ABSTRACT
The antitumor efficacy of 5-fluorouracil (5-FU) in advanced colorectal cancer (CRC) is hindered not only by the low therapeutic index, but also by tumor cell resistance to this cytotoxic drug. Therefore, to enhance the 5-FU antitumor activity, the present research used a novel tumor-targeted therapy based on the co-administration of 5-FU encapsulated in long-circulating liposomes (LCL-5-FU) together with liposomal prednisolone phosphate (LCL-PLP), a formulation with known anti-angiogenic actions on C26 murine colon carcinoma cells. Thus, we assessed the in vivo effects of the combined liposomal drug therapy on C26 carcinoma growth as well as on the production of molecular markers with key roles in tumor development such as angiogenic, inflammatory, and oxidative stress molecules. To get further insight into the polarization state of tumor microenvironment after the treatment, we determined the IL-10/IL-12p70 ratio in tumors. Our results showed that combined liposomal drug therapy inhibited almost totally tumor growth and was superior as antitumor activity to both single liposomal drug therapies tested. The antitumor efficacy of the combined therapy was mainly related to the anti-angiogenic and anti-inflammatory actions on C26 carcinoma milieu, being favored by its controlling effect on intratumor oxidative stress and the skewing of polarization of tumor microenvironmental cells toward their antineoplastic phenotypes. Thus, our study unveils a promising treatment strategy for CRC that should be furthermore considered.
Abbreviation list
| 5-FU | = | 5-Fluorouracil |
| CRC | = | Colorectal cancer |
| LCL | = | Long-circulating liposomes |
| EPR | = | Enhanced permeability and retention |
| PLP | = | Prednisolone disodium phosphate |
| s.c. | = | Subcutaneous administration |
| i.v. | = | Intravenous(ly) |
| AUTC | = | Area under the tumor growth curve |
| DT | = | Doubling time |
| PBS | = | Phosphatebuffered saline |
| IL-9 | = | Interleukin 9 |
| IL-13 | = | Interleukin 13 |
| TPO | = | Thrombopoietin |
| IL-6 | = | Interleukin 6 |
| TNF-á | = | Tumor necrosis factor á |
| VEGF | = | Vascular endothelial growth factor |
| IFN-ã | = | Interferon ã |
| MIG | = | Monokine induced by IFN-ã |
| ANOVA | = | Analysis of variance |
| M-CSF | = | Monocyte-colony stimulating factor |
| MCP-1 | = | Monocyte chemoattractant protein-1 |
| G-CSF | = | Granulocyte-colony stimulating factor |
| IGF-II | = | Insulin growth factor II |
| IL-1á | = | Interleukin 1á |
| IL-1â | = | Interleukin 1â |
| IL-12p40 | = | Interleukin 12 p40 |
| FasL | = | Fas ligand |
| bFGF | = | Basic fibroblast growth factor |
| TIMP-1 | = | Tissue inhibitor of metalloproteinase 1 |
| TIMP-2 | = | Tissue inhibitor of metalloproteinase 2 |
| GM-CSF | = | Granulocyte-macrophage-colony stimulating factor |
| PF-4 | = | Platelet factor 4 |
| IL-10 | = | Interleukin 10 |
| IL-12p70 | = | Interleukin 12 p70 |
| MDA | = | Malondialdehyde |
| DPPC | = | 1,2-Dipalmitoyl-sn-glycero-3-phosphocholine |
| PEG-2,000-DSPE | = | N-(Carbonyl-methoxypolyethyleneglycol-2,000)-1,2-distearoylsn-glycero-3 phosphoethanolamine (Na-salt) |
| CHL | = | Cholesterol |
| PEG | = | Polyethylene glycol |
| SD | = | Standard deviation |
Introduction
Fluoropyrimidine-based therapies (such as the administration of 5-FU) are the main treatment aproaches in metastatic CRC.Citation1 5-FU is an antimetabolite that acts via inhibiting essential processes for tumor cell proliferation such as DNA and RNA synthesis, which lead to cell death.Citation1-3 Nevertheless, the therapeutic efficacy of 5-FU is limited, this drug being highly catabolized (more than 80%) through the activity of the dihydropyrimidine dehydrogenase in the liver.Citation4 Therefore, tumor-targeted delivery of 5-FU might be a valuable strategy for improving the therapeutic index of this cytotoxic agent. Moreover, previous studies have demonstrated that tumor-targeting properties of LCL could enable several cytotoxic drugs such as doxorubicin, paclitaxel, daunorubicin, vincristine, cisplatin, cytarabine, and irinotecan to accumulate into the tumor tissuesCitation5 and acted more efficiently compared with conventional chemotherapy based on the free administration of the same drugs.Citation6-8 Thus, LCL as nanovehicles for 5-FU will ensure its passive accumulation in solid tumors, due to the peculiar features of the tumor vasculature architecture, also known as EPR effect.Citation9,Citation10 Besides high therapeutic response as a result of tumor targeting property of the LCL-encapsulated 5-FU (LCL-5-FU), the drug toxicity on healthy tissues could also be greatly reduced. Moreover, the combination of LCL-5-FU with LCL containing PLP (LCL-PLP) formulation with known inhibitory effects in colon carcinoma in vivoCitation11 via tumor angiogenesis suppressionCitation12 could significantly improve CRC treatment. In tight connection with these findings, in the present article, the antitumor activity of the combination therapy for colon carcinoma in vivo, based on the administration of LCL-PLP and LCL-5-FU was investigated. To this purpose, we evaluated the effects of this novel combined tumor-targeted treatment on s.c. C26 murine colon carcinoma growth, as well as on the main processes that support tumor development, such as tumor inflammation, angiogenesis, and oxidative stress.
Results
The combined liposomal drug therapy inhibited more efficiently the C26 colon carcinoma growth than single liposomal therapy
To compare the antitumor activity of combined liposomal drug therapy based on simultaneous administration of 20 mg/kg LCL-PLP and 1.2 mg/kg LCL-5-FU with that induced by liposomal monotherapy (either 20 mg/kg LCL-PLP or 1.2 mg/kg LCL-5-FU) on the growth of C26 colon carcinoma in vivo, mice were injected i.v. when tumor volumes were about 200 mm3 (at day 8) and at day 11 after tumor induction. The same dosing schedule and treatment schemes were used when the drugs were administered as free forms. The effects of different treatments on the tumor development were presented in and were assessed by means of tumor volume (at day 12) (, , and ), the AUTC (until day 12) (, , and ) and the tumor volume DT (until day 12) ().
Figure 1. Effects of the combined administration of LCL-PLP and LCL-5-FU on the growth of s.c. C26 colon carcinoma. Tumor volumes at day 12 after tumor cell inoculation (when mice were killed) after different treatments were presented in panels A, C, and E. AUTCs after various treatments were presented in panels B, D and F. The results were compared with PBS-treated groups (controls) and expressed as mean ± SD of tumor volumes of 5–6 mice.; ns - not significant (*P > 0.05); **P < 0.01; ***P < 0.001; Control - untreated group; LCL - group treated with empty liposomes; PLP - group treated with 20 mg/kg PLP as free form at days 8 and 11 after tumor cell inoculation; LCL-PLP - group treated with 20 mg/kg PLP as liposomal form at days 8 and 11 after tumor cell inoculation; 5-FU - group treated with 1.2 mg/kg 5-FU as free form at days 8 and 11 after tumor cell inoculation; LCL-5-FU - group treated with 1.2 mg/kg 5-FU as liposomal form at days 8 and 11 after tumor cell inoculation; PLP+5-FU - group treated with 20 mg/kg PLP and 1.2 mg/kg 5-FU, both drugs administered as free forms at days 8 and 11 after tumor cell inoculation; LCL-PLP+LCL-5-FU - group treated with 20 mg/kg LCL-PLP and 1.2 mg/kg LCL-5-FU at days 8 and 11 after tumor cell inoculation.
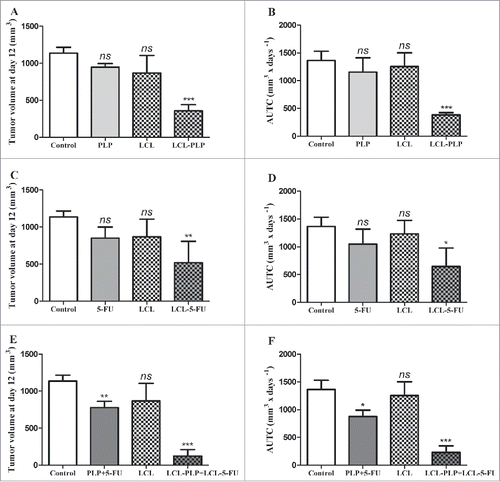
Table 1. The doubling time of C26 colon carcinoma growth after different treatments.
Our data suggested that the growth of C26 colon carcinoma was affected moderately after LCL-5-FU administration (by 53%, P < 0.01) to strongly after LCL-PLP treatment (by 70%) when compared with control tumors (PBS-/LCL-treated groups) growth according to tumor volumes measurements ( and ) as well as AUTC data ( and ) at sacrification day. These inhibitory effects were clearly enabled by the tumor-targeting properties of the liposomal formulations since the same doses of either PLP or 5-FU administered as free forms did not show any suppressive actions on tumor growth (). Nevertheless, none of the monotherapies tested (as free or LCL formulation) had any effect on DT of tumor volumes (), that might suggest the limitations of these tumor-targeted monotherapies. Concurrent administration of 20 mg/kg LCL-PLP with 1.2 mg/kg LCL-5-FU (LCL-PLP+LCL-5-FU) decelerated almost totally (by over 83–85% inhibition compared with control tumors growth, P < 0.001, and ) the growth of C26 tumors, while the combination of the free drugs inhibited only slightly the tumor growth (by 30%, P < 0.01, and ). Noteworthy that DT of tumors treated with LCL-PLP+LCL-5-FU was about 2.5–3.5 times longer than the DT for C26 tumors after any other treatment tested ().
Simultaneous administration of LCL-PLP and LCL-5-FU inhibited stronger tumor growth than their sequential administration
To investigate whether different administration regimens of the liposomal formulations could affect their antitumor efficacy, 20 mg/kg LCL-PLP and 1.2 mg/kg LCL-5-FU were injected sequentially (pretreatment with LCL-PLP with 24 h before LCL-5-FU administration at days 8 and 11 after tumor induction) as well as simultaneously (at days 8 and 11 after tumor induction). The same administration regimens were applied for both active drugs injected as free forms. The antitumor activity of each type of treatment was monitored until the first tumors from control groups reached 2,000 mm3 (at day 18 after tumor cell inoculation). The results were presented in as tumor volumes. Our data revealed that the simultaneous administration of the liposomal drugs exerted stronger suppression of the C26 tumor growth than that induced by their sequential administration (LCL-PLP/LCL-5-FU) (72% vs 50% inhibition compared with control tumors, P = 0.02). The higher anticancer effects of the simultaneous treatment approach compared with the sequential administration regimen was also supported by DT of tumor volumes at day 12 (DT of 5.3 d after LCL-PLP+LCL-5-FU compared with DT of 3.8 d after LCL-PLP/LCL-5-FU). Moreover, simultaneous injection of both free drugs exerted slight anticancer effects (34% inhibition, P < 0.05), while their sequential administration did not affect statistically significantly tumor development.
Figure 2. Effects of 2 different administration regimens of combined therapy (LCL-PLP+LCL-5-FU vs. LCL-PLP/LCL-5-FU) on s.c. C26 colon carcinoma growth. For each liposomal treatment scheme, as well as for the free drugs treatment schemes, tumor volumes at day 18 after tumor cell inoculation (when tumors from control group reached 2,000 mm3) were compared with the tumor volumes from control group (PBS-treated) measured at the same time point. The results were expressed as mean ± SD of tumor volumes of 5–6 mice. ns - not significant (P > 0.05); *P < 0.05; ***P < 0.001; Control - untreated group; LCL-PLP+LCL-5-FU - group treated with 20 mg/kg LCL-PLP and 1.2 mg/kg LCL-5-FU at days 8 and 11 after tumor cell inoculation; LCL - PLP/LCL-5-FU - group pretreated with 20 mg/kg LCL-PLP at days 8 and 11 after tumor cell inoculation with 24 h before administration of 1.2 mg/kg LCL-5-FU.
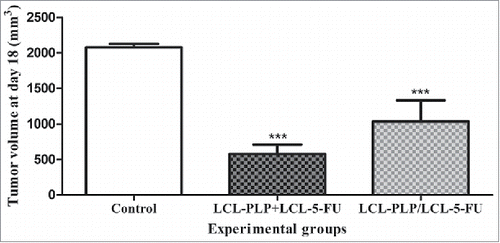
Altogether, these data suggested that the treatment approach based on concurrent administration of LCL-PLP and LCL-5-FU might be exploited for future therapeutic strategies applied in CRC. Therefore, the main mechanisms of the antitumor activity of LCL-PLP+LCL-5-FU in C26 colon carcinoma-bearing mice were further investigated.
Effects of LCL-PLP+LCL-5-FU treatment on tumor inflammatory transcription factors production
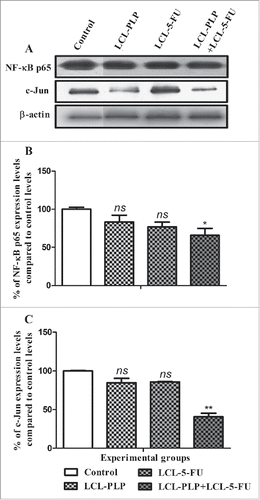
To further investigate the molecular mechanisms of the antitumor activity of LCL-PLP+LCL-5-FU on C26 colon carcinoma we evaluated the intratumor production of 2 key inflammatory factors, NF-κB via western blot analysis of its p65 subunit and AP-1 by testing the production of c-Jun subunit of this factorCitation13-15 and the results were shown in . Among all treatments tested, only combined liposomal drug therapy exerted an inhibitory effect on the production of NF-κB p65 subunit (by 35% inhibition compared with control protein production) ( and ). Moreover, our data indicated that c-Jun subunit levels were suppressed strongly after combined liposomal drug therapy (by 60% suppression compared with the control levels of the same protein) ( and ).
Figure 3. Effects of different treatments on the intratumor levels of p65 subunit of NF-κB and c-Jun subunit of AP-1. (A) Western blot analyses of NF-κB p65 and c-Jun levels in C26 tumor homogenates from each experimental group: Control - untreated group (lane 1); LCL-PLP - group treated with 20 mg/kg PLP as liposomal form at days 8 and 11 after tumor cell inoculation (lane 2); LCL-5-FU - group treated with 1.2 mg/kg 5-FU as liposomal form at days 8 and 11 after tumor cell inoculation (lane 3); LCL-PLP+LCL-5-FU - group treated with 20 mg/kg LCL-PLP and 1.2 mg/kg LCL-5-FU at days 8 and 11 after tumor cell inoculation (lane 4). β-actin was used as loading control. Quantification of western blot data for NF-κB p65 expression levels (B) and for c-Jun expression levels (C). The levels of proteins from each experimental group are compared with the control levels of the same proteins and are expressed as mean ± SD of 2 independent measurements; ns - not significant (P > 0.05); *P < 0.05; **P < 0.01; ***P < 0.001.
Effects of LCL-5-FU+LCL-PLP combination therapy on the intratumor production of inflammatory and angiogenic proteins
To assess the effects of different treatments on tumor angiogenesis, a screening for 24 angiogenic/inflammatory proteins present in C26 tumor tissue was performed using a protein array and the results were shown in . No differences between angiogenic and inflammatory protein production in empty liposome-treated tumors and PBS-treated tumors were noted (P = 0.2109) (data not shown). Except for mice that received LCL-PLP injected either alone or in combination with LCL-5-FU, the average production of tumor proteins tested was not affected statistically significantly by any treatment applied. Nevertheless, for specific angiogenic and inflammatory proteins, PLP, 5-FU, PLP+5-FU, and LCL-5-FU treatments exerted moderate (by 40–100% stimulation compared with their control production) to strong (by 100–200% enhancement compared with their control production) stimulatory effects on their intratumor production (data not shown). As LCL-5-FU exerted the most pronounced stimulatory action on the expression of specific proteins, these results were presented in detail. Thus, LCL-5-FU enhanced moderately the levels of IL-9, IL-13, eotaxin, and TPO to strongly the production of IL-6, TNF-α, VEGF, leptin, IFN-γ, and MIG ().
Figure 4. The effects of different treatments on angiogenic and inflammatory proteins production in s.c. C26 colon carcinoma tissue. The protein levels in tumors after different treatments are compared with the levels of the same proteins in control tumors. Data are expressed as average % of reduction of tumor protein levels ranging from 0% (white) to -100% (black) or stimulation (+) of production of proteins ranging from 0% (white) to +200% (red) compared with the levels of the same proteins in control tumors. LCL-PLP - group treated with 20 mg/kg PLP as liposomal form at days 8 and 11 after tumor cell inoculation; LCL-5-FU - group treated with 1.2 mg/kg 5-FU as liposomal form at days 8 and 11 after tumor cell inoculation; LCL-PLP+LCL-5-FU - group treated with 20 mg/kg LCL-PLP and 1.2 mg/kg LCL-5-FU at days 8 and 11 after tumor cell inoculation.
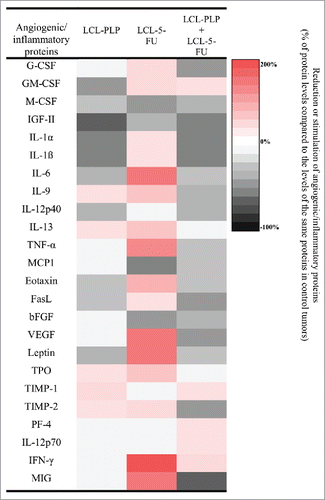
Among all treatments tested, only LCL-PLP administered alone as well as in combination with LCL-5-FU reduced significantly the overall C26 carcinoma production of most of the angiogenic and inflammatory proteins compared with their production in control tumors (). Therefore, to emphasize additional effects of LCL-PLP+LCL-5-FU over LCL-PLP treatment on the intratumor production of angiogenic/inflammatory proteins, the protein array results for these 2 groups were detailed in . Moreover, the 2-way ANOVA analysis with Bonferroni correction for multiple comparisons post-test highlights the statistically stronger reduction for 6 out of 18 pro-angiogenic/pro-inflammatory proteins studied, after combined liposomal drug therapy compared with LCL-PLP (). Thus, LCL-PLP+LCL-5-FU treatment suppressed the production of M-CSF, MCP-1, eotaxin, leptin (by 25–35%), G-CSF, IGF-II, IL-1α, IL-1β, IL-9, IL-12p40, FasL, bFGF, and VEGF (by 35–60%). The levels of the majority of anti-angiogenic/anti-inflammatory proteins were not or only slightly affected by the combined liposomal drug therapy as well as by LCL-PLP administered alone, except for the levels of TIMP-1 which increased by 33% after LCL-PLP treatment and the production of TIMP-2 and MIG that was reduced by 45–65% after the LCL-PLP+LCL-5-FU treatment ().
Table 2. The effects of LCL-PLP administered alone and in combination with LCL-5-FU on angiogenic and inflammatory protein production in s.c. C26 colon carcinoma.
Immunohistochemical examination of tumor tissues after LCL-PLP+LCL-5-FU treatment
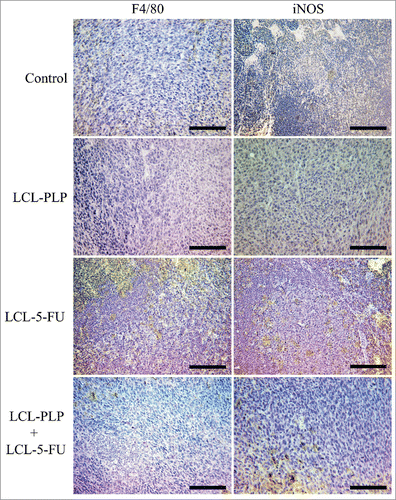
For immunohistochemical examination of macrophage infiltration in the tumor tissue, we stained tumor sections for F4/80 - a widely used marker for murine tissue macrophages (the equivalent of human CD68) and for inducible nitric oxide synthase (iNOS) which is M1 macrophage-specific.Citation16,Citation17 The results are shown in and a 3-score qualitative analysis is displayed in , depending on the overall density of positively stained cells from several slides in each experimental group.Citation18 The results show higher densities of macrophages positive for both proteins tested in tumors treated with LCL-5-FU than in control tumors and tumors treated with LCL-PLP or LCL-PLP+LCL-5-FU ().
Table 3. Immunohistochemical examination of macrophages infiltration in s.c. C26 colon carcinoma tumor tissues after different treatments.
Figure 5. Immunohistochemical analysis of the macrophage antigen F4/80 and iNOS in s.c. C26 colon carcinoma tissue. Positively stained cells appear in brown; size bars = 20 µm. Control - untreated group; LCL-PLP - group treated with 20 mg/kg PLP as liposomal form at days 8 and 11 after tumor cell inoculation; LCL-5-FU - group treated with 1.2 mg/kg 5-FU as liposomal form at days 8 and 11 after tumor cell inoculation; LCL-PLP+LCL-5-FU - group treated with 20 mg/kg LCL-PLP and 1.2 mg/kg LCL-5-FU at days 8 and 11 after tumor cell inoculation.
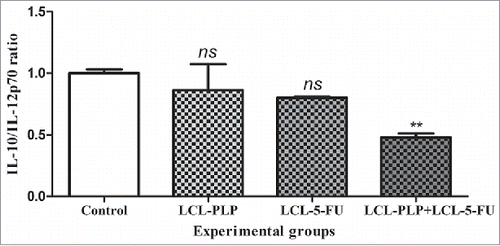
Liposomal combination treatment alters the IL-10/IL-12p70 ratio in tumors
The levels of the antagonist cytokines IL-10 and IL-12p70 were expressed as % of expression compared with the levels of the same proteins in control tumors, and the ratios for each experimental group were plotted as depicted in . When liposomal combination therapy was applied, there was a 2-fold reduction of IL-10/IL-12p70 ratio (P = 0.0037) compared with control ratio for the same cytokine, whereas the single liposomal therapies did not affect this value ().
Figure 6. The effects of different treatments on the production ratio of IL-10/IL-12p70 in s.c. C26 colon carcinoma tissue. Levels of IL-10 and IL-12p70 cytokines are expressed as mean percentage ± SD compared with the expression levels of the same proteins in control tumors. Control - untreated group; LCL-PLP - group treated with 20 mg/kg PLP as liposomal form at days 8 and 11 after tumor cell inoculation; LCL-5-FU - group treated with 1.2 mg/kg 5-FU as liposomal form at days 8 and 11 after tumor cell inoculation; LCL-PLP+LCL-5-FU - group treated with 20 mg/kg LCL-PLP and 1.2 mg/kg LCL-5-FU at days 8 and 11 after tumor cell inoculation; ns - not significant (P > 0.05); **P < 0.01.
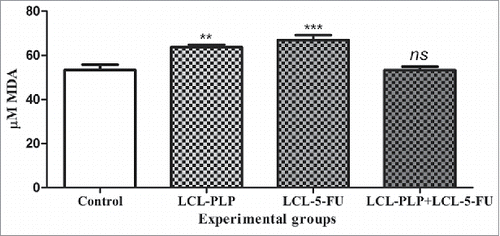
The effects of LCL-PLP+LCL-5-FU therapy on tumor oxidative stress
To investigate whether the combined therapy tested can affect tumor oxidative stress, MDA, a general marker for lipid peroxidation,Citation19 was quantified by HPLC and the results are shown in . Each single liposomal drug therapy affected statistically significantly the MDA levels in tumors (). In this respect, pro-oxidant effects were noted after administration of either LCL-PLP or LCL-5-FU that increased MDA levels by 20–25% compared with its control production (P < 0.001). This finding might suggest the limited antitumor activity of both single liposomal drug therapies since the MDA production was enhanced in the proliferative range of tumor oxidative stress (µM).Citation20 Notably, this protumor effect was counteracted after simultaneous administration of both liposomal formulations, since the levels of MDA after LCL-PLP+LCL-5-FU treatment remained similar to those measured in control tumors ().
Figure 7. The effects of different treatments on MDA levels from C26 tumor homogenates. Data were expressed as mean ± SD of triplicate measurements. Control - untreated group; LCL-PLP - group treated with 20 mg/kg PLP as liposomal form at days 8 and 11 after tumor cell inoculation; LCL-5-FU - group treated with 1.2 mg/kg 5-FU as liposomal form at days 8 and 11 after tumor cell inoculation; LCL-PLP+LCL-5-FU - group treated with 20 mg/kg LCL-PLP and 1.2 mg/kg LCL-5-FU at days 8 and 11 after tumor cell inoculation; ns - not significant (P > 0.05); **P < 0.01; ***P < 0.001.
Discussion
The aim of this study was to investigate the underlying mechanism of action of a novel targeted combination therapy based on the simultaneous administration of LCL-PLP with LCL-5-FU to s.c. C26 colon carcinoma in vivo, which, to our knowledge, has never been described before. Thus, LCL-PLP+LCL-5-FU decelerated almost totally the tumor growth, being superior as antitumor efficacy to both single liposomal drug therapies tested ( and ). To further study the earlier suggested antitumor activities of LCL-PLPCitation11,Citation12,Citation18 as well as of 5-FUCitation21 via modulation of key protumor processes, the effects of the LCL-PLP + LCL-5-FU in C26 colon carcinoma-bearing mice were investigated with regard to intratumor production of inflammatory, angiogenic and oxidative stress markers. Our data provided confirmatory evidence for the anti-inflammatory and anti-angiogenic mode of action of the antitumor activity of the combined liposomal drug therapy in colon carcinoma in vivo. Hence, LCL-PLP+LCL-5-FU treatment exerted a marked suppression of the majority of the pro-angiogenic and pro-inflammatory proteins production (13 out of 18) in tumors, while the expression of most of the anti-angiogenic/anti-inflammatory proteins (4 out of 6) was not or only slightly affected by the treatment ( and ). Probably, the persistent presence of these antitumor proteins might also strengthen the inhibitory effects exerted by this treatment on the C26 tumor production of pro-angiogenic and pro-inflammatory proteins. Furthermore, the anti-angiogenic and anti-inflammatory mode of action of the antitumor activity of LCL-PLP+LCL-5-FU is also supported by the suppressive effects of the combined treatment on the intratumor levels of NF-κB and AP-1 () which are key transcription factors involved in the regulation of tumor inflammation and angiogenesis.Citation13,Citation15,Citation22,Citation23 Moreover, both regulatory factors and certain pro-angiogenic (bFGF, VEGF) and pro-inflammatory proteins (IL-1α, IL-12p40, TNF-α, MCP-1, FasL and TPO) were previously associated with tumor cell proliferation, cancer cells rescue from cell death as well as with the aggressiveness and metastatic potential of cancer cells.Citation13,Citation15,Citation24-30 Therefore, the reduction of their expression in C26 colon carcinoma-bearing mice treated with the combined tumor-targeted therapy might account not only for inhibition of tumor angiogenesis and inflammation but also for altered tumor cell proliferation and induction of apoptosis in these cancer cells.
Furthermore, to determine the efficacy of our therapeutic approach on the polarization of tumor microenvironmental cells toward antitumor phenotypes, we performed immunohistochemical analysis for M1 macrophages and the assessment of IL-10/IL-12p70 ratio in tumors after different treatments. After LCL-5-FU treatment, immunostaining revealed a marked macrophage infiltration into tumor tissue (), most likely on account of the overexpression of pro-angiogenic proteins VEGF and leptin (). Hence, these results are in agreement with the array results and support the fact that low intratumor amounts of 5-FU render tumors more aggressive.Citation31 In contrast, tumors treated with LCL-PLP and LCL-PLP+LCL-5-FU displayed a strong tumor growth inhibition, a strong anti-angiogenic and anti-inflammatory effect ( and ), which potentially led to occasional infiltration of macrophages into tumor tissue as only little or no stainings for both F4/80 and iNOS markers were noted ().
However, the accurate immunohistochemical analysis of tumors is hindered by the lack of specific markers for different cell types of the immune system and because TAM polarization in tumors is not absolute and can fluctuate depending on tumor type and stage.Citation32 Therefore, we estimated the overall polarization of the tumor microenvironment by investigating the production of IL-10 and IL-12p70. In this respect, a large body of evidence suggested IL-12p70 as being mainly an M1-like macrophage marker, as opposed to M2-like macrophages which are characterized by an increased production of IL-10.Citation33 Moreover, IL-10 is an anti-inflammatory cytokine produced by Th2 cells which suppresses Th1-mediated immune responseCitation34 and an increase in the levels of this protein have been strongly associated with progression of CRC and a poor prognosis.Citation35 In contrast with IL-10, IL-12p70 is a pro-inflammatory cytokine produced by Th1 cells and the high level of this protein indicates the shift to antineoplastic phenotypes of tumor microenvironmental cells.Citation34,Citation36 Thus, the 2-fold alteration of the IL-10/IL-12p70 ratio () together with the reduction of M-CSF, IL-6 (), and NF-κB levels ( and ) induced by combined liposomal drug therapy, might suggest the conversion of the immunosuppressed phenotypes of the infiltrated immune cells to their antitumor phenotypes.Citation32,Citation37
Additionally, as previous studies have demonstrated that cancer cells require persistent levels of reactive oxigen species (ROS) for the preservation of tumor cell phenotype,Citation21,Citation38 the effects of different treatements on tumor oxidative stress were tested. Our results suggested that MDA levels were similar in C26 tumors treated with LCL-PLP+LCL-5-FU with those measured in control tumors (). Notably, each liposomal drug monotherapy enhanced significantly the production of MDA in C26 tumors in the proliferative range of tumor oxidative stress (µM) ().Citation20 The pro-oxidant effect exerted by the administration of either LCL-PLP or LCL-5-FU alone might be the principal cause for their lower antitumor activity compared with that determined after simultaneous administration of the liposomal formulations in C26 carcinoma in vivo ( and ). Since our recent data have already proved that tumor oxidative stress potentiated angiogenic capacity of C26 colon carcinoma microenvironment,Citation39 the pro-oxidant effect of the single administration of LCL-5-FU probably determined a more agressive phenotype of C26 colon carcinoma. Thus, after this treatment the levels of the angiogenic (VEGF and leptin) as well as anti-apoptotic (IL-6) and immunosuppressive (IL-13) proteins were strongly enhanced in tumor microenvironment ().Citation40-45 The LCL-PLP pro-oxidant action could also be linked to the less efficient anti-angiogenic and anti-inflammatory activity of this treatment compared with that exerted by LCL-PLP+LCL-5-FU in the same tumor type but in the presence of diminished intratumor levels of ROS ( and ). Our findings might be supported by previous studies that linked the increase in ROS levels in Caco-2 cells after different cytotoxic drug administrations with ROS-induced resistance to these treatments.Citation46 Moreover, probably, this enhancing effect of each liposomal drug monotherapy on tumor oxidative stress could be an explanation for lower antitumor activity of the combined therapy based on the sequential administration of the liposomal formulations compared with the antitumor activity of that based on their simultaneous administration (). Thus, the increased ROS levels induced by the pretreatment with LCL-PLP would diminish the antitumor activity of the subsequently administered LCL-5-FU in this tumor model.
In conclusion, the present study demonstrates the antitumor efficacy of the combined therapy based on the concurrent administration of LCL-PLP and LCL-5-FU compared with single administration of each liposomal formulation in C26 murine colon carcinoma-bearing mice. The antitumor activity of LCL-PLP+LCL-5-FU was based on the inhibition of tumor angiogenesis and inflammation in a C26 colon carcinoma microenvironment that was polarized toward an antineoplastic phenotype.
Material and methods
Preparation of liposomal formulations
Both liposomal formulations were prepared using the lipid film hydration methodCitation11,Citation47 with a lipid molar ratio of 9.5:0.5:1 (DPPC:PEG-2,000-DSPE:CHL) as described previously.Citation12,Citation48 These LCL were PEG-coated liposomes and contained 4.5 mol % PEG 2,000 (as PEG-2,000-DSPE) which increases their circulation time and passive accumulation in tumors.Citation49 The obtained LCL-PLP and LCL-5-FU were further characterized as mean particle size, polydispersity value, drug content, and encapsulation efficiency (EE).Citation12,Citation48 Thus, LCL-PLP had a size of about 100 nm with a polydispersity value < 0.1 and contained a relatively high concentration of PLP (9 mg PLP/ml and EE about 22%).Citation12 The size for LCL-5-FU was around 180 nm (polydispersity index < 0.10) and EE was 1.4% (150 µg/ml).Citation48 The advantage of these nano-sized liposomes that are below tumor vasculature cutoff limits (200–800 nm) enabled their passive accumulation within the tumor tissue.Citation50
Cells
C26 murine colon carcinoma cells (Cell Line Services, 440156) were cultured as a monolayer in complete RPMI 1640 medium (Lonza, 09–774F) supplemented with 10% (v/v) heat-inactivated fetal bovine serum (HyClone, SV30160.03), at 37 ºC in 5% CO2 humidified atmosphere. This cell line was authenticated via Real-Time PCR by the company from where it was purchased and was tested for micoplasma using MycoAlert Mycoplasma Detection Kit (Lonza, LT07).
Murine tumor model
Male BALB/c mice (6–8 weeks of age) were obtained from Cantacuzino Institute (Romania) and kept in standard housing with standard rodent chow and water available ad libitum under a 12-hour light/dark cycle. Experiments were performed according to the national regulations and were approved by the local animal experiments ethical committee (registration no. 31375/06.04.2015). For tumor induction, 1 × 106 C26 cells were inoculated s.c. in the right flank of syngeneic BALB/c mice. C26 tumors became palpable at day 7 after tumor cell inoculation. Tumor size was measured regularly starting with day 7 and the tumor volume was calculated according to the formula V = 0.52 x a2 x b, where a is the smallest and b is the largest superficial diameter (in mm). Each experimental group consisted of 5–6 mice.
Assessment of the antitumor activity of the combined administration of liposomal formulations versus single liposomal drug therapy in C26 colon carcinoma-bearing mice
The effects of the combined administration of liposomal formulations on the tumor growth were compared with those induced by the combined administration of the unencapsulated active agents. Thus, C26 murine colon carcinoma-bearing mice received i.v. 20 mg/kg PLP (as LCL or free form) and 1.2 mg/kg 5-FU (either LCL or free form) at days 8 and 11 after tumor cell inoculation. The antitumor activity of each combined therapy on C26 colon carcinoma-bearing mice was compared with that induced after the single treatment with either PLP or 5-FU administred as liposomal or free form using the same dosing schedule as shown above. The dosing schedule for LCL-PLP was selected based on previous studies on the high therapeutic index (antitumor efficacy vs. side effects) of a similar liposomal formulation on tumor growth.Citation51 The dose of 5-FU administered as LCL-5-FU was 100-fold lower than clinically applied doses for 5-FU in conventional chemotherapy of CRC.Citation52-54 Control groups were either injected with PBS or empty liposomes. Animals were killed at day 12 after tumor cell inoculation and tumors were isolated and frozen in liquid nitrogen.
Assessment of the antitumor activity of the 2 different combined administrations of liposomal formulations on tumor growth
To compare the inhibitory effects of different dosing schedules of the proposed combined tumor-targeted therapy on tumor growth, the mice received 2 i.v. injections of the indicated formulations administered either simultaneously at days 8 and 11 or sequentially, animals being pretreated with PLP (either LCL or free form at days 7 and 10) with 24 h before 5-FU (either LCL or free form) administration at days 8 and 11 after tumor induction. The antitumor activity of the sequential treatment scheme was selected to be tested based on previous studies that suggested the enhancement of the conventional chemotherapeutic efficacy after corticosteroid pretreatment of various animal tumor models including colon cancer.Citation55,Citation56 Mice were killed at day 18 after tumor induction, when the first tumors from control groups reached 2,000 mm3.
Determination of key inflammatory transcription factors production
Tumors from mice sacrificed at day 12 after C26 tumor induction were isolated, weighed, and then pooled to obtain tumor tissue lysates for each group.Citation57 The protein content of the tumor tissue lysates was assessed by biuret method.Citation58 To determine the effects of lipsomal drugs combination vs. the combination of the same free drugs on the levels of NF-κB and AP-1 key transcription factors for tumor inflammation,Citation13-15 western blot analysis was performed as described previously.Citation21,Citation47 Primary antibodies for NF-κB p65 (sc-56735), c-Jun (sc-45) or β-actin (sc-130656), and HRP-labeled goat anti-rabbit (sc-2004) and goat anti-mouse (sc-2005) antibodies (Santa Cruz Biotechnology) were used. The immunocomplexes were developed using Clarity™ Western ECL (Bio-Rad, 170–5061) and the blots were exposed to an X-ray film (Kodak, Z358487) for about 1–2 min. Results represent mean ± SD of 2 independent experiments.
Assessment of the tumor production of angiogenic/inflammatory proteins
To investigate the effects of combined liposomal drug therapy vs. free PLP+5-FU treatment on the expression levels of angiogenic/inflammatory proteins in whole tumor lysates we performed a screening for 24 proteins involved in angiogenesis and inflammation by using RayBio® Mouse Angiogenic Cytokine Antibody Array kit (RayBiotech, AAM-ANG-1–8) as described previously.Citation47 For this assay 250 µg of protein from whole tumor lysates were used. The protein expression levels were quantified by measuring the intensity of each spot on the membranes, in comparison to the positive control spots already bound to the membranes, using TotalLab Quant Software version 12 for Windows. The production of each protein in every experimental group was determined in duplicate and final results represent the mean ± SD of 2 independent measurements.
Immunohistochemical examination of tumor tissue after LCL-PLP + LCL-5-FU treatment
To assess the effects of the liposomal combination of LCL-PLP + LCL-5-FU over those of single agent liposomal therapy compared with control, tumors were fixed in 10% neutral buffered formalin and furthermore embedded in paraffin. For immunohistochemical studies, 4-µm-thick tumor sections were dewaxed and rehydrated. Heat-mediated epitope retrieval was achieved by samples immersion in boiling citrate buffer pH 6, using a pressure-cooker. Sections were cooled in citrate buffer at room temperature and washed in triphosphate-buffered saline. Endogenous peroxidase activity was blocked by applying 3% hydrogen peroxide in methanol for 10 minutes. Sections were incubated for 1 hour at room temperature with primary antibodies: monoclonal rat anti-mouse F4/80 diluted 1:50 (MCA497, Serotec, Oxford, UK) and rabbit policlonal anti-mouse iNOS diluted 1:100, (ab15323, Abcam, Newcastle, UK). For detection Novolink Max-Polymer detection system (Novocastra, Newcastle, UK) was used according to the manufacturer instructions. Sections were further incubated with 3,3’- diaminobenzidine (DAB) substrate to visualize positive reactions and counterstained with Gill 2 haematoxylin, dehydrated and mounted. The slides were examined under a microscope Olympus BX 51. The images were taken with Olympus UC 30 digital camera and processed by using Olympus Stream Basic image acquisition and processing program.
The numbers of F4/80 and iNOS positive cells in each experimental group were assessed by counting labeled cells on several non-overlapping fields of each slide and categorized into 3-score cathegories depending on the abundance of positively stained cells.Citation18
Effects of the LCL-PLP+LCL-5-FU treatment on the IL-10/IL-12p70 production ratio
For the detection of IL-10 protein levels from whole tumor lysates, mouse inflammatory cytokines multi-analyte ELISArray kit (Qiagen, MEM-004A) was used. For this assay, each IL-10 antibody-coated well was incubated with 200 µg of protein and every step was performed according to the manufacturer instructions. The IL-10 expression values from duplicate measurements were expressed as mean percentage ± SD compared with the expression levels of the same protein in control tumors. IL-12p70 was determined via protein array analysis and was expressed as % compared with its control levels.
Determination of malondialdehyde levels in C26 colon carcinoma tumors
To investigate the effects of different treatments on the tumor oxidative stress, the amount of MDA in tumor tissue was determined by HPLC as described previously.Citation21,Citation47 The retention time for MDA was around 6.4 minutes. Triplicate measurements were expressed as µM MDA and were normalized to the protein concentration from tumor homogenates.
Statistical analysis
All statistical analysis were performed by using GraphPad Prism version 6 for Windows (GraphPad Software, San Diego, CA, USA) and expressed as mean ± SD. The overall effects of different treatments on tumor growth were analyzed by one-way ANOVA with Bonferroni post-test for multiple comparisons. The DT of tumor volumes was estimated by using an exponential tumor growth equation. The differences between the effects of various treatments on the production of angiogenic/inflammatory proteins were analyzed by 2-way ANOVA with Bonferroni correction for multiple comparisons. A P value of < 0.05 was considered significant.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the UEFISCDI (Romanian Ministry of Education, Research and Innovation) under Grant PN-II-PTPCCA-2011–3–2–1060 (No. 95/2012) and Grant PN-II-RU-TE-2014–4–1191 (No. 235/01.10.2015).
References
- Zhang N, Yin Y, Xu SJ, Chen WS. 5-Fluorouracil: Mechanisms of resistance and reversal strategies. Molecules 2008; 13:1551-69; PMID:18794772; https://doi.org/10.3390/molecules13081551
- Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: Mechanisms of action and clinical strategies. Nat Rev Cancer 2003; 3:330-8; PMID:12724731; https://doi.org/10.1038/nrc1074
- Noordhuis P, Holwerda U, Van der Wilt CL, Van Groeningen CJ, Smid K, Meijer S, Pinedo HM, Peters GJ. 5-Fluorouracil incorporation into RNA and DNA in relation to thymidylate synthase inhibition of human colorectal cancers. Ann Oncol 2004; 15:1025-32; PMID:15205195; https://doi.org/10.1093/annonc/mdh264
- Diasio RB, Beavers TL, Carpenter JT. Familial deficiency of dihydropyrimidine dehydrogenase. Biochemical basis for familial pyrimidinemia and severe 5-fluorouracil-induced toxicity. J Clin Invest 1988; 81:47-51; PMID:3335642; https://doi.org/10.1172/JCI113308
- Pillai G, Ceballos-Coronel ML. Science and technology of the emerging nanomedicines in cancer therapy: A primer for physicians and pharmacists. SAGE Open Med 2013; 1:2050312113513759; PMID:26770691; https://doi.org/10.1177/2050312113513759
- Albanese A, Tang PS, Chan WC. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu Rev Biomed Eng 2012; 14:1-16; PMID:22524388; https://doi.org/10.1146/annurev-bioeng-071811-150124
- Gabizon AA. Liposomal drug carrier systems in cancer chemotherapy: Current status and future prospects. J Drug Target 2002; 10:535-8; PMID:12683720; https://doi.org/10.1080/1061186021000043061
- Schiffelers RM, Storm G. Liposomal nanomedicines as anticancer therapeutics: Beyond targeting tumor cells. Int J Pharm 2008; 364:258-64; PMID:18773947; https://doi.org/10.1016/j.ijpharm.2008.08.005
- Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J Control Release 2000; 65:271-84; PMID:10699287; https://doi.org/10.1016/S0168-3659(99)00248-5
- Torchilin V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv Drug Deliv Rev 2011; 63:131-5; PMID:20304019; https://doi.org/10.1016/j.addr.2010.03.011
- Schiffelers RM, Fens MH, Janssen AP, Molema G, Storm G. Liposomal targeting of angiogenic vasculature. Curr Drug Deliv 2005; 2:363-8; PMID:16305439; https://doi.org/10.2174/156720105774370186
- Sylvester B, Porfire A, Muntean DM, Vlase L, Luput L, Licarete E, Sesarman A, Alupei MC, Banciu M, Achim M, et al. Optimization of prednisolone-loaded long-circulating liposomes via application of Quality by Design (QbD) approach. J Liposome Res 2016:1-13; PMID:27788618; https://doi.org/10.1080/08982104.2016.1254242. [Epub ahead of print].
- Ashida R, Tominaga K, Sasaki E, Watanabe T, Fujiwara Y, Oshitani N, Higuchi K, Mitsuyama S, Iwao H, Arakawa T. AP-1 and colorectal cancer. Inflammopharmacology 2005; 13:113-25; PMID:16259733; https://doi.org/10.1163/156856005774423935
- Bossy-Wetzel E, Bakiri L, Yaniv M. Induction of apoptosis by the transcription factor c-Jun. EMBO J 1997; 16:1695-709; PMID:9130714; https://doi.org/10.1093/emboj/16.7.1695
- Sakamoto K, Maeda S, Hikiba Y, Nakagawa H, Hayakawa Y, Shibata W, Yanai A, Ogura K, Omata M. Constitutive NF-kappaB activation in colorectal carcinoma plays a key role in angiogenesis, promoting tumor growth. Clin Cancer Res 2009; 15:2248-58; PMID:19276252; https://doi.org/10.1158/1078-0432.CCR-08-1383
- Austyn JM, Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol 1981; 11:805-15; PMID:7308288; https://doi.org/10.1002/eji.1830111013
- Kou XX, Li CS, He DQ, Wang XD, Hao T, Meng Z, Zhou YH, Gan YH. Estradiol promotes M1-like macrophage activation through cadherin-11 to aggravate temporomandibular joint inflammation in rats. J Immunol 2015; 194:2810-8; PMID:25681337; https://doi.org/10.4049/jimmunol.1303188
- Banciu M, Metselaar JM, Schiffelers RM, Storm G. Antitumor activity of liposomal prednisolone phosphate depends on the presence of functional tumor-associated macrophages in tumor tissue. Neoplasia 2008; 10:108-17; PMID:18283332; https://doi.org/10.1593/neo.07913
- Del Rio D, Stewart AJ, Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis 2005; 15:316-28; PMID:16054557; https://doi.org/10.1016/j.numecd.2005.05.003
- Licarete E, Sesarman A, Banciu M. Exploitation of pleiotropic actions of statins by using tumour-targeted delivery systems. J Microencapsul 2015; 32:619-31; PMID:26299551; https://doi.org/10.3109/02652048.2015.1073383
- Patras L, Sesarman A, Licarete E, Luca L, Alupei MC, Rakosy-Tican E, Banciu M. Dual role of macrophages in the response of C26 colon carcinoma cells to 5-fluorouracil administration. Oncol Lett 2016; 12:1183-91; PMID:27446416; https://doi.org/10.3892/ol.2016.4708
- Lind DS, Hochwald SN, Malaty J, Rekkas S, Hebig P, Mishra G, Moldawer LL, Copeland EM 3rd, Mackay S. Nuclear factor-kappa B is upregulated in colorectal cancer. Surgery 2001; 130:363-9; PMID:11490372; https://doi.org/10.1067/msy.2001.116672
- Wang H, Birkenbach M, Hart J. Expression of Jun family members in human colorectal adenocarcinoma. Carcinogenesis 2000; 21:1313-7; PMID:10874008; https://doi.org/10.1093/carcin/21.5.313
- Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell 2005; 8:299-309; PMID:16226705; https://doi.org/10.1016/j.ccr.2005.09.005
- Igney FH, Krammer PH. Tumor counterattack: Fact or fiction? Cancer Immunol Immunother 2005; 54:1127-36; PMID:15889255; https://doi.org/10.1007/s00262-005-0680-7
- Nai YJ, Jiang ZW, Wang ZM, Li N, Li JS. Prevention of cancer cachexia by pyrrolidine dithiocarbamate (PDTC) in colon 26 tumor-bearing mice. JPEN J Parenter Enteral Nutr 2007; 31:18-25; PMID:17202436; https://doi.org/10.1177/014860710703100118
- Nowis D, McConnell EJ, Dierlam L, Palamarchuk A, Lass A, Wojcik C. TNF potentiates anticancer activity of bortezomib (Velcade) through reduced expression of proteasome subunits and dysregulation of unfolded protein response. Int J Cancer 2007; 121:431-41; PMID:17373661; https://doi.org/10.1002/ijc.22695
- Salven P, Ruotsalainen T, Mattson K, Joensuu H. High pre-treatment serum level of vascular endothelial growth factor (VEGF) is associated with poor outcome in small-cell lung cancer. Int J Cancer 1998; 79:144-6; PMID:9583728; https://doi.org/10.1002/(SICI)1097-0215(19980417)79:2<144::AID-IJC8>3.0.CO;2-T
- Takahashi Y, Kitadai Y, Bucana CD, Cleary KR, Ellis LM. Expression of vascular endothelial growth factor and its receptor, KDR, correlates with vascularity, metastasis, and proliferation of human colon cancer. Cancer Res 1995; 55:3964-8; PMID:7664263. Available from: http://cancerres.aacrjournals.org/content/55/18/3964.full-text.pdf
- Tammali R, Ramana KV, Singhal SS, Awasthi S, Srivastava SK. Aldose reductase regulates growth factor-induced cyclooxygenase-2 expression and prostaglandin E2 production in human colon cancer cells. Cancer Res 2006; 66:9705-13; PMID:17018629; https://doi.org/10.1158/0008-5472.CAN-06-2105
- Mantovani A, Allavena P. The interaction of anticancer therapies with tumor-associated macrophages. J Exp Med 2015; 212:435-45; PMID:25753580; https://doi.org/10.1084/jem.20150295
- Cook J, Hagemann T. Tumour-associated macrophages and cancer. Curr Opin Pharmacol 2013; 13:595-601; PMID:23773801; https://doi.org/10.1016/j.coph.2013.05.017
- Liu CY, Xu JY, Shi XY, Huang W, Ruan TY, Xie P, Ding JL. M2-polarized tumor-associated macrophages promoted epithelial-mesenchymal transition in pancreatic cancer cells, partially through TLR4/IL-10 signaling pathway. Lab Invest 2013; 93:844-54; PMID:23752129; https://doi.org/10.1038/labinvest.2013.69
- Michielsen AJ, Hogan AE, Marry J, Tosetto M, Cox F, Hyland JM, Sheahan KD, O'Donoghue DP, Mulcahy HE, Ryan EJ, et al. Tumour tissue microenvironment can inhibit dendritic cell maturation in colorectal cancer. PLoS One 2011; 6:e27944; PMID:22125641; https://doi.org/10.1371/journal.pone.0027944
- Stanilov N, Miteva L, Stankova N, Jovchev J, Deliyski T, Stanilova S. Role of IL-12P40 and IL-10 in progression of colorectal cancer. Khirurgiia (Sofiia) 2010; 4-5:26-9; PMID:21972680. Available from: http://tru.uni-sz.bg/tsj/vol8,Suppl.2,2010/N.Stanilov.pdf.
- Bien E, Krawczyk M, Izycka-Swieszewska E, Trzonkowski P, Kazanowska B, Adamkiewicz-Drozynska E, Balcerska A. Deregulated systemic IL-10/IL-12 balance in advanced and poor prognosis paediatric soft tissue sarcomas. Biomarkers 2013; 18:204-15; PMID:23557126; https://doi.org/10.3109/1354750X.2013.764351
- Rolny C, Mazzone M, Tugues S, Laoui D, Johansson I, Coulon C, Squadrito ML, Segura I, Li X, Knevels E, et al. HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PlGF. Cancer Cell 2011; 19:31-44; PMID:21215706; https://doi.org/10.1016/j.ccr.2010.11.009
- Qi XF, Kim DH, Yoon YS, Kim SK, Cai DQ, Teng YC, Shim KY, Lee KJ. Involvement of oxidative stress in simvastatin-induced apoptosis of murine CT26 colon carcinoma cells. Toxicol Lett 2010; 199:277-87; PMID:20883752; https://doi.org/10.1016/j.toxlet.2010.09.010
- Luput L, Licarete E, Sesarman A, Laura P, Alupei MC, Banciu M. Tumor-associated macrophages favor C26 murine colon carcinoma cell proliferation in an oxidative stress-dependent manner. Oncol Rep 2017; 37:2472-80; PMID:28260079; https://doi.org/10.3892/or.2017.5466
- Becker C, Fantini MC, Wirtz S, Nikolaev A, Lehr HA, Galle PR, Rose-John S, Neurath MF. IL-6 signaling promotes tumor growth in colorectal cancer. Cell Cycle 2005; 4:217-20; PMID:15655344; https://doi.org/10.4161/cc.4.2.1413
- Bendardaf R, Buhmeida A, Hilska M, Laato M, Syrjanen S, Syrjanen K, Collan Y, Pyrhönen S. VEGF-1 expression in colorectal cancer is associated with disease localization, stage, and long-term disease-specific survival. Anticancer Res 2008; 28:3865-70; PMID:19192642. Available from: http://ar.iiarjournals.org/content/28/6B/3865.long.
- Gonzalez-Perez RR, Lanier V, Newman G. Leptin's pro-angiogenic signature in breast cancer. Cancers (Basel) 2013; 5:1140-62; PMID:24202338; https://doi.org/10.3390/cancers5031140
- Gordon S, Martinez FO. Alternative activation of macrophages: Mechanism and functions. Immunity 2010; 32:593-604; PMID:20510870; https://doi.org/10.1016/j.immuni.2010.05.007
- Guerriero E, Capone F, Rusolo F, Colonna G, Castello G, Costantini S. Dissimilar cytokine patterns in different human liver and colon cancer cell lines. Cytokine 2013; 64:584-9; PMID:24064000; https://doi.org/10.1016/j.cyto.2013.09.002
- Pucci S, Mazzarelli P, Sesti F, Boothman DA, Spagnoli LG. Interleukin-6 affects cell death escaping mechanisms acting on Bax-Ku70-Clusterin interactions in human colon cancer progression. Cell Cycle 2009; 8:473-81; PMID:19177010; https://doi.org/10.4161/cc.8.3.7652
- Boonyong C, Pattamadilok C, Suttisri R, Jianmongkol S. Benzophenones and xanthone derivatives from Garcinia schomburgkiana-induced P-glycoprotein overexpression in human colorectal Caco-2 cells via oxidative stress-mediated mechanisms. Phytomedicine 2017; 27:8-14; PMID:28314481; https://doi.org/10.1016/j.phymed.2017.01.011
- Alupei MC, Licarete E, Patras L, Banciu M. Liposomal simvastatin inhibits tumor growth via targeting tumor-associated macrophages-mediated oxidative stress. Cancer Lett 2015; 356:946-52; PMID:25444912; https://doi.org/10.1016/j.canlet.2014.11.010
- Achim M, Tomuta I, Muntean D, Porfire A, Tefas LR, Patras L, Licarete E, Alupei MC, Vlase L, Banciu M. Optimization and in vitro evaluation of 5-fluorouracil – loaded long – circulating liposomes. Farmacia 2017; 65:82-91. Available from: http://www.revistafarmacia.ro/201701/issue12017art13.html
- Torchilin VP, Omelyanenko VG, Papisov MI, Bogdanov AA Jr., Trubetskoy VS, Herron JN, Gentry CA. Poly(ethylene glycol) on the liposome surface: On the mechanism of polymer-coated liposome longevity. Biochim Biophys Acta 1994; 1195:11-20; PMID:7918551; https://doi.org/10.1016/0005-2736(94)90003-5
- Porfire A, Tomuta I, Muntean D, Luca L, Licarete E, Alupei MC, Achim M, Vlase L, Banciu M. Optimizing long-circulating liposomes for delivery of simvastatin to C26 colon carcinoma cells. J Liposome Res 2015; 25:261-9; PMID:25487170; https://doi.org/10.3109/08982104.2014.987787
- Banciu M, Fens MH, Storm G, Schiffelers RM. Antitumor activity and tumor localization of liposomal glucocorticoids in B16 melanoma-bearing mice. J Control Release 2008; 127:131-6; PMID:18291548; https://doi.org/10.1016/j.jconrel.2008.01.008
- Kuebler JP, Wieand HS, O'Connell MJ, Smith RE, Colangelo LH, Yothers G, Petrelli NJ, Findlay MP, Seay TE, Atkins JN, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: Results from NSABP C-07. J Clin Oncol 2007; 25:2198-204; PMID:17470851; https://doi.org/10.1200/JCO.2006.08.2974
- Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm 2016; 7:27-31; PMID:27057123; https://doi.org/10.4103/0976-0105.177703
- Tournigand C, Andre T, Achille E, Lledo G, Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: A randomized GERCOR study. J Clin Oncol 2004; 22:229-37; PMID:14657227; https://doi.org/10.1200/JCO.2004.05.113
- Rayburn ER, Ezell SJ, Zhang R. Anti-inflammatory agents for cancer therapy. Mol Cell Pharmacol 2009; 1:29-43; PMID:20333321; https://doi.org/10.4255/mcpharmacol.09.05
- Wang H, Li M, Rinehart JJ, Zhang R. Pretreatment with dexamethasone increases antitumor activity of carboplatin and gemcitabine in mice bearing human cancer xenografts: In vivo activity, pharmacokinetics, and clinical implications for cancer chemotherapy. Clin Cancer Res 2004; 10:1633-44; PMID:15014014; https://doi.org/10.1158/1078-0432.CCR-0829-3
- Alupei MC, Licarete E, Cristian FB, Banciu M. Cytotoxicity of lipophilic statins depends on their combined actions on HIF-1alpha expression and redox status in B16.F10 melanoma cells. Anticancer Drugs 2014; 25:393-405; PMID:24441744; https://doi.org/10.1097/CAD.0000000000000065
- Gornall AG, Bardawill CJ, David MM. Determination of serum proteins by means of the biuret reaction. J Biol Chem 1949; 177:751-66; PMID:18110453. Available from: http://www.jbc.org/content/177/2/751.short.
