSCC in vitro. Two HNSCC cell lines (FaDu and HLaC-78) were treated with 0.1, 1 or 10 μM Cetuximab as well as 0, 0.1 or 1 μg/ml ZnO-NP. Qualitative assessment of ZnO-NP was conducted via transmission electron microscopy (TEM) and immunofluorescence staining. Evaluation was done via the MTT-assay after 24, 48 and 72 hours of incubation with Cetuximab and ZnO-NPs. ZnO-NPs were shown to antagonize the anti-tumor effects of Cetuximab in a time-dependent as well as dose-dependent way. These findings suggest an inhibitory interaction of ZnO-NPs with Cetuximab, which warrants further investigation.
Introduction
HNSCC are a major cause of cancer morbidity and mortality. 5 % of all cancer diagnoses in the US are related to HNSCC.Citation1 Despite multi-modal treatment strategies, the survival rates have not improved significantly over the last 40 y.Citation2 For pharyngeal and oral squamous cell carcinoma, the 5-year survival rate is around 60% in the US in 2011.Citation1,3
Apart from classical cytostatic chemotherapy, advances have been achieved by the addition of molecular targeting agents. Cetuximab is a monoclonal antibody of the immunoglobulin G1 subclass (IgG1), which is directed against the epidermal growth factor receptor (EGFR).Citation4,5 Many studies have shown a benefit of adding Cetuximab to an induction chemotherapy regime before radiotherapy for advanced HNSCC.Citation6-8 Cetuximab was also demonstrated to increase overall survival when combined with radiotherapy alone.Citation9 It has been used as an additional agent to a platin-based concurrent chemoradiotherapy (CRT) for locally advanced HNSCC,Citation10-12 although the additional toxicity proves to be problematic. The most frequent use of Cetuximab today, however, is in palliative chemotherapeutic regimes for recurrent or metastatic HNSCC, improving overall survival compared with standard chemotherapy alone.Citation13,14 As part of the EXTREME protocol, it is considered the state-of-the-art therapy in HNSCC studies.Citation15,16
Zinc oxide nanoparticles (ZnO-NPs) are used in a variety of industrial products like cosmetics, paints and medical supplies. Because of their small size of less than 100 nm in diameter, NPs have an extended surface with regard to their mass compared with their bulk substance, thus exhibiting specific physicochemical properties and functions.Citation17,18 ZnO-NPs have been shown to degrade and mineralize environmental pollutants,Citation19 or generate reactive oxygen species (ROS) in combination with UV exposure.Citation20 For titanium dioxide nanoparticles (TiO2-NPs), a photocatalytic elimination of tumor cells was described in colorectal cancer cell linesCitation21 as well as melanoma cell lines.Citation22 ZnO-NPs have also been shown to induce photocatalytic cell death in HNSCC cell lines in vitro.Citation23 This photocatalytical antitumor activity of ZnO-NPs against HNSCC in vitro has been shown to be associated to autophagy.Citation24 Moreover, photo-stimulated ZnO-NPs have been demonstrated to synergistically enhance the cytotoxic effects of chemotherapeutic agents like paclitaxel and cisplatin against HNSCC cell lines in vitro.Citation25 That is why metal oxide nanoparticles are considered as potential and promising adjuvant agents in HNSCC therapy. However, nanoparticles in general are known to interact with protein structures and thereby may influence the functionality of protein structures.
Yet, until now no studies have analyzed the interactions between photoactive NPs and Cetuximab. Therefore, the aim of this study was to investigate possible effects of ZnO-NPs on the Cetuximab-induced cell death of HNSCC in vitro.
Results
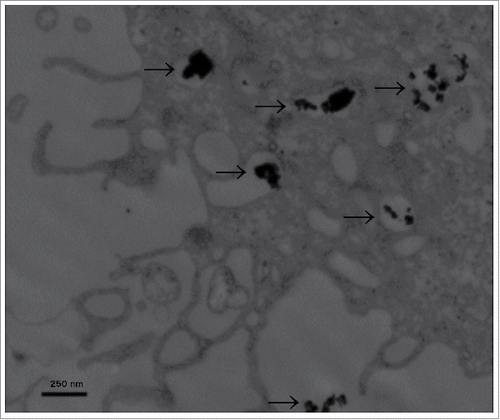
Particle characterization and intracellular distribution. Nanoparticle size distribution was evaluated by dynamic light scattering. The mean diameter of ZnO-NP aggregates was 354 nm. The zeta potential of the NP suspension was −11.2 mV. The ultrastructural evaluation of the intracellular distribution and shape of nanoparticles was done via TEM at a magnification of 40.000x (). ZnO-NPs were spherically shaped and could be seen in small aggregates in cellular compartments like vesicles or organelles as well as free in the cytoplasm. Particle deposition inside of the nucleus was not observed.
Figure 1. Transmission electron microscopy. ZnO-NPs typically clotted together in various intracellular patches of different amounts (indicated by black arrows). Scale bar represents 250 nm, image was taken at 40.000x magnification.
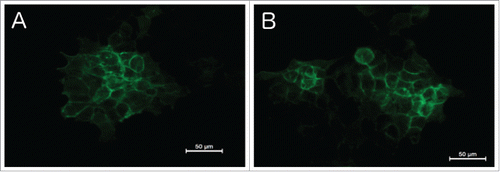
Effect of ZnO-NPs on EGF-receptors. The qualitative antibody staining showed a high expression of the EGF-receptor on the cell membrane of FaDu and HLaC tumor cells in the absence of ZnO-NPs (). After addition of ZnO-NPs, no difference in staining regarding the EGF-receptor intensity could be detected on a qualitative basis (). Since it should only provide a generell overview, quantification of the immunofluorescence staining was not conducted.
Figure 2. Immunofluorescence microscopy of the EGF-Receptor. Regular staining of EGFR on the cell membrane without Zn-NPs (A) as well as after addition of Zn-NPs (B), showing no significant difference.
MTT assay. The MTT assay showed a consecutive decline in tumor cell viability with increased concentration of Cetuximab as well as with increased incubation time.
After 24 hours of incubation with Cetuximab for both cell lines and after 48 hours for HLaC cells, no significant differences were seen after addition of ZnO-NPs (, , , respectively). For FaDu cells, a significantly increased tumor cell survival could be demonstrated at 48 hours when adding 1 µg/ml ZnO-NP to the wells containing 1µM Cetuximab compared with 0 µg/ml (p = 0.0009) and 0.1 µg/ml (p = 0.0003) ZnO-NP (). Two-way ANOVA revealed an overall p-value for the interaction of both substances for FaDu at 48 hours of 0.0182. After 72 hours of incubation, both cell lines showed significant increase in tumor cell viability at 1 µM Cetuximab when adding 1 µg/ml ZnO-NP compared with 0 µg/ml (p = 0.0005 for FaDu; p = 0.0001 for HLaC) and 0.1 µg/ml (p = 0.0007 for FaDu; p = 0.030 for HLaC) ZnO-NP (, respectively). Overall p-values for interaction of Cetuximab and ZnO-NPs at 72 hours were determined at 0.0182 (FaDu) and 0.0545 (HLaC).
Figure 3. MTT-assay for FaDu cell line. Time points were 24 hours (A), 48 hours (B) and 72 hours (C). At 48 and 72 hours, ZnO-NPs increased tumor cell survival at 1 µM Cetuximab, whereas no significant influence was found at 0.1 µM and 10 µM Cetuximab or after 24 hours of incubation.
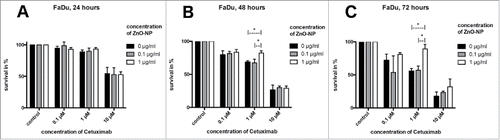
Figure 4. MTT-assay for HLaC cell line. Time points were 24 hours (A), 48 hours (B) and 72 hours (C). At 72 hours, ZnO-NPs increased tumor cell survival at 1 µM Cetuximab, whereas no significant influence was found at 0.1 µM and 10 µM Cetuximab or after 24 and 48 hours of incubation.
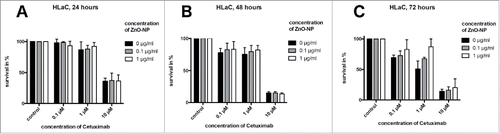
With 0.1 µM and 10 µM Cetuximab, no differences were seen at any time point.
Discussion
The aim of the present study was to evaluate an interaction between zinc oxide nanoparticles and the monoclonal EGFR-antibody Cetuximab in human head and neck squamous cell carcinoma cell lines.
Nanoparticles are being used for various industrial, cosmetical or biomedical applications. Recently, metal oxide nanoparticles have been shown to be promising candidates for topical cancer therapy.Citation26 Due to their larger percentage of molecules at the exposed surface, penetration into tumor sites is enhanced.Citation27 In vitro studies showed a preferential killing of cancer cells for certain metal oxide nanoparticles with few effects on non-tumor cells.Citation28
Zinc oxide nanoparticles in particular have been demonstrated to possess anti-proliferative effects on lymphoblastoidCitation29 and myoblastoma cells.Citation30 The mechanism behind these effects, however, is still largely unknown. Some authors state that ZnO-NPs lead to a rapid intracellular release of zinc ions,Citation31 thereby causing lysosomal and mitochondrial damage.Citation32 Nagajyothi et al. reported of an antioxidant effect of ZnO-NPs to be the mechanism involved in its anti-tumor properties,Citation33 while other studies instead demonstrated an increase in reactive oxygen species (ROS) inside of the cells.Citation34 Regarding the effect of ZnO-NPs on HNSCC, the studies conducted so far proved a photocatalytic cell death of HNSCC cancer cells when exposed to ZnO-NPs and UVA-1.Citation23 The present study was conducted without UV-stimulation of ZnO-NPs and showed no reduction in tumor cell survival with increasing concentrations of ZnO-NPs. Thus, we could show that ZnO-NPs alone, without photo-stimulation, seem to have no anti-tumorigenic effects on HNSCC cell lines in vitro within the applied concentrations. As compared with the literature, the applied NP dosage was lower in this study than in other publications. Often, cytotoxic effects of ZnO-NPs occur at concentrations between 5 and 10 µg/ml.Citation35 Since we aimed to characterize the interactions of ZnO NPs with the tumortoxic agent Cetuximab, we chose a non-toxic concentration of ZnO NPs and a toxic concentration of Cetuximab. Since with photoactivation even those non-toxic concentrations of ZnO-NP have been shown to reduce tumor cell viability (data not shown), we decided against photoactivation to prevent it from masking the desired antagonistic effect on cetuximab. However, a concentration of 1 µg/ml would fit into the effective concentration range of photocatalytic cell killing of ZnO NPs. Thus, the chosen dosages are clinically relevant.
Several studies have shown synergistic effects of UV-stimulated ZnO-NPs and different chemotherapeutic drugs on cancer cells in vitro. Guo et al. demonstrated synergies between photo-stimulated ZnO-NPs and Daunorubicin on human leukemia cell lines,Citation36 while our own group observed synergistic effects with Paclitaxel and Cisplatin on HNSCC cell lines.Citation25 In the latter study, these synergistic effects could only be shown for UVA-treated ZnO-NPs and not for non-photoactivated ZnO-NPs, indicating that photoactivation is a crucial requirement for synergistic effects of ZnO NPs with cytostatic drugs.Citation25
For targeted drugs, few studies containing nanoparticles of any sort have been published so far. Most of these studies address the conjugation of antibodies to iron-oxide or gold nanoparticles to allow in vivo imaging of tumors.Citation37,38 Cetuximab in particular has been conjugated to iron oxide nanoparticles to enhance delivery of the targeted therapy to the tumor cells via magnetic resonance imaging-guidance for glioblastomaCitation37 and EGFR-overexpressing cell lines A431 and 32D/EGFR.Citation39 However, to our knowledge no studies regarding interactions of nanoparticles with Cetuximab on tumor cells have been published so far. In the present study, Cetuximab showed a consecutive decrease in tumor cell survival with increasing concentrations, as was to be expected. In the presence of 1µg/ml ZnO-NPs, the anti-proliferative effects of 1µM Cetuximab were antagonized after 72 hours (for HLaC) and after 48 and 72 hours (for FaDu). These findings suggest a time- and dose-dependent antagonistic effect of ZnO-NPs and Cetuximab on HNSCC cell lines. The cytotoxic effect of Cetuximab at 0.1 µM seemed too weak for an antagonistic effect of ZnO-NPs to show. Cetuximab at 10µM, on the other hand, showed no antagonistic effects with ZnO-NPs either. An interference with the EGFR could be excluded as a mechanism of effects by fluorescence staining. Whether ZnO-NPs interfere with Cetuximab by binding to the Fab part of the molecule or mediate an antagonistic effect in another way remains unclear. Among different biomolecules, proteins are the most important factors which regulate biodistribution of NPs throughout the body as their binding can act as ligand that may favor NP internalization. ZnO NPs are able to bind proteins with important biologic functions, including immunoglobulins, lipoproteins and several others.Citation40 Various factors such as electrostatic or hydrophobic interactions as well as specific chemical processes contribute to these interactions.Citation41
This study must be seen as an example for the sensitive interactions of targeted drugs with nanomaterials. For future applications of nanotechnological therapeutic approaches, such interactions have to be taken into account. As shown in the present study, an exact definition of the relevant concentrations of both agents is required. Further studies are warranted to elucidate these interactions.
In conclusion, the present study could demonstrate an antagonizing effect of ZnO-NPs for Cetuximab-treated HNSCC cell lines in vitro at a certain range of concentrations. These effects seem to be time dependent as well as dose-dependent. Higher concentrations of Cetuximab could negate this effect. To further elucidate which mechanisms are involved in these findings and which implications they have for ZnO-NPs in oncologic research, further investigation is warranted.
Material and methods
Reagent preparation. ZnO-NPs with a diameter <100 nm and a surface area of 15–25 m2/g were obtained as a powder from Sigma-Aldrich (Steinheim, Germany). Particles were suspended in sterilized distilled water before inoculation into the wells. The stock suspension of 50 μg/ml was sonicated (Bandelin, Sonopuls HD 60, Berlin, Germany) for 60 sec at an energy level of 4.2 × 105 kJ/m3 using a continuous mode to create a high grade of dispersion. Bovine serum albumin (BSA) was added at an end concentration of 1.5 mg/ml to stabilize the suspension. Then, 10 X concentrated phosphate-buffered saline (PBS) was added to achieve a physiologic salt concentration and pH 7.4. This stock suspension was subsequently diluted with RPMI-1640 medium (Biochrom AG, Berlin, Germany) supplemented with 10% fetal calf serum (FCS), 100 U/ml penicillin, 100 μg/ml streptomycin, 1% 100 mM sodium pyruvate (Biochrom AG) and 1% of a 100-fold concentration of non-essential amino acids (Biochrom AG).
Characterization of nanoparticles. Size distribution of NP aggregates in RPMI 1640 was evaluated by dynamic light scattering (Malvern Instruments Ltd., Herrenberg, Germany). The NP stock dispersion was prepared as described above and diluted with RPMI 1640 at 1:10 to receive an end concentration of 1 μg/ml. The measurement was performed approximately 1 h after sonication. The surface zeta-potential of the dispersion in the above-mentioned cell culture medium (pH 7.4) was assessed by a ZetaSizer 3000HSA (Malvern Instruments Ltd.).
Cell lines and cell cultures. Two HNSCC cell lines were investigated: The human head and neck squamous carcinoma cell line HLaC 78, established from the lymph node metastasis of a laryngeal squamous cell carcinoma,Citation42 and FaDu cell line, established from a hypopharyngeal carcinoma.Citation43 Cells were cultivated in RPMI-1640 medium (Biochrom AG, Berlin, Germany) supplemented with 10% fetal calf serum (FCS), 100 U/ml penicillin, 100 μg/ml streptomycin, 1% 100 mM sodium pyruvate (Biochrom AG) and 1% of a 100-fold concentration of non-essential amino acids (Biochrom AG). The cells were incubated at 37°C/5% CO2 in 75 or 150 cm2 flasks. Media were replaced every second day and passaging was done before reaching 80% of cell confluence by trypsinization (0.25% trypsin; Gibco, Eggenstein, Germany). Afterwards, cells were washed and seeded in new flasks or treatment wells.
Cell preparation for TEM. For the ultrastructural study of the intracellular distribution of nanoparticles, cell pellets were obtained after 24 h of NP exposure. They were fixed in a fresh solution of 0.1 M sodium cacodylate containing 2.5% glutaraldehyde and 2% formaldehyde followed by a 2-h fixation at 4°C with 2% osmium tetroxide in 50 mM sodium cacodylate (pH 7.2). Staining was performed overnight with 0.5% aqueous uranyl acetate. Specimens were dehydrated, embedded in Epon 812 and ultrathin sections were prepared. Examination of the sections was performed with an EM900 electron microscope (Carl Zeiss, Jena, Germany) and photographic negatives were digitalized by scanning and processed using Adobe Photoshop.
EGF-receptor-staining. Cells were grown on cover slides in 6 well tissue culture plates (Greiner Bio-One GmbH, Frickenhausen, Germany) until 50% confluent, fixed with methanol (−20°C) for 30 min and stained with the mouse-anti-human-EGFR antibody (Amersham, Hamburg, Germany, 1:200) in the presence of 3%BSA/0.3%NP40/PBS for 1 h. Afterwards, the cells were washed in the same buffer (3%BSA/0.3%NP40/PBS) and incubated with a secondary FITC-coupled anti-mouse-antibody (Amersham, Hamburg, Germany, 1:250) for additional 45 min. Fluorescent mounting medium was from DakoCytomation GmbH (Hamburg, Germany). The resulting FITC-signal was visualized by fluorescense microscopy (Leica DMI 4000B Inverted Microscope, Wetzlar, Germany).
Exposure to Cetuximab and ZnO-NP. The anti-human EGFR-specific chimeric monoclonal antibody Cetuximab (Erbitux, Merck Serono GmbH, Darmstadt, Germany) was delivered from the University of Wuerzburg Pharmacy. Cetuximab was diluted to 0.1, 1 or 10 μM and subsequently applied to the regarding wells containing the tumor cells. ZnO-NPs were diluted to 0, 0.1 or 1 μg/ml and subsequently applied to the specific wells. The effects of Cetuximab alone, of ZnO-NPs alone as well as the effects of both combined on tumor cells were evaluated for the different concentrations of both substances. One third of the wells was evaluated after 24 hours, another third after 48 hours and finally the last third after 72 hours, respectively. All experiments were performed in triplicates.
MTT assay. The MTT (Sigma-Aldrich) colorimetric staining method was performed after 1, 2 or 3 d of culture, according to Mosmann,Citation44 to determine cell viability. Cells were seeded at 10,000 cells per well in a 12-well plate. Wells were incubated with 1 ml MTT (1 mg/ml) for 5 h at 37°C and 5% CO2. MTT was then removed and 1 ml isopropanol was added, followed by another incubation period of 1 h at 37°C and 5% CO2. The color conversion of the blue formazan dye was measured using a multi-plate reader (Titertek Multiskan PLUS MK II; Thermo Labsystems, Thermo Fisher Scientific, Inc.) at a wavelength of 570 nm.
Statistical analysis. The data collected was transferred to standard spreadsheets and statistically analyzed using GraphPad Prism software (version 6.0; GraphPad Software, Inc., San Diego, CA, USA). Data are presented as the mean ± standard deviation of 3 experiments, unless otherwise stated. Gaussian distribution was tested via first column analysis. Two-way analysis of variance (ANOVA) followed by Tukey's multiple comparison test was used and multiplicity adjusted p-values were determined, as well as overall significance for interaction between rows and columns. P<0.05 was used to indicate a statistically significant difference.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This study was supported by the Rudolf Bartling Foundation.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016; 66:7-30; PMID:28055103; https://doi.org/10.3322/caac.21387
- Chan GG, Tai BC, Liang S, Lim DT, Soo KC. Squamous cell carcinoma of the head and neck (HNSCC)–multi-modality treatment and impact on survival. Asian J Surg 2002; 25:35-40; PMID:17585443
- Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, et al. Cancer treatment and survivorship statistics, 2016. CA: a cancer journal for clinicians 2016; 66:271-89; PMID:27253694; https://doi.org/10.3322/caac.21349
- Li S, Schmitz KR, Jeffrey PD, Wiltzius JJ, Kussie P, Ferguson KM. Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell 2005; 7:301-11; PMID:15837620; https://doi.org/10.1016/j.ccr.2005.03.003
- Sunada H, Magun BE, Mendelsohn J, MacLeod CL. Monoclonal antibody against epidermal growth factor receptor is internalized without stimulating receptor phosphorylation. Proc Natl Acad Sci U S A 1986; 83:3825-9; PMID:2424012; https://doi.org/10.1073/pnas.83.11.3825
- Kies MS, Holsinger FC, Lee JJ, William WN, Jr., Glisson BS, Lin HY, Lewin JS, Ginsberg LE, Gillaspy KA, Massarelli E, et al. Induction chemotherapy and cetuximab for locally advanced squamous cell carcinoma of the head and neck: results from a phase II prospective trial. J Clin Oncol 2010; 28:8-14; PMID:19917840; https://doi.org/10.1200/JCO.2009.23.0425
- Rampino M, Bacigalupo A, Russi E, Schena M, Lastrucci L, Iotti C, Reali A, Musu A, Balcet V, Piva C, et al. Efficacy and feasibility of induction chemotherapy and radiotherapy plus cetuximab in head and neck cancer. Anticancer Res 2012; 32:195-9; PMID:22213307; http://ar.iiarjournals.org/content/32/1/195.long
- Argiris A, Heron DE, Smith RP, Kim S, Gibson MK, Lai SY, Branstetter BF, Posluszny DM, Wang L, Seethala RR, et al. Induction docetaxel, cisplatin, and cetuximab followed by concurrent radiotherapy, cisplatin, and cetuximab and maintenance cetuximab in patients with locally advanced head and neck cancer. J Clin Oncol 2010; 28:5294-300; PMID:21079141; https://doi.org/10.1200/JCO.2010.30.6423
- Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 2006; 354:567-78; PMID:16467544; https://doi.org/10.1056/NEJMoa053422
- Ma BB, Kam MK, Leung SF, Hui EP, King AD, Chan SL, Mo F, Loong H, Yu BK, Ahuja A, et al. A phase II study of concurrent cetuximab-cisplatin and intensity-modulated radiotherapy in locoregionally advanced nasopharyngeal carcinoma. Ann Oncol 2012; 23:1287-92; PMID:21948811; https://doi.org/10.1093/annonc/mdr401
- Kuhnt T, Sandner A, Wendt T, Engenhart-Cabillic R, Lammering G, Flentje M, Grabenbauer G, Schreiber A, Pirnasch A, Dunst J. Phase I trial of dose-escalated cisplatin with concomitant cetuximab and hyperfractionated-accelerated radiotherapy in locally advanced squamous cell carcinoma of the head and neck. Ann Oncol 2010; 21:2284-9; PMID:20427347; https://doi.org/10.1093/annonc/mdq216
- Pfister DG, Su YB, Kraus DH, Wolden SL, Lis E, Aliff TB, Zahalsky AJ, Lake S, Needle MN, Shaha AR. Concurrent cetuximab, cisplatin, and concomitant boost radiotherapy for locoregionally advanced, squamous cell head and neck cancer: a pilot phase II study of a new combined-modality paradigm. J Clin Oncol 2006; 24:1072-8; PMID:16505426; https://doi.org/10.1200/JCO.2004.00.1792
- Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer HR, Cupissol D, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 2008; 359:1116-27; PMID:18784101; https://doi.org/10.1056/NEJMoa0802656
- Burtness B, Goldwasser MA, Flood W, Mattar B, Forastiere AA, Eastern Cooperative Oncology G. Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: an Eastern Cooperative Oncology Group study. J Clin Oncol 2005; 23:8646-54; PMID:16314626; https://doi.org/10.1200/JCO.2005.02.4646
- Rivera F, Garcia-Castano A, Vega N, Vega-Villegas ME, Gutierrez-Sanz L. Cetuximab in metastatic or recurrent head and neck cancer: the EXTREME trial. Expert Rev Anticancer Ther 2009; 9:1421-8; PMID:19828002; https://doi.org/10.1586/era.09.113
- Vermorken JB, Trigo J, Hitt R, Koralewski P, Diaz-Rubio E, Rolland F, Knecht R, Amellal N, Schueler A, Baselga J. Open-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapy. J Clin Oncol. 2007 Jun 1; 25(16):2171-7; PMID:17538161; https://doi.org/10.1200/JCO.2006.06.7447
- Oberdorster G, Oberdorster E, Oberdorster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect 2005; 113:823-39; PMID:16002369; https://doi.org/10.1289/ehp.7339
- Colvin VL. The potential environmental impact of engineered nanomaterials. Nat Biotechnol 2003; 21:1166-70; PMID:14520401; https://doi.org/10.1038/nbt875
- Yeber MC, Rodriguez J, Freer J, Duran N, Mansilla HD. Photocatalytic degradation of cellulose bleaching effluent by supported TiO2 and ZnO. Chemosphere 2000; 41:1193-7; PMID:10901246; https://doi.org/10.1016/S0045-6535(99)00551-2
- Li D, Haneda H. Morphologies of zinc oxide particles and their effects on photocatalysis. Chemosphere 2003; 51:129-37; PMID:12586145; https://doi.org/10.1016/S0045-6535(02)00787-7
- Zhang AP, Sun YP. Photocatalytic killing effect of TiO2 nanoparticles on Ls-174-t human colon carcinoma cells. World J Gastroenterol 2004; 10:3191-3; PMID:15457572; https://doi.org/10.3748/wjg.v10.i21.3191
- Seo JW, Chung H, Kim MY, Lee J, Choi IH, Cheon J. Development of water-soluble single-crystalline TiO2 nanoparticles for photocatalytic cancer-cell treatment. Small 2007; 3:850-3; PMID:17385208; https://doi.org/10.1002/smll.200600488
- Hackenberg S, Scherzed A, Kessler M, Froelich K, Ginzkey C, Koehler C, Burghartz M, Hagen R, Kleinsasser N. Zinc oxide nanoparticles induce photocatalytic cell death in human head and neck squamous cell carcinoma cell lines in vitro. Int J Oncol 2010; 37:1583-90; PMID:21042728; https://doi.org/10.3892/ijo_00000812
- Hackenberg S, Scherzed A, Gohla A, Technau A, Froelich K, Ginzkey C, Koehler C, Burghartz M, Hagen R, Kleinsasser N. Nanoparticle-induced photocatalytic head and neck squamous cell carcinoma cell death is associated with autophagy. Nanomedicine (Lond) 2014; 9:21-33; PMID:23731458; https://doi.org/10.2217/nnm.13.41
- Hackenberg S, Scherzed A, Harnisch W, Froelich K, Ginzkey C, Koehler C, Hagen R, Kleinsasser N. Antitumor activity of photo-stimulated zinc oxide nanoparticles combined with paclitaxel or cisplatin in HNSCC cell lines. J Photochem Photobiol B, Biol 2012; 114:87-93; PMID:22722055; https://doi.org/10.1016/j.jphotobiol.2012.05.014
- Rasmussen JW, Martinez E, Louka P, Wingett DG. Zinc oxide nanoparticles for selective destruction of tumor cells and potential for drug delivery applications. Expert Opin Drug Deliv 2010; 7:1063-77; PMID:20716019; https://doi.org/10.1517/17425247.2010.502560
- Mo R, Jiang T, Gu Z. Recent progress in multidrug delivery to cancer cells by liposomes. Nanomedicine (Lond) 2014; 9:1117-20; PMID:25118703; https://doi.org/10.2217/nnm.14.62
- Hanley C, Layne J, Punnoose A, Reddy KM, Coombs I, Coombs A, Feris K, Wingett D. Preferential killing of cancer cells and activated human T cells using ZnO nanoparticles. Nanotechnology 2008; 19:295103; PMID:18836572; https://doi.org/10.1088/0957-4484/19/29/295103
- Yin H, Casey PS, McCall MJ, Fenech M. Size-dependent cytotoxicity and genotoxicity of ZnO particles to human lymphoblastoid (WIL2-NS) cells. Environ Mol Mutagen 2015; 56:767-76; PMID:26248212; https://doi.org/10.1002/em.21962
- Chandrasekaran M, Pandurangan M. In Vitro Selective Anti-Proliferative Effect of Zinc Oxide Nanoparticles Against Co-Cultured C2C12 Myoblastoma Cancer and 3T3-L1 Normal Cells. Biol Trace Elem Res 2016; 172:148-54; PMID:26563419; https://doi.org/10.1007/s12011-015-0562-6
- Brun NR, Lenz M, Wehrli B, Fent K. Comparative effects of zinc oxide nanoparticles and dissolved zinc on zebrafish embryos and eleuthero-embryos: importance of zinc ions. Sci Total Environ 2014; 476–477:657-66; PMID:24508854; https://doi.org/10.1016/j.scitotenv.2014.01.053
- Xia T, Kovochich M, Liong M, Madler L, Gilbert B, Shi H, Yeh JI, Zink JI, Nel AE. Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS Nano 2008; 2:2121-34; PMID:19206459; https://doi.org/10.1021/nn800511k
- Nagajyothi PC, Cha SJ, Yang IJ, Sreekanth TV, Kim KJ, Shin HM. Antioxidant and anti-inflammatory activities of zinc oxide nanoparticles synthesized using Polygala tenuifolia root extract. J Photochem Photobiol B 2015; 146:10-7; PMID:25777265; https://doi.org/10.1016/j.jphotobiol.2015.02.008
- Wang C, Hu X, Gao Y, Ji Y. ZnO Nanoparticles Treatment Induces Apoptosis by Increasing Intracellular ROS Levels in LTEP-a-2 Cells. Biomed Res Int 2015; 2015:423287; PMID:26339612; https://doi.org/10.1155/2015/423287
- Vandebriel RJ, De Jong WH. A review of mammalian toxicity of ZnO nanoparticles. Nanotechnol Sci Appl 2012; 5:61-71; PMID:24198497; https://doi.org/10.2147/NSA.S23932
- Guo D, Wu C, Jiang H, Li Q, Wang X, Chen B. Synergistic cytotoxic effect of different sized ZnO nanoparticles and daunorubicin against leukemia cancer cells under UV irradiation. J Photochem Photobiol B 2008; 93:119-26; PMID:18774727; https://doi.org/10.1016/j.jphotobiol.2008.07.009
- Hadjipanayis CG, Machaidze R, Kaluzova M, Wang L, Schuette AJ, Chen H, Wu X, Mao H. EGFRvIII antibody-conjugated iron oxide nanoparticles for magnetic resonance imaging-guided convection-enhanced delivery and targeted therapy of glioblastoma. Cancer Res 2010; 70:6303-12; PMID:20647323; https://doi.org/10.1158/0008-5472.CAN-10-1022
- Karmani L, Labar D, Valembois V, Bouchat V, Nagaswaran PG, Bol A, Gillart J, Levêque P, Bouzin C, Bonifazi D, et al. Antibody-functionalized nanoparticles for imaging cancer: influence of conjugation to gold nanoparticles on the biodistribution of 89Zr-labeled cetuximab in mice. Contrast Media Mol Imaging 2013; 8:402-8; PMID:23740810; https://doi.org/10.1002/cmmi.1539
- Tseng SH, Chou MY, Chu IM. Cetuximab-conjugated iron oxide nanoparticles for cancer imaging and therapy. Int J Nanomedicine 2015; 10:3663-85; PMID:26056447; https://doi.org/10.2147/IJN.S80134
- Deng ZJ, Mortimer G, Schiller T, Musumeci A, Martin D, Minchin RF. Differential plasma protein binding to metal oxide nanoparticles. Nanotechnology 2009; 20:455101; PMID:19822937; https://doi.org/10.1088/0957-4484/20/45/455101
- Roy R, Das M, Dwivedi PD. Toxicological mode of action of ZnO nanoparticles: Impact on immune cells. Mol Immunol 2015; 63:184-92; PMID:25193324; https://doi.org/10.1016/j.molimm.2014.08.001
- Zenner HP, Lehner W, Herrmann IF. Establishment of carcinoma cell lines from larynx and submandibular gland. Arch Otorhinolaryngol 1979; 225:269-77; PMID:548013; https://doi.org/10.1007/BF00455679
- Rangan SR. A new human cell line (FaDu) from a hypopharyngeal carcinoma. Cancer 1972; 29:117-21; PMID:4332311; https://doi.org/10.1002/1097-0142(197201)29:1<117::AID-CNCR2820290119>3.0.CO;2-R
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983; 65:55-63; PMID:6606682; https://doi.org/10.1016/0022-1759(83)90303-4
