ABSTRACT
The procoagulant status of patients with non-small cell lung cancer (NSCLC) after chemotherapy is poorly characterized and the role of platelets in hypercoagulative state of NSCLC is unknown. The aim of this study was to evaluate the procoagulant activity (PCA) of platelets in NSCLC before and after chemotherapy. The subjects were 52 patients newly diagnosed with NSCLC. The patients had decreased clotting time compared with healthy subjects, and the thrombin-antithrombin complex increased 2.5-fold after chemotherapy. Platelets in the patients after chemotherapy had enhanced phosphatidylserine (PS) exposure, and shortened coagulation time as well as increased thrombin and fibrin formation of platelets compared with those before chemotherapy. Platelet-derived microparticles increased 2-fold at day 1 and peaked at day 2 post-chemotherapy. Treatment of cisplatin in vitro also resulted in upregulated intrinsic FXa and thrombin formation on platelets with a dose-dependent manner. Platelets treated with aspirin significantly decreased PCA. However, lactadherin blocked PS and inhibited the PCA approximately by 70%. Seven days after chemotherapy, PCA of platelets restored to the baseline as that before chemotherapy, indicating that within a week of chemotherapy patient platelets are highly procoagulant and effective intervention should be taken in case of thrombosis. Our results suggested that platelets after chemotherapy had elevated PCA and may contribute to the hypercoagulative state of NSCLC. Prophylactic anti-coagulant combined with anti-platelet therapy may play an inhibitory role in thrombotic complications in NSCLC.
Abbreviations
| NSCLC | = | non-small cell lung cancer |
| MPs | = | microparticles |
| PCA | = | procoagulant activity |
| PS | = | phosphatidylserine |
| PMP | = | platelet-derived microparticle |
| TF | = | tissue factor |
Introduction
Cancer-associated thrombosis causes significant morbidity and mortality in cancer patients. Hypercoagulative state and venous thromboembolism (VTE) associated with lung cancer has been documented.Citation1-3 In a retrsospective analysis of a lung cancer, VTE occurred in 13.9% of the lung cancer cohort and 1.4% of the control cohort. About 3% patients newly diagnosed with lung cancer have a tendency to develop VTE within 1 year. Non-small cell lung cancer (NSCLC) accounts for more than 80% lung cancers, and recent studies have shown that increased arterial and venous thrombosis occur in NSCLC and may predict poor prognosis.Citation4,5
Nadir Y et al showed heparanase procoagulant activity (PCA) elevated in NSCLC.Citation6,7 The hypercoagulable state in lung cancer involves several complex interdependent mechanisms, and the current studies focus on the roles of tissue factor (TF), anticoagulant/fibrinolytic system, and platelets.Citation1,3 Tumor cells express TF and spontaneously release TF-positive microparticles into the blood, which may initiate the extrinsic coagulation pathway and contribute to systemic hypercoagulability. The disorders in anticoagulant/fibrinolytic system are also involved in the coagulation abnormalities in NSCLC. Recently, Koldas M et al reported thrombin-activatable fibrinolysis inhibitor activity, F1+2 and tissue factor pathway inhibitor significantly increased in NSCLC.Citation8 A low plasmin inhibitor-plasmin complex/thrombin-antithrombin complex (TAT) was observed in advanced NSCLC.Citation9
Platelets are primary cellular components contributing to coagulation and thrombogenesis, however, the role platelets play in coagulation in NSCLC is not fully understood. Previous studies show that elevated platelet count and platelet/lymphocyte ratio have clinical significance in prognosis of NSCLC.Citation10,11 However, the alterations of platelets in morphology, PCA as well as cell count before and after chemotherapy in NSCLC are unclear. The aim of this study was to evaluate the changes of platelets in morphology and PCA in NSCLC before and after chemotherapy. Chemotherapy is applied in anti-cancer treatment, however, can also damage blood cells especially platelets that are easy to activate. Recent studies show that increased phosphatidylserine (PS) exposed on endothelial cells with the treatment of daunorubicin, resulting in reduced coagulation time and elevated thrombin formation.Citation12 Thus we speculated that drugs in chemotherapy may induce platelets' activation even apoptosis leading to the enhanced PCA in NSCLC.
Materials and methods
Patients
Peripheral venous blood was obtained upon informed consent from 52 healthy subjects who had not received any medication in the past 2 weeks, and from 52 patients newly diagnosed with NSCLC between Dec. 2015 and Jan. 2017. Chemotherapy was applied according to the NCCN guidelines.Citation13 The patients received combined treatment with gemcitabine and cisplatin. Exclusion criteria were age <18 years, cardiovascular disease, diabetes, liver or renal dysfunction, platelets and/or blood coagulation disorders and prior treatment with anticoagulant and/or antiplatelet therapy. Study was performed with the approval of the Ethics Committee of Harbin Medical University in accordance with the Declaration of Helsinki.
Reagents
Bovine serum albumin (BSA), poly-d-lysine, ethylenediaminetetraacetic acid (EDTA), cisplatin were obtained from Sigma-Aldrich (St Louis, MO, USA). Lactadherin was prepared in our laboratory. Human factors Va, VIIa, VIII, IXa, X, Xa, prothrombin and thrombin were all from Haematologic Technologies (Burlington, VT, USA). The Chromogenix substrates S-2238 and S-2765 were from DiaPharma Group (West Chester, OH, USA). Monoclonal antibodies against CD31, CD41a, CD142 were from Becton Dickinson Biosciences (San Jose, CA, USA).
Cell preparation
Peripheral venous blood was drawn with a 21 gauge needle and collected into a 5-ml tube containing 3.8% sodium citrate. Specimens were centrifuged for 15 minutes (min) at 200 g at room temperature (RT); platelet-rich plasma (PRP) was isolated and diluted with modified Tyrode's buffer (137 mM NaCl, 2.7 mM KCl, 11.9 mM NaHCO3, 0.42 mM NaH2PO4, 1 mM MgCl2, 5.5 mM glucose, 5 mM HEPES and 0.35% BSA, pH 7.4) supplemented with 1 mM EDTA. Platelets were pelleted by centrifugation at 1000 g for 5 min and resuspended in Tyrode's buffer. Platelet count was calculated with a blood cell counter (Cellometer, Nexcelom, America).
Microparticles (MPs) were isolated with the method described previously.Citation14 Briefly, samples were centrifuged for 20 min at 1,500 g, and plasma was harvested and re-centrifuged for 2 min at 13,000 g to remove all residual platelets. Platelet-free plasma (PFP) was obtained and snap-frozen in liquid nitrogen, and then stored at −80°C until use. To isolate the MPs, 250 μl of PFP was thawed on ice for 60 min and then centrifuged at 20,000 g for 45 min at 20°C. Subsequently, 225 μl of supernatant (i.e. MP-free plasma) was aspirated and the remaining 25 μl MPs pellet was washed once by centrifugation and resuspended in 75 μl of Tyrode 's buffer (MP enriched suspension).
Flow cytometry
To quantify PS and tissue factor (TF) expression, platelets were adjusted to 1 × 106 cells/ml and incubated with Alexa 647-anti-CD142 (5 nM) and Alexa 488-labeled lactadherin (2 nM) for 15 min in the dark at RT. The MPs-enriched suspension (5 μl) was resuspended in 35 μl Tyrode 's buffer and incubated for 15 min at 4°C in dark independently stained with Alexa 488-anti-CD31 (5 nM) and Alexa 647-anti-CD41a (5 nM). Cells were analyzed on a flow cytometer (FACSA CantoII, Becton Dickinson, USA). Platelet-derived microparticles (PMPs) were identified by anti CD41a.
The number of platelet-derived microparticles were measured as previously descrived with modification.Citation31 Five µl of MPs-enriched suspension was resuspended in 35 µl Tyrode's buffer and incubated for 15 min at 4°C in the dark with Alexa Fluro 488-conjugated CD41a (5 µl)/ Alexa Fluro 647-conjugated CD31 (5 µl) together with beads (1 µm) in a Truecount Tube. Then, the suspension was diluted in 1 ml of Tyrode's buffer and analyzed immediately by flow cytometer (FACSA CantoII, Becton Dickinson, USA). The number of MPs per µl was calculated by Truecount Tube (with a precise number of fluorescent beads 49254 to determine the number of MPs in a sample) after accumulation of 10,000 gated events.
Confocal microscopy
Platelets from healthy subjects and patients were incubated with Alexa Fluro 488 labeled lactadherin (4 nM) to detect PS exposure. Cells were washed to remove unbound proteins and evaluated immediately. The samples were excited with 488 nm emission line of a krypton-argon laser, and narrow bandpass filters were used to restrict emission wavelength overlap. Images were captured with Zeiss LSM 510 Meta confocal microscope (Carl Zeiss Jena GmbH, Jena, Germany).
Electron microscopy
For scanning electron microscopy, platelets were prepared on glass coverslips. Samples were fixed in 2 steps: first with 2.5% glutaraldehyde fixative and stored at 4°C until processed. Following several rinses in 0.1 M Na-cacodylate HCl buffer, samples were then fixed with 1% OsO4 before dehydrated in various concentrations of ethanol. A layer of platinum, approximately 10 nm thick, was sprayed on the samples. Images were examined with a S-3400N Scanning Electron Microscope (Hitachi Ltd., Tokyo, Japan) using an ultra-high-resolution mode.
Thrombin/anti-thrombin complexes
Blood collected was spun twice at 1250 g for 5 min to separate plasma, aliquoted, and stored at −80°C. TAT complexes were analyzed using the Enzygnost TAT ELISA (Siemens Healthcare Diagnostics, Deerfield, IL, USA) according to the manufacturer's protocol. Supplied human TAT at known concentrations was used as a standard.
Clotting times of platelets
Platelets (2 × 106) were suspended in 100 µl Tyrode's buffer. Clotting time was determined by a one-stage recalcification time assay in a STart4-coagulometer (Diagnostica Stago).Citation15 Briefly, 100 µl cell suspension was incubated with 100 µl platelet-poor plasma. After 180 seconds incubation at 37°C, 100 µl of warmed CaCl2 (1.5 mM, final) was added to start the reaction and the clotting time was recorded immediately.
Intrinsic, extrinsic FXa and prothrombinase assays
The formation of intrinsic, extrinsic FXa and prothrombinase was detected with the method described previously with some modifications.Citation16 To evaluate the production of intrinsic FXa, cells were incubated with 1 nM factor IXa, 5 nM factor VIII, 0.2 nM thrombin, 130 nM factor X and 1.5 mM CaCl2 in factor Xa buffer (TBS with 0.2% BSA) at RT for 5 min. The reaction was then stopped by the addition of EDTA to 7 mM final concentration. After the addition of 10 μl S-2765 (0.8 mM, final), generation of factor Xa was quantified immediately at 405 nm on a automatic microplate reader (Tecan Infinite M200) in kinetic mode. The activation of extrinsic FXa was performed with 130 nM factor X, 1 nM factor VIIa and 1.5 mM CaCl2 adding to the samples. Measurement of extrinsic FXa was similar to that of intrinsic FXa. Results were evaluated against the rate of substrate cleavage of a standard dilution of FXa.
For the thrombin generation, cells were incubated with 1 nM factor Va, 0.05 nM factor Xa, 1 μM prothrombin and 1.5 mM CaCl2 in prothrombinase buffer (TBS with 0.05% BSA) for 5 min at RT. Thrombin production was measured with 10 μl S-2238 (0.8 mM, final) after the addition of EDTA at 405 nm in the kinetic microplate reader. Fibrin clots were evaluated as described previously.Citation17 Briefly, isolated platelets were added to re-calcified (1.5 mM, final), pooled platelet free plasma (86.7% plasma, final). Peak turbidities (A405) of fibrin clots were quantified using the Tecan microplate reader.
Statistical analysis
Results are presented as mean ± standard deviation (SD) of at least triplicate measurements. Statistical analysis was performed with Student t-test or ANOVA as appropriate. P<0.05 was considered statistically significant.
Results
Increased PCA after chemotherapy in NSCLC patients
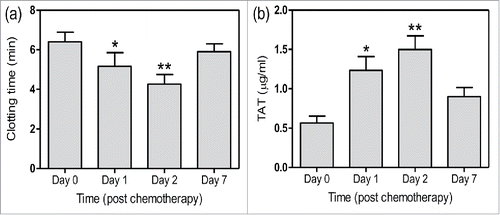
Patients' characteristics before chemotherapy were shown in and . Compared with healthy subjects, patents had decreased aPTT and increased D-dimer as well as TAT generation. Furthermore, we observed the PCA of patients 1, 2 and 7 d after chemotherapy. We found that clotting time of whole blood significantly shortened at day 1, which aggravated at day 2 and nearly restored at day 7 (). TAT in plasm had significant increase at day 1 and 2, and reduced at day 7 (). Platelet counts after chemotherapy were (82.5 ± 32.1) × 109/L (day 1), (116.3 ± 26.5) × 109/L (day 2) and (197.2 ± 33.8) × 109/L (day 7). These results indicate that cellular components are involved in the high PCA and increased thrombin generation in NSCLC patients.
Table 1. Characters of patients with NSCLC before chemotherapy.
Table 2. Characters of platelets in healthy controls and NSCLC patients before chemotherapy.
Elevated PS exposure, PMP generation and PCA of platelets after chemotherapy in NSCLC patients
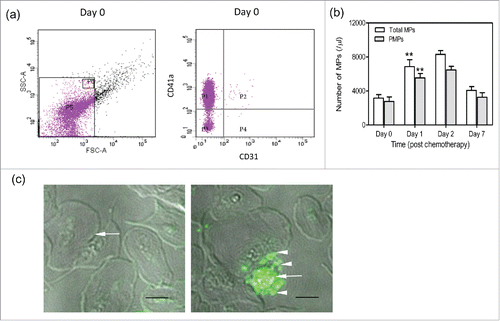
We evaluated the alterations in morphology and PCA of platelets before and after chemotherapy. Platelets were isolated from patients 0, 1, 2, and 7 d after chemotherapy. PS and TF on platelets were identified with lactadherin and anti CD142 antibody by flow cytometry. We found that PS expression on platelets significantly increased at day 1 compared with that at day 0 by flow cytometry (). Confocal microscopy images further showed greater PS exposure on platelets at day 1 and even apoptotic vesicle formation ( right). The quiescent platelets were as control ( left). In addition, patients had significantly increased PS exposure before chemotherapy compared with healthy subjects (data not shown). MPs in serum were isolated and the platelet-derived ones were identified with anti CD41, which accounted for more than 80% of the total MPs (). Results showed that the number of PMPs was also elevated at day 1 to more than 2-fold and peaked at day 2 (). However, we showed little expression of CD142 (TF) on platelets before and after chemotherapy. PCA of platelets peaked at day 1 with significantly reduced coagulation time and increased thrombin and fibrin formation (). At day 7, thrombin and fibrin formation decreased and had no significance compared with those before chemotherapy, leading to the restored coagulation time. These results suggest that platelets have high PCA after chemotherapy, which will significantly reduce within a week.
Figure 2. PS expression and PCA of platelets after chemotherapy. Platelets were isolated from patients before and after chemotherapy. (a) PS and TF expression on platelets were measured respectively with Alexa 488-lactadherin (green) and Alexa 647-anti CD142 (red) by flow cytometry. (b) Coagulation time was measured by coagulometer and thrombin and fibrin formation were evaluated with microplate reader. Data are present as mean ± SD, *P < 0.05 vs day 0, **P < 0.01 vs day 0.
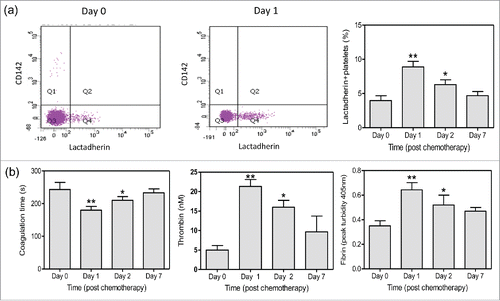
Figure 3. The number of PMPs after chemotherapy. Platelets were isolated from patients before and after chemotherapy. (a,b) The number of total MPs and PMPs were analyzed by flow cytometry. PMPs were characterized by CD41a expression. Data are present as mean ± SD, **P < 0.01 vs day 0. (c) Confocal microscopy images showed quiescent platelets (left, arrow) and apoptotic platelets positive for lactadherin staining (right, arrow) with MPs (right, arrowheads) at day 1. Bars represent 2 µm.
Effects of cisplatin on platelets
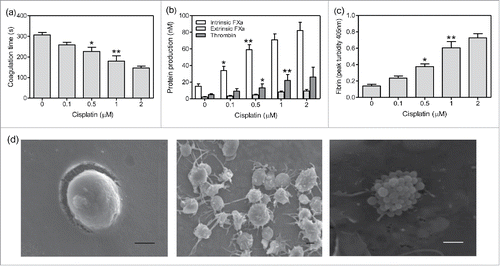
To further illustrate the effect of chemotherapeutic agents on the PCA of platelets, experiments in vitro were performed. Platelets isolated from healthy subjects were treated with cisplatin of different concentrations. Intrinsic FXa significantly increased at 0.1 µM cisplatin (). Increased intrinsic FXa led to elevated thrombin formation at 0.5 µM cisplatin, resulting in increased fibrin and decreased coagulation time (). However, extrinsic FXa on platelets had little increase after treatment with cisplatin (). PCA of platelets elevated in a dose-dependent manner of cisplatin. Scan microscopy further showed that platelets were activated extending pseudopodia and even apoptotic with MP formation at 1 µM cisplatin ( middle and right), which was consistent with the confocal microscopy results. The inactivated platelet without pseudopodia was as control ( left). Furthermore, the expression of PS on platelets significantly increased in a concentration dependent manner of cisplatin, which reached about 26% at 1 µM cisplatin. The number of PMPs was also elevated after the treatment of cisplatin compared with that in untreated group (data not shown). These results indicate that cisplatin induces platelet activation or even apoptosis, leading to the high PCA.
Figure 4. The effect of cisplatin on PCA of platelets. Platelets were isolated from healthy subjects and treated with cisplatin of different concentrations for 1 h in vitro. Coagulation time (a), intrinsic/extrinsic FXa and thrombin (b) as well as fibrin (c) formation were evaluated. Data are present as mean ± SD, *P < 0.05 vs untreated, **P < 0.01 vs untreated. (c) After 1µM cisplatin treatment of 1 h, quiescent platelets (left), activated platelets (middle) and apoptotic platelets with MPs (right) were shown by scanning microscopy images. Bars represent 1 µm.
Effects of anti-platelet agents and lactadherin on the PCA
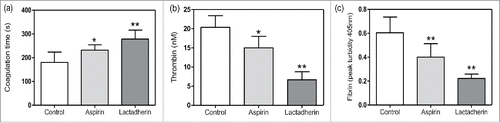
As the enhanced PCA of platelets after the treatment of cisplatin was shown in , we evaluated the effects of anti-platelet agents and lactadherin on reducing the high PCA. Platelets treated with 1 µM cisplatin were as control and other groups with additional treatment of aspirin or lactadherin were experimental groups. Results showed that aspirin could significantly reduce the thrombin and fibrin formation () and prolonged coagulation time (). In addition, lactadherin inhibited the PCA more than 60% through blocking PS on platelets. These results suggest that cisplatin induced high PCA could be reduced by anti-platelet agents and even inhibited by lactadherin.
Figure 5. The effect of anti-platelet agents and lactadherin on the PCA. After 1 µM cisplatin treatment of 1 h, platelets were treated with aspirin (100 nM) and lactadherin (2 nM). Coagulation time (a), intrinsic/extrinsic FXa and thrombin (b) as well as fibrin (c) formation of platelets were measured. Data are present as mean ± SD, *P < 0.05 vs control, **P < 0.01 vs control.
Discussion
Platelets have multiple functions in the development of lung cancer, such as secreting growth factors and chemotactic factors, promoting angiogenesis and accelerating cancer growth.Citation18-20 However, the role platelets play in hypercoagulability of NSCLC is largely unknown. This study for the very first time not only evaluates alterations of platelets in PS/TF expression, morphology, MP formation, cell count within a week after chemotherapy, but also emphasizes on the dynamic changes of procoagulant activity of platelets as well as the inhibitory effects of aspirin and lactadherin after the treatment of chemotherapeutic agents, which all provide evidence for the prophylactic treatment of thrombotic complications in NSCLC.
Cancer-associated thrombosis is a common but dangerous complication in patients with malignant disease, and lung cancer has a high incidence of venous thrombotic events.Citation21 Though increasing attention has been paid to the phenomenon of thrombotic complications in NSCLC in recent years, studies mainly focus on the serum components and little is known about the role of blood cells in the hypercoagulative state. Recent studies show that blood cells in gastric and colon cancer had high PCA and contribute to the hypercoagulative state.Citation22,23 We, for the first time show that platelets are activated and even apoptotic with increased intrinsic FXa, thrombin and fibrin formation after chemotherapy in patients with NSCLC. FXa combined with FVa stimulates prothrombin into thrombin which activates fibrinogen and finally forms fibrin.Citation24 Thus increased FXa contributes to more fibrin leading to rapid clotting formation and thus the coagulation time shortens. Therefore platelets after chemotherapy may contribute to the reduced clotting time and increased TAT of NSCLC patients.
Previous studies show that chemotherapeutic agents increase cancer PCA that will be downregulated by differentiation drugs like arsenic trioxide and is closely associated with PS exposure.Citation25,26 In this study, platelets after chemotherapy or treated with cisplatin in vitro have significantly elevated PS exposure, and some platelets are even apoptotic. Blockade of PS with lactadherin on platelets inhibits thrombin and fibrin formation by approximately 70%. Though the platelets were isolated gently, they may be slightly activated during the preparation, which may have an effect on the PS expression. Platelets from healthy subjects and patients before and after chemotherapy were treated in the same way to reduce the deviation. We also find little CD142 (TF) expresses on platelets and extrinsic FXa has no significant increase after chemotherapy, indicating that TF has little effect on PCA of NSCLC platelets. MPs are formed when cells undergo apoptosis, and they express membrane antigens that reflect their cellular origin.Citation27 Here, our data show that the number of PMPs is significantly enhanced 1 day after chemotherapy with persistent increase at day 2, indicating platelets undergo apoptosis after the treatment of chemotherapy and a great number of PMPs may not be efficiently cleared by the body. MPs express abundant PS on the membrane and the PCA of MPs has been well demonstrated.Citation28,29 Thus activated/apoptotic platelets and accumulated PMPs after chemotherapy are important sources to contribute the hypercoagulative state and are closely associated to thrombotic complications in NSCLC.
Precise and effective prophylactic therapy against the thrombotic events is urgently needed due to the increased mortality rate in NSCLC.Citation30 In this study, we show that aspirin which can inhibit TXA2 formation of platelets reduces thrombin and fibrin generation of platelets after cisplatin treatment, suggesting that anti-platelet agents can downregulate PCA through suppressing platelet release and aggregation. Furthermore, the PCA reduced to 30% with the addition of lactadherin to block PS on platelets after the treatment of cisplatin, which is significantly better than aspirin. Our data suggest that PS exposure on platelets could be the main cause for hypercoagulability and play a vital role in thrombotic complications in NSCLC. Blockade of PS seems crucial in prevention of the hypercoagulable state. PS provides binding sites for FXa and prothrombinase complexes and promotes thrombin and fibrin formation. Therefore, therapies directly targeting FXa and prothrombinase complexes may achieve the desired effect. Antiplatelet agents are not sufficient to entirely prevent PCA activity. Early application of anti-platelet drugs combined with anticoagulant therapy after chemotherapy may effectively protect thrombosis in NSCLC.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
Contribution: R.M. designed the research, performed experiments, analyzed results, made the figures and wrote the paper; J.S. obtained funding, designed the study, performed some experiments, analyzed results, made the figures, and revised the manuscript; Y.B., J.K. performed some experiments; J.Z. analyzed data and revised the manuscript.
Funding
This work was funded by grants from the National Science Foundation of China (81470301, 81670128) and Graduate Innovation Fund of Harbin Medical University (YJSCX2014–02HYD).
References
- Walker AJ, Baldwin DR, Card TR, Powell HA, Hubbard RB, Grainge MJ. Risk of venous thromboembolism in people with lung cancer: A cohort study using linked UK healthcare data. Br J Cancer 2016; 115(1):115-21; PMID:27253177; https://doi.org/10.1038/bjc.2016.143
- Jara-Palomares L, Otero R, Jimenez D, Carrier M, Tzoran I, Brenner B, Margeli M, Praena-Fernandez JM, Grandone E, Monreal M, et al. Development of a risk prediction score for occult cancer in patients with venous thromboembolism. Chest 2017 Nov 1. pii: S0012-3692(16)62282-1; 151(3):564-71; PMID:27815153; https://doi.org/10.1016/j.chest.2016.10.025
- Elyamany G, Alzahrani AM, Bukhary E. Cancer-associated thrombosis: an overview. Clin Med Insights Oncol 2014; 8:129-37; PMID:25520567; https://doi.org/10.4137/CMO.S18991
- Zer A, Moskovitz M, Hwang DM, Hershko-Klement A, Fridel L, Korpanty GJ, Dudnik E, Peled N, Shochat T, Leighl NB, et al. ALK-rearranged non-small-cell lung cancer is associated with a high rate of venous thromboembolism. Clin Lung Cancer. 2017; 18(2):156-61;PMID:27913214; https://doi.org/10.1016/j.cllc.2016.10.007
- Li R, Hermann G, Baldini E, Chen A, Jackman D, Kozono D, Nguyen P, Nohria A, Powell G, Mak R. Advanced nodal stage predicts venous thromboembolism in patients with locally advanced non-small cell lung cancer. Lung Cancer 2016; 96:41-7; PMID:27133748; https://doi.org/10.1016/j.lungcan.2016.03.004
- Nadir Y, Brenner B. Heparanase procoagulant activity in cancer progression. Thromb Res 2016; 140(Suppl 1):S44-8; PMID:27067977; https://doi.org/10.1016/S0049-3848(16)30097-4
- Nadir Y, Sarig G, Axelman E, Meir A, Wollner M, Shafat I, Hoffman R, Brenner B, Vlodavsky I, Haim N. Heparanase procoagulant activity is elevated and predicts survival in non-small cell lung cancer patients. Thromb Res 2014; 134(3):639-42; PMID:25065557; https://doi.org/10.1016/j.thromres.2014.07.006
- Koldas M, Gummus M, Seker M, Seval H, Hulya K, Dane F, Kural A, Gumus A, Salepci T, Turhal NS. Thrombin-activatable fibrinolysis inhibitor levels in patients with non-small-cell lung cancer. Clin Lung Cancer 2008; 9(2):112-5; PMID:18501098; https://doi.org/10.3816/CLC.2008.n.017
- Masago K, Fujita S, Mio T, Togashi Y, Kim YH, Hatachi Y, Fukuhara A, Irisa K, Sakamori Y, Mishima M. Clinical significance of the ratio between the alpha 2 plasmin inhibitor-plasmin complex and the thrombin-antithrombin complex in advanced non-small cell lung cancer. Med Oncol 2011; 28(1):351-6; PMID:20300980; https://doi.org/10.1007/s12032-010-9454-y
- Kim S-H, Lee HW, Go S-I, Lee SI, Lee G-W. Clinical signifiance of the preoperative platelet count and platelet-to-lymphocyte ratio (PLT-PLR) in patients with surgically resected non-small cell lung cancer. Oncotarget 2016; 7(24):36198-206; PMID:27105529; https://doi.org/10.18632/oncotarget.8809
- Omar M, Tanriverdi O, Cokmert S, Oktay E, Yersal O, Pilancı KN, Menekse S, Kocar M, Sen CA, Ordu C, et al. Role of increased mean platelet volume (MPV) and decreased MPV/platelet count ratio as poor prognostic factors in lung cancer. Clin Respir J 2016 Dec 27; PMID:28026133; https://doi.org/10.1111/crj.12605
- Fu Y, Zhou J, Li H, Cao F, Su Y, Fan S, Li Y, Wang S, Li L, Gilbert GE, et al. Daunorubicin induces procoagulant activity of cultured endothelial cells through phosphatidylserine exposure and microparticles release. Thromb Haemost 2010; 104(6):1235-41; PMID:20886178; https://doi.org/10.1160/TH10-02-0102
- Ettinger DS, Wood DE, Akerley W, Bazhenova LA, Borghaei H, Camidge DR, Cheney RT, Chirieac LR, D'Amico TA, Dilling TJ, et al. NCCN guidelines insights: Non-small cell lung cancer, Version 4.2016. J Natl Compr Canc Netw 2016; 14(3):255-64; PMID:26957612; https://doi.org/10.6004/jnccn.2016.0031
- Ding W, Kou J, Shi J, Kou Y, He Z, Cao M, Wang L, Bi Y, Thatte HS, Shi J. Procoagulant activity induced by transcatheter closure of atrial septal defects is associated with exposure of phosphatidylserine on microparticles, platelets and red blood cells. Thromb Res 2015; 136(2):354-60; PMID:26099643; https://doi.org/10.1016/j.thromres.2015.06.015
- Wang L, Bi Y, Shi J, Ma R, Wu X, Zhang Y, Ding W, Liu Y, Yu Q, Zhang Y, et al. Microparticles and blood cells induce procoagulant activity via phosphatidylserine exposure in NSTEMI patients following stent implantation. Int J Cardiol 2016; 223:121-8; PMID:27537737; https://doi.org/10.1016/j.ijcard.2016.07.260
- Yao Z, Wang L, Shi J, Zhao L, Chi C, Guo L, Tong D, Yang X, Dong Z, Deng R, et al. Enhanced procoagulant activity on blood cells after acute ischemic stroke. Transl Stroke Res 2017; 8(1):83-91; PMID:27650774; https://doi.org/10.1007/s12975-016-0501-7
- Campbell RA, Overmyer KA, Selzman CH, Sheridan BC, Wolberg AS. Contributions of extravascular and intravascular cells to fibrin network formation, structure, and stability. Blood 2009; 114(23):4886-96; PMID:19797520; https://doi.org/10.1182/blood-2009-06-228940
- Kee NLA, Krause J, Blatch GL, Muramoto K, Sakka K, Sakka M, Naudé RJ, Wagner L, Wolf R, Rahfeld JU, et al. The Proteolytic Profile of Human Cancer Procoagulant Suggests That It Promotes Cancer Metastasis at the Level of Activation Rather Than Degradation. Protein J 2015; 34(5):338-48; PMID:26341972; https://doi.org/10.1007/s10930-015-9628-8
- Lysov Z, Swystun LL, Kuruvilla S, Arnold A, Liaw PC. Lung cancer chemotherapy agents increase procoagulant activity via protein disulfide isomerase-dependent tissue factor decryption. Blood Coagul Fibrinolysis 2015; 26(1):36-45; PMID:24911456; https://doi.org/10.1097/MBC.0000000000000145
- Gay LJ, Felding-Habermann B. Contribution of platelets to tumourmetastasis. Nat Rev Cancer 2011; 11(2):123-34; PMID:21258396; https://doi.org/10.1038/nrc3004
- Vitale C, D'Amato M, Calabrò P, Stanziola AA, Mormile M, Molino A. Venous thromboembolism and lung cancer: a review. Multidiscip Respir Med 2015; 10(1):28; PMID:26380084; https://doi.org/10.1186/s40248-015-0021-4
- Yang C, Shi J, Zou X, Cao M, Zhao L, Bi Y, Kou J, Shi J, Zou X. Contributions of phosphatidylserine-positive platelets and leukocytes and microparticles to hypercoagulable state in gastric cancer patients. Tumour Biol 2016; 37(6):7881-91; PMID:26700666; https://doi.org/10.1007/s13277-015-4667-5
- Zhao L, Bi Y, Kou J, Shi J, Piao D. Phosphatidylserine exposing-platelets and microparticles promote procoagulant activity in colon cancer patients. J Exp Clin Cancer Res 2016; 35:54; PMID:27015840; https://doi.org/10.1186/s13046-016-0328-9
- Stacy ZA, Call WB, Hartmann AP, Peters GL, Richter SK. Edoxaban: A comprehensive review of the pharmacology and clinical data for the management of atrial fibrillation and venous thromboembolism. Cardiol Ther 2016; 5(1):1-18; PMID:26935434; https://doi.org/10.1007/s40119-016-0058-2
- Hoffan EA, Gizelska K, Mirowski M, Mielicki W. Arsenic trioxide downregulates cancer procoagulant activity in MCF-7 and WM-115 cell lines in vitro. Contemp Oncol (Pozn) 2015; 19(2):108-12; PMID:26034387; https://doi.org/10.5114/wo.2014.41390
- Zhou J, Shi J, Hou J, Cao F, Zhang Y, Rasmussen JT, Heegaard CW, Gilbert GE. Phosphatidylserine exposure and procoagulant activity in acute promyelocytic leukemia. J Thromb Haemost 2010; 8(4):773-82; PMID:20102487; https://doi.org/10.1111/j.1538-7836.2010.03763.x
- Zubairova LD, Nabiullina RM, Nagaswami C, Zuev YF, Mustafin IG, Litvinov RI, Weisel JW. Circulating microparticles alter formation, structure, and properties of fibrin clots. Sci Rep 2015; 5:17611; PMID:26635081; https://doi.org/10.1038/srep17611
- van Doormaal F, Kleinjan A, Berckmans RJ, Mackman N, Manly D, Kamphuisen PW, Richel DJ, Büller HR, Sturk A, Nieuwland R. Coagulation activation and microparticle-associated coagulant activity in cancer patients. An exploratory prospective study. Thromb Haemost 2012; 108(1):160-5; PMID:22535219; https://doi.org/10.1160/TH12-02-0099
- Chen W, Zhang Y, Yang Y, Zhai Z, Wang C. Prognostic significance of arterial and venous thrombosis in resected specimens for non-small cell lung cancer. Thromb Res 2015; 136(2):451-5; PMID:26099644; https://doi.org/10.1016/j.thromres.2015.06.014
- Kourelis TV, Wysokinska EM, Wang Y, Yang P, Mansfield AS, Tafur AJ. Early venous thromboembolic events are associated with worse prognosis in patients with lung cancer. Lung Cancer 2014; 86(3):358-62; PMID:25453848; https://doi.org/10.1016/j.lungcan.2014.10.003
- Gao C, Xie R, Yu C, Wang Q, Shi F, Yao C, Xie R, Zhou J, Gilbert GE, Shi J. Procoagulant activity of erythrocytes and platelets through phosphatidylserine exposure and microparticles release in patients with nephrotic syndrome. Thromb Haemost 2012; 107(4):681-9; PMID:22370875; https://doi.org/10.1160/TH11-09-0673