ABSTRACT
Therapy of colorectal cancer (CRC), especially a subset known as locally advanced rectal cancer, is challenged by progression and recurrence. Sphingolipids, a lipid subtype with vital roles in cellular function, play an important role in CRC and impact on therapeutic outcomes. In this review we discuss how dietary sphingolipids or the gut microbiome via alterations in sphingolipids influence CRC carcinogenesis. In addition, we discuss the expression of sphingolipid enzymes in the gastro-intestinal tract, their alterations in CRC, and the implications for therapy responsiveness. Lastly, we highlight some novel therapeutics that target sphingolipid signaling and have potential applications in the treatment of CRC. Understanding how sphingolipid metabolism impacts cell death susceptibility and drug resistance will be critical toward improving therapeutic outcomes.
Treatment challenges in colorectal cancer
As of 2016, colorectal cancer (CRC) remains the third most frequently diagnosed cancer with an estimated 140,000 new cases per year.Citation1 Of concern are the findings of a recent study by the American Cancer Society that the incidence of CRC is rapidly increasing among younger adults.Citation2 The incidence of rectal cancer, a subset of CRC located in the pelvis within 12 cm from the anus, has doubled between 1989–90 to 2012–13 in adults under 55 year old.Citation2 Locally advanced rectal cancer (LARC), either a muscle-invasive primary tumor (AJCC Stage 2) or involved lymph nodes (AJCC Stage 3), is particularly challenging to treat and has high rates of disease recurrence. Neoadjuvant 5-fluorouracil (5FU) chemoradiation (5FU/RT) is the most effective treatment approach for patients with LARC. The landmark randomized German Rectal Cancer Trial (CAO/ARO/AIO-94) showed that LARC patients treated with neoadjuvant 5FU/RT had fewer treatment-associated complications and less local disease recurrence compared with patients receiving surgery followed by 5FU/RT.Citation3 Tumor 5FU/RT pathologic response (AJCC tumor regression grading (TRG)) has since become an established surrogate marker of long-term survival and a useful oncologic benchmark.Citation4,5 Approximately 20% of the LARC patients have a pathologic complete response (TRG0) to 5FU/RT, with exceptional long-term outcomes.Citation5,6 Unfortunately in ∼80% of cases, therapeutic resistance is evident and contributes to surgical failure, disease recurrence, and ultimately, death of the patient. Further, while additional agents have shown promise in cancer cell lines, therapies combining these agents with 5FU/RT have significantly increased toxicity in patients without improvement in clinical response.Citation7-10 To change the therapeutic paradigm, LARC inter-patient heterogeneity must be integrated into clinical algorithms tailoring therapy for individual patients by either identifying more effective strategies or by omitting ineffective treatments to avoid unnecessary toxicity. Improving therapeutic response rates to preoperative therapy should ultimately translate into better outcomes associated with CRC. Given the high rate of resistance, highlighted by the lack of complete response in the majority of rectal cancer patients, exploring novel molecular strategies to enhance conventional therapy for CRC is desperately needed.
Molecular mechanisms of therapeutic resistance in CRC continue to be under intense investigation. Recently, investigations have demonstrated that bioactive sphingolipids play an important role in CRC and impact oncologic therapies such as chemotherapy and radiation. Therefore, dissecting the relationship between bioactive sphingolipids and chemoradiation resistance should provide insight into tumor survival mechanisms and suggest potential novel targets to improve CRC treatment strategies.
Sphingolipid metabolism
Sphingolipids represent a lipid subtype with vital roles in cellular function.Citation11 As structural components, sphingolipids influence the physical properties of membranes, which impacts on cellular signaling.Citation12 In addition, sphingolipids affect cellular signaling by acting as secondary messengers. An increasing body of literature supports the role of sphingolipids in regulating biologically critical processes such as cell growth and death, autophagy, migration and invasion, and angiogenesis.Citation13,14 Central to sphingolipid metabolism is ceramide, which can be generated de novo, through hydrolysis of complex sphingolipids, or via the salvage pathway (). De novo synthesis of ceramide occurs in the endoplasmic reticulum, where serine palmitoyl transferase (SPT) mediates the condensation of serine and palmitoyl-CoA to generate 3-ketosphinganine, which is then reduced to dihydrosphingosine. Next, ceramide synthases (CerS) add a fatty acid moiety generating dihydroceramide and lastly dihydroceramide desaturase (DEGS) introduces a double bond to generate ceramide. Ceramide serves as a building block for complex sphingolipids such as sphingomyelin and glycosphingolipids. Hydrolysis of these complex sphingolipids is the second metabolic pathway by which ceramide can be generated. Ceramide can also be hydrolyzed by ceramidases into sphingosine. In the salvage pathway, ceramide synthases utilize sphingosine and fatty acids as substrates to regenerate ceramide. Thus ceramide can be generated through 3 metabolic pathways, an apparent redundancy that may be necessary since lipid molecules do not freely diffuse throughout the cytoplasm. Distribution of sphingolipid enzymes across various subcellular compartments can thus fulfill the need for site-specific generation of ceramide. Ceramide can also be shuttled between compartments by ceramide transporters, such as CERT.
Figure 1. Sphingolipid metabolism. Central to sphingolipid metabolism is ceramide, which can be generated de novo and is used as a building block for complex sphingolipids. It can also be phosphorylated or further metabolized into sphingosine-1-phosphate. See text for more details. Abbreviations: SPT = serine palmityoltransferase; CerS = ceramide synthase; DEGS = dihydroceramide desaturase; SMS = sphingomyelin synthase; SMase = sphingomyelinase; GCS = glucosylceramide synthase; CDase = ceramidase; CK = ceramide kinase.
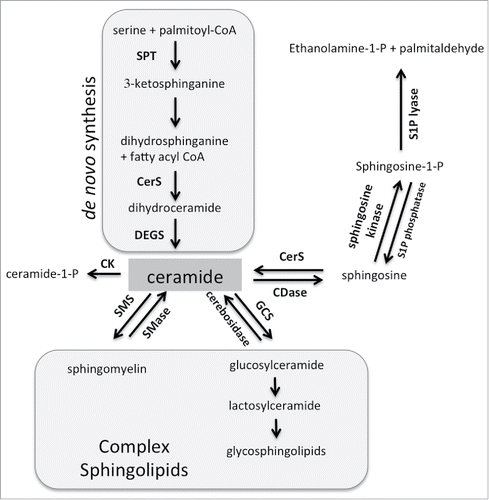
In contrast to ceramide, sphingosine and sphingosine-1-phosphate are soluble mediators. Sphingosine is generated by hydrolysis of ceramide by ceramidases and serves as a substrate for sphingosine kinases, which through the addition of a phospho-group generate sphingosine-1-phosphate (S1P). The phospho-group of S1P can be removed by S1P phosphatase or S1P lyase, which results in regeneration of sphingosine or irreversible degradation into ethanolamine-1-P and palmitaldehyde, respectively. The latter step constitutes the exit from sphingolipid metabolism. As shown in , the majority of enzymatic reactions in the sphingolipid pathway are reversible, which endows cells with flexibility in responding to cellular stimuli. Consequently, cellular stimuli can induce a flux in sphingolipids that involve rapid conversion between ceramide, complex sphingolipids, sphingosine and S1P.
Additional complexity derives from multiple isoforms of sphingolipid enzymes that can vary in subcellular location and pH requirements. For example, there are at least 3 ceramidases and sphingomyelinases with different pH requirements and 2 sphingosine kinases with different subcellular locations. Furthermore, the 6 ceramide synthases (CerS1-CerS6) have preferential substrate utilization and can generate ceramides that vary in acyl chain length ranging from 14–30 (or more) carbons.Citation15 CerS1 preferentially generates ceramide with 18-carbon fatty acids (C18-Cer) while CerS5 or CerS6 primarily generate ceramide with 16-carbon fatty acids (C16-Cer). Phenotypes observed in CerS-deficient mice suggest that ceramides with different fatty acid chain lengths have distinct biologic roles. For example, CerS3 or CerS4-deficient mice develop problems with skin barrier function and alopecia, respectively,Citation16,17 while CerS1- and CerS2-deficient mice exhibit abnormalities in the central nervous system.Citation18 In addition, CerS2-deficiency (inability to generate C24-Cer) results in a compensatory generation of C16-Cer, which in the liver leads to apoptosis and subsequent development of hepatocellular cancer.Citation19,20 Similarly, shifting ceramide composition in cancer cell lines through targeting specific CerS changes cellular signaling and responses.Citation21 The tissue distribution of CerS varies and likely reflects the need of specific ceramide species for proper signaling and sphingolipid homeostasis in any given tissue. Several comprehensive reviews on CerS are available.Citation18,21,22
The two isoforms of sphingosine kinase, SK1 and SK2, both utilize sphingosine and generate S1P, but have significant differences in subcellular localization and function.Citation23 Upon activation, SK1 migrates to the plasma membrane where it releases S1P extracellularly. Extracellular S1P exert its actions in an autocrine or paracrine fashion by binding to 5 different S1P-receptors that via G-proteins regulate a broad range of cellular functions. In contrast to SK1, SK2 appears to have both pro- and anti-apoptotic functions depending on cell type, stimuli, and subcellular localization.Citation23 Targeting SK2 in malignancies reduces tumor growth and suggests an anti-apoptotic role for this enzyme in cancer.Citation23
Ceramide has been associated with cell death, growth inhibition and differentiation whereas S1P regulates proliferation, motility, angiogenesis and inflammation. Sphingolipid homeostasis is critical for normal cellular function, which it evident from rare mutations that result in lipid storage diseases as well as phenotypes of various sphingolipid gene knockout mice. Alterations in bioactive sphingolipids have been associated with a variety of human diseases, including cancer.
The role of dietary sphingolipids in CRC carcinogenesis
The per capita consumption in the United States from sources rich in sphingolipids such as dairy products or soy is estimated at 0.3–0.4 g/day.Citation24 Considering this significant intake of dietary sphingolipids, the impact of dietary sphingolipids on intestinal carcinogenesis has been investigated in different animal models. Models of intestinal carcinogenesis include treatment with chemical carcinogens such as 1,2-dimethylhydrazine (DMH) or azoxymethane (AOM) and APCmin mice, which serve as genetic model for inactivating mutations of the Adenomatous Polyposis Coli (APC) gene. Inactivation of APC, a negative regulator of the Wnt signaling pathway is the most common gene abnormality in CRC and mutations are found in 81% of the CRC cases in the Cancer Genome Atlas. Mutation of APC results in accumulation of β-catenin, which acts as a transcriptional co-activator of TCF/LEF gene family and promotion of CRC.Citation25 APCmin mice, which express a truncation mutation of the APC gene, develop up to 100 polyps in the small intestine as well as colon tumors. Multiple studies have confirmed that dietary sphingolipids, including sphingodienes, glucosylceramide, and sphingomyelin reduce carcinogenesis in both APCmin mice as well as in DMH treated mice.Citation26-29 Adding soy glucosylceramide to the diet of DMH treated mice reduced colonic cell proliferation by about 50% and significantly diminished the number of aberrant colonic crypt foci.Citation27 Similarly, sphingomyelin supplementation reduced the number of aberrant crypt foci by 70% and diminished the number of colonic adenocarcinomas.Citation30 These studies suggested a role for intestinal metabolism of dietary sphingolipids as a critical step in protection from colon carcinogenesis and supported the hypothesis that elucidating signaling events may provide a foundation for possible therapeutic approaches in CRC.
Dietary sphingolipids are metabolized throughout the intestinal tract.Citation31 The metabolism of dietary sphingomyelin, which is found in dairy products,Citation30 is initiated by the release of cholecystokinin from intestinal endocrine cells, which stimulates the release of alkaline sphingomyelinase from the gallbladder and the secretion of trypsin from the pancreas. Trypsin cleaves alkaline sphingomyelinase releasing it from the intestinal mucosa and enhancing enzymatic activity in the lumen.Citation32 Ceramide generated by the activity of alkaline sphingomyelinase is then further hydrolyzed by neutral ceramidase into sphingosine, which is absorbed into enterocytes, where it can be converted into S1P. In the DMH model, dietary sphingomyelin resulted in increased mRNA, protein expression and activity of alkaline sphingomyelinase,Citation33 which presumably increases the generation of ceramide, although this was not directly evaluated in the study. In the APCmin model, 3 types of sphingolipid-supplemented diets (ceramide, milk sphingolipids similar to proportions found in dairy products, and a mixture of 60% complex sphingolipids and 40% ceramide) were compared with the control diet. While all sphingolipid-supplemented diets significantly reduced the tumor burden per animal, the mixture of complex sphingolipid and ceramide was the most effective and resulted in the redistribution of β-catenin from a diffuse pattern into localization to intercellular junctions between intestinal epithelial cells.Citation29 Moreover, when CRC cell lines that harbor APC mutations (SW480 and T84) were treated with sphingosine or ceramide, cytosolic and nuclear β-catenin levels were reduced, which ultimately lead to cell death.Citation29 In the APCmin model, sphingodienes reduce Wnt signaling via a protein phosphatase 2A/Akt/GSK3β-dependent mechanism.Citation26 Glucosylceramide, which reduced tumor burden in the APCmin and DMH models, significantly alters the expression of 96 genes, including decreased expression of the TCF/LEF family member TCF4.Citation27 Taken together, these studies suggest that dietary sphingolipids may inhibit intestinal carcinogenesis through negative regulation of Wnt pathway signaling. The protective effects of sphingolipids in the setting of APC mutations provide supportive evidence of targeting sphingolipid metabolism for therapeutic intervention.
The role of the gut microbiome in CRC
Symbiosis between the host and the gut microbiome is critical for intestinal health. Metagenomic studies show that patients with CRC have altered gut microbiomes when compared with healthy controls and some studies have linked specific microbes to increased tumorigenesis.Citation34,35 While sphingolipids have been primarily studied in eukaryotes, microbes such as the soil dwelling Sphingomonas as well as human commensals including Bacteroides, Porphyromonas, and Prevetella from the Bacteroidetes family contain sphingolipids.Citation36,37 Bacteroides fragilis, a prevalent gram-negative microbe within the intestine, encounters a significant amount of stress within the constantly changing host environment. B. fragilis sphingolipids do not seem to play a role in growth of the microbe but rather are essential for survival under stressful conditions.Citation38 Interestingly, germ-free mice or mice colonized with B. fragilis lacking the bacterial equivalent of SPT were found to have an increase in invariant natural killer T cells (iNKT), which play a key role in innate and adaptive immunity.Citation39 This increase in iNKT cells was unique to the gastro-intestinal niche and facilitated an increased inflammatory response following induction of colitis.Citation39 B. fragilis sphingolipids were found to inhibit the proliferation of iNKT cells during neonatal development, which suggested a potential role of these lipids in the maintenance of gut homeostasis.Citation39 A proof-of principle experiment demonstrated that oral administration of bacterial glycosphingolipids to young mice resulted in reduced iNKT cells and protection from colitis into adulthood.Citation39
A subset of B. fragilis known as enterotoxigenic B. fragilis (ETBF) is distinguished by its ability to secrete a pathogenic enterotoxin. ETBF are increased in patients with inflammatory bowel disease and CRC.Citation40,41 Recently it was shown that ETBF induce the release of intestinal mouse derived exosome-like nanoparticles (IDEN) that contain S1P and mediate tumor promotion through induction of pro-inflammatory Th17 cells.Citation42 Comparing the effect of secreted particles from nontoxigenic (NTBF) and ETBF, researchers found that NTBF particles induced immunosuppressive Treg cells (CD4+FoxP3+) and exerted a protective effect in the DSS-induced colitis model, whereas particles from ETBF induced inflammatory Th17 cells.Citation42 The effect of ETBF was recapitulated with NTBF engineered to express the enterotoxin. Further studies led to a model in which particles from ETBF induced intestinal epithelial cells to secrete S1P-containing IDENs. These IDENs then had a 2-fold function in promoting the inflammatory response: (i) IDENs recruit inflammatory T cells (CD4+IL17A+CCR6+) from the periphery into the intestinal tissue and (ii) IDENs were taken up by intestinal macrophages resulting in enhanced production of prostaglandin E2, which promotes the proliferation of the recruited pro-inflammatory CD4+IL17A+CCR6+ T cells.Citation42 Since the risk of CRC in patients with inflammatory bowel disease is increased by approximately 2–3-fold,Citation43 these studies suggest that toxin-induced S1P release may contribute to colon tumorigenesis.
Certain probiotic bacteria can also influence host sphingolipids and/or their enzymes. Lactobacillus rhamnosus GG reduces levels of lysophosphatidylcholines, sphingomyelins, and glycerophosphatidylcholines, whereas Lactobacillus brevis and Streptococcus thermophilus increase neutral sphingomyelinase levels, which may be involved in ceramide induced immune cell apoptosis.Citation44,45 Taken together these studies show that microbes can either directly, through their own sphingolipids, or indirectly, through modulation of host sphingolipids, impact intestinal inflammation, which has been associated with CRC carcinogenesis. These findings suggest novel opportunities to utilize microbes or nano-particle mediated delivery as preventative or possibly as therapeutic strategies in CRC.
Expression of sphingolipid enzymes in the gastro-intestinal tract and alterations in CRC
Ceramide synthases
Ceramide synthases (CerS1-CerS6) vary in mRNA distribution across tissues and preferentially generate ceramides with specific chain lengths. The first mammalian ceramide synthase was discovered in 2002 and subsequent overexpression and knockout experiments have indicating that altering the composition of ceramide species influences cell physiology and pathology.Citation21,46 CerS-deficient mice are viable and do not have any obvious defects in the gastrointestinal tract, suggesting that CerS family members have the ability to functionally compensate under normal physiologic conditions.Citation21,46
CerS mRNA expression varies among tissues but is not necessarily reflective of protein expression or activity. The biscistronic mRNA for CerS1, a CerS that preferentially generates C18-Cer, is detected in the small and large intestine.Citation47 CerS1 protein however was only detected in CRC cell lines and not in colonic biopsies,Citation47 which is consistent with data in the protein atlas http://www.proteinatlas.org/ENSG00000223802-CERS1/tissue. CerS2, which preferentially generates very long chain ceramides such as C24-ceramide, is ubiquitously expressed and has been suggested to function as a housekeeping gene.Citation48,49 CerS3 appears to primarily play a role in the skin and testis and although its mRNA has been detected in the gastrointestinal tract, it is unclear whether the protein is expressed. CerS4 has been implicated in folate stress, yet RNAi targeting of this protein did not protect cells (including HCT116 colon cancer cells) from methotrexate toxicity nor did it noticeably affect proliferation.Citation50 Similar to CerS2, CerS5 and CerS6, which both preferentially generate C16-Cer, are ubiquitously expressed.Citation51 CerS5 and CerS6 appear to be involved in cell stress responses.Citation21 CerS6 mRNA is highly expressed in the intestinal tract and the C16-Cer content in CerS6-deficient mice is reduced to 25%.Citation22,52 Reduced CerS6 expression has been associated with epithelial to mesenchymal transition in a panel of cancer cells that included several CRC cell lines.Citation53
Sphingomyelinases
In healthy human colon tissue sphingomyelinase activity is detected at acidic, neutral, and alkaline pH, indicating that 3 sphingomyelinases (SMase) known as acid SMase (aSMase), neutral SMase (nSMase) and alkSMase, are expressed. AlkSMase plays an important role in digestion with expression highest in the ascending colon and lowest in the rectum.Citation54 A similar gradient was found for nSMase but not for aSMase.Citation54 In comparison to surrounding normal tissue, SMase activity in colorectal cancer is reduced by 75%, 50%, and 30% for alkSMase, nSMase and aSMase, respectively.Citation54 These results are consistent with immunohistochemical staining for alkSMase described in the protein atlas, although results are considered uncertain as they are based on only one antibody (http://www.proteinatlas.org/ENSG00000182156-ENPP7/tissue#gene_information).
Ceramidases
Five different ceramidase genes encode enzymes that hydrolyze ceramide at different pH optimums. Little is known about alkaline ceramidases but interestingly ACER3-deficiency has recently been associated with colitis-induced colon cancer in mice due to an increase in C18:1-Cer and increased expression of proinflammatory cytokines.Citation55 Acid (ASAH1) and neutral ceramidase (ASAH2) have been more extensively studied and both have been identified as potential therapeutic targets in malignancies.
ASAH1 is primarily found in the lysosomal compartment and mutations in its gene result in rare genetic disorders as well as other diseases.Citation56,57 Knockout of ASAH1 results in death early during embryonic development, indicating that other ceramidases are unable to compensate for its enzymatic activity.Citation58 Although ASAH1 expression is essential for development, expression in the normal colon is relatively low.Citation59 Ceramide has been shown to transcriptionally induce ASAH1 expression either in response to radiation or as a consequence of CerS6 (and possibly CerS3–5) overexpression.Citation60,61 Thus it is possible that expression of ASAH1 increases in response to cell stresses that elevate intracellular ceramide within the intestinal system.
ASAH2 is the enzyme that hydrolyses ceramide generated by alkSMase during digestion of dietary sphingolipids. ASAH2-deficient mice develop normally but tissues have elevated ceramide and reduced levels of sphingosine.Citation62 RNAi targeting of ASAH2 in HT29 and HCT116 colon cancer cell lines increases ceramide, leading to 50% loss in viability in vitro and delayed xenograft growth in vivo.Citation63 In the AOM carcinogenesis model, ASAH2-deficient mice developed significantly fewer tumors with a 93% reduction in adenocarcinomas and an 82% decrease in total colon tumors compared with wild type mice.Citation63
Sphingosine kinases
Both sphingosine kinases play important roles in CRC and are potential therapeutic targets. The SK1/S1P pathway was found to positively regulate cyclooxygenase-2 (COX-2), an enzyme involved in inflammatory pathways that is overexpressed in several epithelial cancers. SK1 expression is elevated in the majority of human CRC specimen (78–89%).Citation64,65 A similar increase in SK1 expression was detected in the AOM carcinogenesis model.Citation64 Elevated expression of SK1 also increases the susceptibility to colitis-associated cancer.Citation66 Conversely, SK1-deficient mice had significantly less aberrant crypt foci formation and reduced colon cancer development.Citation64 Recently, it was shown that targeting SK1 with RNAi in CRC cell lines SW480 and HCT116 increases E-cadherin expression, decreases vimentin expression and reduces viability and migration.Citation65 These observations suggest a role of SK1 in epithelial to mesenchymal transition, proliferation, and migration in the absence of inflammatory signaling.
SK2 also increases in CRC (51/64; 80%), although 21/64 apparently normal tissues also stained positive for SK2.Citation67 In serum-free cultures of HCT116 CRC cells, SK2 has been suggested to function as a survival factor.Citation68 Recently targeting of SK2 by RNAi was shown to decrease expression of cMyc in the CRC cell line LoVo, which is consistent with cMyc as a downstream target of SK2.Citation67,69 cMyc plays an important role as a target gene of TCF-4 during aberrant signaling of mutated APC,Citation70 which highlights SK2 as a potential novel target in CRC.
Sphingolipids and radiation sensitivity
With the central role of sphingolipids in mediating apoptosis, a growing body of evidence implicates sphingolipids as critical mediators of anti-neoplastic treatment approaches such as chemotherapy and radiation. Given the importance of radiation in the therapeutic armamentarium for LARC, understanding the close association between sphingolipid metabolism and radiation may open opportunities to define novel therapeutic strategies for the future.
Early studies supported a direct link between radiation sensitivity and sphingolipids by demonstrating that radiation activates aSMase to generate ceramide with resultant formation of ceramide-rich domains in the plasma and mitochondrial membranes.Citation71-73 Investigations definitively demonstrated that endothelial derived aSMase was a central mediator of the radiation efficacy highlighting the importance of the tumor stromal compartment in mediating the ceramide response.Citation73 An elegant study using a model of radiation-induced apoptosis in Caenorhabditis elegans germ cells confirmed a central role of ceramide in the radiation response.Citation74 Investigators demonstrated that loss-of-function mutations in CerS genes, hyl-1 and lagr-1, completely inhibited the apoptotic response that could be rescued with exogenous delivery of C16-Cer.Citation74
Recent investigations have demonstrated that radiation efficacy is driven in part by the resulting accumulation of ceramides to enhance apoptosis mediated by multiple pathways including protein phosphatases PP1 and PP2A, cathepsin D and protein kinase Cζ.Citation75 Studies in cancer cell lines demonstrated the complex regulation of radiation-induced ceramide generation and apoptosis.Citation76 In contrast to CerS2 overexpression that resulted in partial protection from apoptosis, CerS5 and 6 enhanced radiation-induced apoptosis and increased C16-Cer.Citation76 Further, many agents and stimuli that lead to cell death require CerS, particularly CerS6, activity for effect, suggesting a critical role in radiation response.Citation77-84
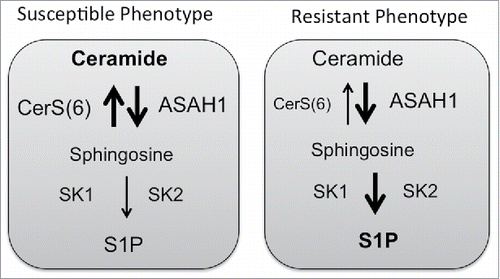
The generation of ceramide following therapy induces apoptosis but can also transcriptionally activate ASAH1. In prostate cancer cells, ceramide generated following radiation therapy transcriptionally activates ASAH1 expression via an AP-1 mediated mechanism.Citation61 The increase in ASAH1 resulted in elevation of S1P and therapy resistance and was also associated with relapse following radiation therapy.Citation61 These results are consistent with several other studies that have associated elevated expression of ASAH1 with proliferation and drug resistance.Citation85-87 In colon cancer cells, overexpression of CerS6, which shifts the cellular ceramide profile toward C16-Cer, also results in transcriptional activation of ASAH1.Citation60 However, in contrast to prostate cancer cells, the increase in ASAH1 as a consequence of CerS6 overexpression resulted in increased susceptibility to 5FU chemotherapy, despite transcriptional activation of ASAH1.Citation60 Interestingly, ASAH1 expression has been positively correlated with a better prognosis in luminal subtypes of breast cancer and epithelial ovarian cancer.Citation88,89 Thus elevated ASAH1 may not be exclusively associated with resistance. The key difference between the prostate and colon cancer models appears to be the metabolic flux of the ASAH1 product sphingosine. While S1P levels increased in prostate cancer cells, analysis of metabolic flux in HT29 cells overexpressing CerS6 yielded minimal generation of S1P compared with HT-GFP control cells.Citation60,61 This suggests that in cells with high levels of CerS6, the ASAH1 product sphingosine may be preferentially used in the salvage pathway to regenerate ceramide whereas in cells where ASAH1 is associated with resistance, sphingosine maybe preferentially used by SK to generate S1P. The influence sphingolipid metabolic flux may have on cell death susceptibility is illustrated in .
Sphingolipid metabolism as a therapeutic target
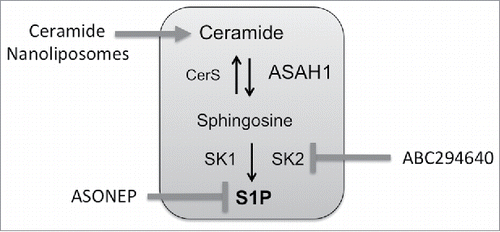
As ceramide has emerged as an important mediator of cell death responses, therapies to directly target sphingolipid metabolism have been developed. Strategies include antibodies that bind S1P, inhibitors of enzymes involved in driving sphingolipid metabolism toward S1P, and the use of ceramide or ceramide analogs.Citation90-93 The S1P antibody Sphingomab slowed tumor growth in a preclinical study of renal cell cancer and appeared to exert this effect by targeting the vasculature, since blood flow to tumors was reduced when measured by MRI.Citation94 The humanized S1P antibody ASONEP was well tolerated but overall progression-free survival in patients with advanced renal cell carcinoma was less than 2 months (http://www.prnewswire.com/news-releases/lpath-reports-results-for-asonep-phase-2a-study-in-renal-cell-carcinoma-300053872.html).
Inhibitors that interfere with generation of S1P include inhibitors of ceramidases and sphingosine kinases. Ceramidase inhibitors have been developed for ASAH1 and ASAH2.Citation63,95 The ASAH1 inhibitor LCL-521 has shown promising results in head and neck squamous carcinoma and prostate cancer modelsCitation61,96 but has not been evaluated in CRC. The ASAH2 inhibitor C6-urea ceramide preferentially reduced viability in a panel of human colon cancer cell lines compared with non-malignant rat intestinal epithelial cells.Citation63 C6-urea ceramide did not significantly impact on levels of sphingosine or S1P but increased ceramide, which enhanced apoptosis and increased autophagic flux.Citation63 Neither LCL-521 nor C6-urea ceramide have yet been evaluated in clinical trials.
SK are also a key targets in cancer therapy and both SK1 and SK2 inhibitors have been developed.Citation97-99 Of particular interest to CRC is the SK2 inhibitor ABC294640, which protects from colitis-driven CRC in the AOM/DSS model.Citation100 ABC294640 also enhances the efficacy of 5FU and significantly reduced the growth of HT29 xenografts.Citation101 The Phase I trial for ABC294640 was successfully completedCitation102 and additional clinical trials in hematological malignancies (NCT02229981, NCT02757326) and hepatocellular carcinoma (NCT02939807) have been initiated. As an inhibitor of SK2, treatment with ABC294640 decreases cMyc, which is a target gene of TCF-4 during aberrant signaling of mutated APC, and may therefore be a promising agent in the treatment of CRC.Citation69,70,103-105
Cationic ceramides, developed based on structure-function and targeting properties, induce anti-proliferative responses, including autophagic cell death, apoptosis and growth inhibition.Citation93,106,107 Among the cationic ceramides, LCL-30 has been investigated in CRC.Citation108,109 Due to the cationic charge LCL-30 preferentially localizes to the mitochondria and triggers the apoptotic pathway.Citation109 Treatment of mice bearing CT26 tumors with LCL-30 mono-therapy resulted in a highly significant reduction in tumor burden.Citation108 The anti-tumor efficacy of LCL-30 was greater than doxorubicin although combination of LCL-30 and doxorubicin did not result in a benefit over LCL-30 alone.Citation108
Another approach to utilize ceramides as therapeutics involves the encapsulation within non-toxic, non-aggregating, nanoscale (<100 nm) vehicles. Nanotechnology has the potential to improve the pharmacokinetics, biodistribution, and toxicological profiles of exogenously administered bioactive ceramide. In addition, drug encapsulation within nanotechnology can address inherent issues of poor solubility, precipitation, non-tumor cell delivery (liver cells or macrophages) and immunogenicity of bioactive lipids. Ceramide has been encapsulated within nanoscale liposomes, calcium phosphosilicate nanocolloids, nanoemulsions, polyethyleneoxide-modified polyepsilon-caprolactone nanoparticles (PEO-PCL) and linear-dendritic nanoparticles.Citation110,111 One of these preclinical approaches, the C6-ceramide nanoliposome (CNL) has just entered the clinic for solid tumors under FDA IND 109471 (NCT 02834611). CNL is a homogeneous 85nM, −8mV, nanotechnology that intercalates 30 molar percent C6-ceramide within a 12 molar percent pegylated liposome.Citation112 Compared to positively charged ceramide analogs or vehicles, these anionic particles intravenously deliver therapeutic doses of ceramide to tumors, without inducing liver or renal toxicities in rodent and canine models. Biological efficacy has been observed in models of CRC, hepatocellular carcinoma, head and neck squamous cell carcinoma, melanoma, breast cancer, pancreatic cancer as well as LGL, BCLL and AML leukemias.Citation112 Alluding to this efficacy, CNL is engineered to exploit the enhanced permeability and retention (EPR) properties of tumors, due to a calculated biologic half-life of between 18–21 hours compared with just minutes for free ceramide. Recently, CNL were shown to inhibit STAT3 signaling in chronic lymphocytic leukemia (Doshi, et al. under review). The STAT3 pathway is constitutively activated in colon-cancer initiating cells (ALDH+CD133+ subpopulation) and targeting STAT3 through genetic (shRNA) or pharmacological (LLL12) approaches significantly inhibited tumor growth.Citation113 Thus incorporating CNL into therapeutic regimen in notoriously difficult to treat LARC could be a powerful approach. Moreover, second generation CNL or other nanoscale approaches are already being developed that can be actively targeted to cancer cells through bio-conjugation of small molecules, antibody fragments or aptamers or that can co-deliver synergistic drugs within the ceramide nanoscale delivery platform.
Conclusions
In over a decade, since the landmark randomized German Rectal Cancer Trial, neoadjuvant chemoradiation has remained the gold standard approach for LARC. As our understanding and appreciation of the complex sphingolipid regulatory pathways grow in the context of cancer biology, we have learned that the imbalance of bioactive sphingolipids can have a major impact the therapeutic efficacy of chemotherapy and radiation. In the future, harnessing these critical metabolic pathways through novel therapies such as ABC294640 or CNL, which are currently under evaluation in clinical trials involving cancer patients, holds significant potential to replace or synergize with traditional therapies like radiation and to improve care for patients with this devastating malignancy.
Disclosure of potential conflicts of interest
Penn State Research Foundation has licensed ceramide nanoliposomes to Keystone Nano, Inc. (University Park, PA). MK is co-founder and CSO of Keystone Nano.
Funding
This work was supported by the National Institutes of Health under grant P30GM103339, P01CA203628. Dr. Mark Kester was supported by grants from (R01 CA 207396,R01 CA 167535, P01 CA 171983).
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016; 66:7-30; PMID:26742998; https://doi.org/10.3322/caac.21332
- Siegel RL, Fedewa SA, Anderson WF, Miller KD, Ma J, Rosenberg PS, Jemal A. Colorectal cancer incidence patterns in the United States, 1974–2013. J Natl Cancer Institute 2017; 109; PMID:28376186; https://doi.org/10.1093/jnci/djw322
- Sauer R, Fietkau R, Wittekind C, Rodel C, Martus P, Hohenberger W, Tschmelitsch J, Sabitzer H, Karstens JH, Becker H, et al. Adjuvant vs. neoadjuvant radiochemotherapy for locally advanced rectal cancer: the German trial CAO/ARO/AIO-94. Colorectal Dis 2003; 5:406-15; PMID:12925071; https://doi.org/10.1046/j.1463-1318.2003.00509.x
- Rodel C, Martus P, Papadoupolos T, Fuzesi L, Klimpfinger M, Fietkau R, Liersch T, Hohenberger W, Raab R, Sauer R, et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol 2005; 23:8688-96; PMID:16246976; https://doi.org/10.1200/JCO.2005.02.1329
- Park IJ, You YN, Agarwal A, Skibber JM, Rodriguez-Bigas MA, Eng C, Feig BW, Das P, Krishnan S, Crane CH, et al. Neoadjuvant treatment response as an early response indicator for patients with rectal cancer. J Clin Oncol 2012; 30:1770-6; PMID:22493423; https://doi.org/10.1200/JCO.2011.39.7901
- Bouzourene H, Bosman FT, Seelentag W, Matter M, Coucke P. Importance of tumor regression assessment in predicting the outcome in patients with locally advanced rectal carcinoma who are treated with preoperative radiotherapy. Cancer 2002; 94:1121-30; PMID:11920483; https://doi.org/10.1002/cncr.10327
- Willett CG, Duda DG, di Tomaso E, Boucher Y, Ancukiewicz M, Sahani DV, Lahdenranta J, Chung DC, Fischman AJ, Lauwers GY, et al. Efficacy, safety, and biomarkers of neoadjuvant bevacizumab, radiation therapy, and fluorouracil in rectal cancer: a multidisciplinary phase II study. J Clin Oncol 2009; 27:3020-6; PMID:19470921; https://doi.org/10.1200/JCO.2008.21.1771
- Czito BG, Willett CG, Bendell JC, Morse MA, Tyler DS, Fernando NH, Mantyh CR, Blobe GC, Honeycutt W, Yu D, et al. Increased toxicity with gefitinib, capecitabine, and radiation therapy in pancreatic and rectal cancer: phase I trial results. J Clin Oncol 2006; 24:656-62; PMID:16446337; https://doi.org/10.1200/JCO.2005.04.1749
- Dewdney A, Cunningham D, Tabernero J, Capdevila J, Glimelius B, Cervantes A, Tait D, Brown G, Wotherspoon A, Gonzalez de Castro D, et al. Multicenter randomized phase II clinical trial comparing neoadjuvant oxaliplatin, capecitabine, and preoperative radiotherapy with or without cetuximab followed by total mesorectal excision in patients with high-risk rectal cancer (EXPERT-C). J Clin Oncol 2012; 30:1620-7; PMID:22473163; https://doi.org/10.1200/JCO.2011.39.6036
- Velenik V, Ocvirk J, Music M, Bracko M, Anderluh F, Oblak I, Edhemovic I, Brecelj E, Kropivnik M, Omejc M. Neoadjuvant capecitabine, radiotherapy, and bevacizumab (CRAB) in locally advanced rectal cancer: results of an open-label phase II study. Radiation Oncol 2011; 6:105; PMID:21880132; https://doi.org/10.1186/1748-717X-6-105
- Hannun YA, Obeid LM. The Ceramide-centric universe of lipid-mediated cell regulation: stress encounters of the lipid kind. J Biol Chem 2002; 277:25847-50; PMID:12011103; https://doi.org/10.1074/jbc.R200008200
- Castro BM, Prieto M, Silva LC. Ceramide: a simple sphingolipid with unique biophysical properties. Prog Lipid Res 2014; 54:53-67; PMID:24513486; https://doi.org/10.1016/j.plipres.2014.01.004
- Ogretmen B. Sphingolipids in cancer: regulation of pathogenesis and therapy. FEBS Lett 2006; 580:5467-76; PMID:16970943; https://doi.org/10.1016/j.febslet.2006.08.052
- Ogretmen B, Hannun YA. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer 2004; 4:604-16; PMID:15286740; https://doi.org/10.1038/nrc1411
- Mullen TD, Hannun YA, Obeid LM. Ceramide synthases at the centre of sphingolipid metabolism and biology. Biochem J 2012; 441:789-802; PMID:22248339; https://doi.org/10.1042/BJ20111626
- Ebel P, Imgrund S, Vom Dorp K, Hofmann K, Maier H, Drake H, Degen J, Dörmann P, Eckhardt M, Franz T, et al. Ceramide synthase 4 deficiency in mice causes lipid alterations in sebum and results in alopecia. Biochem J 2014; 461:147-58; PMID:24738593; https://doi.org/10.1042/BJ20131242
- Jennemann R, Rabionet M, Gorgas K, Epstein S, Dalpke A, Rothermel U, Bayerle A, van der Hoeven F, Imgrund S, Kirsch J, et al. Loss of ceramide synthase 3 causes lethal skin barrier disruption. Hum Mol Genet 2012; 21:586-608; PMID:22038835; https://doi.org/10.1093/hmg/ddr494
- Grosch S, Schiffmann S, Geisslinger G. Chain length-specific properties of ceramides. Prog Lipid Res 2012; 51:50-62; PMID:22133871; https://doi.org/10.1016/j.plipres.2011.11.001
- Pewzner-Jung Y, Brenner O, Braun S, Laviad EL, Ben-Dor S, Feldmesser E, Horn-Saban S, Amann-Zalcenstein D, Raanan C, Berkutzki T, et al. A critical role for ceramide synthase 2 in liver homeostasis: II. insights into molecular changes leading to hepatopathy. J Biol Chem 2010; 285:10911-23; PMID:20110366; https://doi.org/10.1074/jbc.M109.077610
- Pewzner-Jung Y, Park H, Laviad EL, Silva LC, Lahiri S, Stiban J, Erez-Roman R, Brügger B, Sachsenheimer T, Wieland F, et al. A critical role for ceramide synthase 2 in liver homeostasis: I. alterations in lipid metabolic pathways. J Biol Chem 2010; 285:10902-10; PMID:20110363; https://doi.org/10.1074/jbc.M109.077594
- Wegner MS, Schiffmann S, Parnham MJ, Geisslinger G, Grosch S. The enigma of ceramide synthase regulation in mammalian cells. Prog Lipid Res 2016; 63:93-119; PMID:27180613; https://doi.org/10.1016/j.plipres.2016.03.006
- Levy M, Futerman AH. Mammalian ceramide synthases. IUBMB Life 2010; 62:347-56; PMID:20222015; https://doi.org/10.1002/iub.319
- Neubauer HA, Pitson SM. Roles, regulation and inhibitors of sphingosine kinase 2. FEBS J 2013; 280:5317-36; PMID:23638983; https://doi.org/10.1111/febs.12314
- Vesper H, Schmelz EM, Nikolova-Karakashian MN, Dillehay DL, Lynch DV, Merrill AH Jr. Sphingolipids in food and the emerging importance of sphingolipids to nutrition. J Nutrition 1999; 129:1239-50; PMID:10395583
- Markowitz SD, Bertagnolli MM. Molecular origins of cancer: Molecular basis of colorectal cancer. N Eng J Med 2009; 361:2449-60; PMID:20018966; https://doi.org/10.1056/NEJMra0804588
- Kumar A, Pandurangan AK, Lu F, Fyrst H, Zhang M, Byun HS, Bittman R, Saba JD. Chemopreventive sphingadienes downregulate Wnt signaling via a PP2A/Akt/GSK3beta pathway in colon cancer. Carcinogenesis 2012; 33:1726-35; PMID:22581840; https://doi.org/10.1093/carcin/bgs174
- Symolon H, Schmelz EM, Dillehay DL, Merrill AH Jr. Dietary soy sphingolipids suppress tumorigenesis and gene expression in 1,2-dimethylhydrazine-treated CF1 mice and ApcMin/+ mice. J Nutrition 2004; 134:1157-61; PMID:15113963
- Schmelz EM, Merrill AH, Jr. Ceramides and ceramide metabolites in cell regulation: evidence for dietary sphingolipids as inhibitors of colon carcinogenesis. Nutrition 1998; 14:717-9; PMID:9760598
- Schmelz EM, Roberts PC, Kustin EM, Lemonnier LA, Sullards MC, Dillehay DL, Merrill AH Jr. Modulation of intracellular beta-catenin localization and intestinal tumorigenesis in vivo and in vitro by sphingolipids. Cancer Res 2001; 61:6723-9; PMID:11559543
- Berra B, Colombo I, Sottocornola E, Giacosa A. Dietary sphingolipids in colorectal cancer prevention. Euro J Cancer Prev 2002; 11:193-7; PMID:11984139; https://doi.org/10.1097/00008469-200204000-00013
- Schmelz EM, Crall KJ, Larocque R, Dillehay DL, Merrill AH Jr. Uptake and metabolism of sphingolipids in isolated intestinal loops of mice. J Nutrition 1994; 124:702-12; PMID:8169662
- Wu J, Liu F, Nilsson A, Duan RD. Pancreatic trypsin cleaves intestinal alkaline sphingomyelinase from mucosa and enhances the sphingomyelinase activity. Am J Physiol Gastrointest Liver Physiol 2004; 287:G967-73; PMID:15205117; https://doi.org/10.1152/ajpgi.00190.2004
- Zhang P, Li B, Gao S, Duan RD. Dietary sphingomyelin inhibits colonic tumorigenesis with an up-regulation of alkaline sphingomyelinase expression in ICR mice. Anticancer Res 2008; 28:3631-5; PMID:19189644
- Baxter NT, Zackular JP, Chen GY, Schloss PD. Structure of the gut microbiome following colonization with human feces determines colonic tumor burden. Microbiome 2014; 2:20; PMID:24967088; https://doi.org/10.1186/2049-2618-2-20
- Weir TL, Manter DK, Sheflin AM, Barnett BA, Heuberger AL, Ryan EP. Stool microbiome and metabolome differences between colorectal cancer patients and healthy adults. PloS One 2013; 8:e70803; PMID:23940645; https://doi.org/10.1371/journal.pone.0070803
- Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human intestinal microbial flora. Science 2005; 308:1635-8; PMID:15831718; https://doi.org/10.1126/science.1110591
- Olsen I, Jantzen E. Sphingolipids in Bacteria and Fungi. Anaerobe 2001; 7:103-12; https://doi.org/10.1006/anae.2001.0376
- An D, Na C, Bielawski J, Hannun YA, Kasper DL. Membrane sphingolipids as essential molecular signals for Bacteroides survival in the intestine. Proc Natl Acad Sci U S A 2011; 108(Suppl 1):4666-71; PMID:20855611; https://doi.org/10.1073/pnas.1001501107
- An D, Oh SF, Olszak T, Neves JF, Avci FY, Erturk-Hasdemir D, Lu X, Zeissig S, Blumberg RS, Kasper DL. Sphingolipids from a symbiotic microbe regulate homeostasis of host intestinal natural killer T cells. Cell 2014; 156:123-33; PMID:24439373; https://doi.org/10.1016/j.cell.2013.11.042
- Boleij A, Hechenbleikner EM, Goodwin AC, Badani R, Stein EM, Lazarev MG, Ellis B, Carroll KC, Albesiano E, Wick EC, et al. The Bacteroides fragilis toxin gene is prevalent in the colon mucosa of colorectal cancer patients. Clin Infect Dis 2015; 60:208-15; PMID:25305284; https://doi.org/10.1093/cid/ciu787
- Toprak NU, Yagci A, Gulluoglu BM, Akin ML, Demirkalem P, Celenk T, Soyletir G. A possible role of Bacteroides fragilis enterotoxin in the aetiology of colorectal cancer. Clin Microbiol Infect 2006; 12:782-6; PMID:16842574; https://doi.org/10.1111/j.1469-0691.2006.01494.x
- Deng Z, Mu J, Tseng M, Wattenberg B, Zhuang X, Egilmez NK, Wang Q, Zhang L, Norris J, Guo H, et al. Enterobacteria-secreted particles induce production of exosome-like S1P-containing particles by intestinal epithelium to drive Th17-mediated tumorigenesis. Nat Commun 2015; 6:6956; PMID:25907800; https://doi.org/10.1038/ncomms7956
- Bernstein CN, Blanchard JF, Kliewer E, Wajda A. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer 2001; 91:854-62; PMID:11241255; https://doi.org/10.1002/1097-0142(20010215)91:4%3c854::AID-CNCR1073%3e3.0.CO;2-Z
- Angulo S, Morales A, Danese S, Llacuna L, Masamunt MC, Pultz N, Cifone MG, De Simone C, Delgado S, Vila J, et al. Probiotic sonicates selectively induce mucosal immune cells apoptosis through ceramide generation via neutral sphingomyelinase. PloS One 2011; 6:e16953; PMID:21408067; https://doi.org/10.1371/journal.pone.0016953
- Kekkonen RA, Sysi-Aho M, Seppanen-Laakso T, Julkunen I, Vapaatalo H, Oresic M, Korpela R. Effect of probiotic Lactobacillus rhamnosus GG intervention on global serum lipidomic profiles in healthy adults. World J Gastroenterol 2008; 14:3188-94; PMID:18506924; https://doi.org/10.3748/wjg.14.3188
- Park JW, Park WJ, Futerman AH. Ceramide synthases as potential targets for therapeutic intervention in human diseases. Biochim Biophys Acta 2014; 1841:671-81; PMID:24021978; https://doi.org/10.1016/j.bbalip.2013.08.019
- Derer S, Till A, Haesler R, Sina C, Grabe N, Jung S, Nikolaus S, Kuehbacher T, Groetzinger J, Rose-John S, et al. mTNF reverse signalling induced by TNFalpha antagonists involves a GDF-1 dependent pathway: implications for Crohn's disease. Gut 2013; 62:376-86; PMID:22535372; https://doi.org/10.1136/gutjnl-2011-300384
- Mizutani Y, Mitsutake S, Tsuji K, Kihara A, Igarashi Y. Ceramide biosynthesis in keratinocyte and its role in skin function. Biochimie 2009; 91:784-90; PMID:19364519; https://doi.org/10.1016/j.biochi.2009.04.001
- Laviad EL, Albee L, Pankova-Kholmyansky I, Epstein S, Park H, Merrill AH Jr, Futerman AH. Characterization of ceramide synthase 2: tissue distribution, substrate specificity, and inhibition by sphingosine 1-phosphate. J Biol Chem 2008; 283:5677-84; PMID:18165233; https://doi.org/10.1074/jbc.M707386200
- Fekry B, Esmaeilniakooshkghazi A, Krupenko SA, Krupenko NI. Ceramide Synthase 6 Is a Novel Target of Methotrexate Mediating Its Antiproliferative Effect in a p53-Dependent Manner. PloS One 2016; 11:e0146618; PMID:26783755; https://doi.org/10.1371/journal.pone.0146618
- Riebeling C, Allegood JC, Wang E, Merrill AH Jr, Futerman AH. Two mammalian longevity assurance gene (LAG1) family members, trh1 and trh4, regulate dihydroceramide synthesis using different fatty acyl-CoA donors. J Biol Chem 2003; 278:43452-9; PMID:12912983; https://doi.org/10.1074/jbc.M307104200
- Ebel P, Vom Dorp K, Petrasch-Parwez E, Zlomuzica A, Kinugawa K, Mariani J, Minich D, Ginkel C, Welcker J, Degen J, et al. Inactivation of ceramide synthase 6 in mice results in an altered sphingolipid metabolism and behavioral abnormalities. J Biol Chem 2013; 288:21433-47; PMID:23760501; https://doi.org/10.1074/jbc.M113.479907
- Edmond V, Dufour F, Poiroux G, Shoji K, Malleter M, Fouque A, Tauzin S, Rimokh R, Sergent O, Penna A, et al. Downregulation of ceramide synthase-6 during epithelial-to-mesenchymal transition reduces plasma membrane fluidity and cancer cell motility. Oncogene 2015; 34:996-1005; PMID:24632610; https://doi.org/10.1038/onc.2014.55
- Hertervig E, Nilsson A, Nyberg L, Duan RD. Alkaline sphingomyelinase activity is decreased in human colorectal carcinoma. Cancer 1997; 79:448-53; PMID:9028353
- Wang K, Xu R, Snider AJ, Schrandt J, Li Y, Bialkowska AB, Li M, Zhou J, Hannun YA, Obeid LM, et al. Alkaline ceramidase 3 deficiency aggravates colitis and colitis-associated tumorigenesis in mice by hyperactivating the innate immune system. Cell Death Dis 2016; 7:e2124; PMID:26938296; https://doi.org/10.1038/cddis.2016.36
- Koch J, Gartner S, Li CM, Quintern LE, Bernardo K, Levran O, Schnabel D, Desnick RJ, Schuchman EH, Sandhoff K, et al. Molecular cloning and characterization of a full-length complementary DNA encoding human acid ceramidase. Identification Of the first molecular lesion causing Farber disease. J Biol Chem 1996; 271:33110-5; PMID:8955159; https://doi.org/10.1074/jbc.271.51.33110
- Park JH, Schuchman EH. Acid ceramidase and human disease. Biochim Biophys Acta 2006; 1758:2133-8; PMID:17064658; https://doi.org/10.1016/j.bbamem.2006.08.019
- Eliyahu E, Park JH, Shtraizent N, He X, Schuchman EH. Acid ceramidase is a novel factor required for early embryo survival. FASEB J 2007; 21:1403-9; PMID:17264167; https://doi.org/10.1096/fj.06-7016com
- Seelan RS, Qian C, Yokomizo A, Bostwick DG, Smith DI, Liu W. Human acid ceramidase is overexpressed but not mutated in prostate cancer. Genes Chromosomes Cancer 2000; 29:137-46; PMID:10959093; https://doi.org/10.1002/1098-2264(2000)9999:9999%3c::AID-GCC1018%3e3.0.CO;2-E
- Tirodkar TS, Lu P, Bai A, Scheffel MJ, Gencer S, Garrett-Mayer E, Bielawska A, Ogretmen B, Voelkel-Johnson C, et al. Expression of Ceramide Synthase 6 Transcriptionally Activates Acid Ceramidase in a c-Jun N-terminal Kinase (JNK)-dependent Manner. J Biol Chem 2015; 290:13157-67; PMID:25839235; https://doi.org/10.1074/jbc.M114.631325
- Cheng JC, Bai A, Beckham TH, Marrison ST, Yount CL, Young K, Lu P, Bartlett AM, Wu BX, Keane BJ, et al. Radiation-induced acid ceramidase confers prostate cancer resistance and tumor relapse. J Clin Invest 2013; 123:4344-58; PMID:24091326; https://doi.org/10.1172/JCI64791
- Kono M, Dreier JL, Ellis JM, Allende ML, Kalkofen DN, Sanders KM, Bielawski J, Bielawska A, Hannun YA, Proia RL. Neutral ceramidase encoded by the Asah2 gene is essential for the intestinal degradation of sphingolipids. J Biol Chem 2006; 281:7324-31; PMID:16380386; https://doi.org/10.1074/jbc.M508382200
- Garcia-Barros M, Coant N, Kawamori T, Wada M, Snider AJ, Truman JP, Wu BX, Furuya H, Clarke CJ, Bialkowska AB, et al. Role of neutral ceramidase in colon cancer. FASEB J 2016; 30:4159-71; PMID:27609772; https://doi.org/10.1096/fj.201600611R
- Kawamori T, Kaneshiro T, Okumura M, Maalouf S, Uflacker A, Bielawski J, Hannun YA, Obeid LM. Role for sphingosine kinase 1 in colon carcinogenesis. FASEB J 2009; 23:405-14; PMID:18824518; https://doi.org/10.1096/fj.08-117572
- Long J, Xie Y, Yin J, Lu W, Fang S. SphK1 promotes tumor cell migration and invasion in colorectal cancer. Tumour Biol 2016; 37:6831-6; PMID:26662312; https://doi.org/10.1007/s13277-015-4542-4
- Liang J, Nagahashi M, Kim EY, Harikumar KB, Yamada A, Huang WC, Hait NC, Allegood JC, Price MM, Avni D, et al. Sphingosine-1-phosphate links persistent STAT3 activation, chronic intestinal inflammation, and development of colitis-associated cancer. Cancer Cell 2013; 23:107-20; PMID:23273921; https://doi.org/10.1016/j.ccr.2012.11.013
- Zhang L, Liu X, Zuo Z, Hao C, Ma Y. Sphingosine kinase 2 promotes colorectal cancer cell proliferation and invasion by enhancing MYC expression. Tumour Biol 2016; 37:8455-60; PMID:26733171; https://doi.org/10.1007/s13277-015-4700-8
- Mizutani N, Omori Y, Tanaka K, Ito H, Takagi A, Kojima T, Nakatochi M, Ogiso H, Kawamoto Y, Nakamura M, et al. Increased SPHK2 transcription of human colon cancer cells in serum-depleted culture: The involvement of CREB transcription factor. J Cell Biochem 2015; 116:2227-38; PMID:25808826; https://doi.org/10.1002/jcb.25173
- Schrecengost RS, Keller SN, Schiewer MJ, Knudsen KE, Smith CD. Downregulation of critical oncogenes by the selective SK2 inhibitor ABC294640 hinders prostate cancer progression. Mol Cancer Res 2015; 13:1591-601; PMID:26271487; https://doi.org/10.1158/1541-7786.MCR-14-0626
- He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science 1998; 281:1509-12; PMID:9727977; https://doi.org/10.1126/science.281.5382.1509
- Siskind LJ, Kolesnick RN, Colombini M. Ceramide channels increase the permeability of the mitochondrial outer membrane to small proteins. J Biol Chem 2002; 277:26796-803; PMID:12006562; https://doi.org/10.1074/jbc.M200754200
- Santana P, Pena LA, Haimovitz-Friedman A, Martin S, Green D, McLoughlin M, Cordon-Cardo C, Schuchman EH, Fuks Z, Kolesnick R. Acid sphingomyelinase-deficient human lymphoblasts and mice are defective in radiation-induced apoptosis. Cell 1996; 86:189-99; PMID:8706124; https://doi.org/10.1016/S0092-8674(00)80091-4
- Paris F, Fuks Z, Kang A, Capodieci P, Juan G, Ehleiter D, Haimovitz-Friedman A, Cordon-Cardo C, Kolesnick R. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science 2001; 293:293-7; PMID:11452123; https://doi.org/10.1126/science.1060191
- Deng X, Yin X, Allan R, Lu DD, Maurer CW, Haimovitz-Friedman A, Fuks Z, Shaham S, Kolesnick R. Ceramide biogenesis is required for radiation-induced apoptosis in the germ line of C. elegans. Science 2008; 322:110-5; PMID:18832646; https://doi.org/10.1126/science.1158111
- Kolesnick R, Fuks Z. Radiation and ceramide-induced apoptosis. Oncogene 2003; 22:5897-906; PMID:12947396; https://doi.org/10.1038/sj.onc.1206702
- Mesicek J, Lee H, Feldman T, Jiang X, Skobeleva A, Berdyshev EV, Haimovitz-Friedman A, Fuks Z, Kolesnick R. Ceramide synthases 2, 5, and 6 confer distinct roles in radiation-induced apoptosis in HeLa cells. Cell Signal 2010; 22:1300-7; PMID:20406683; https://doi.org/10.1016/j.cellsig.2010.04.006
- Yacoub A, Hamed HA, Allegood J, Mitchell C, Spiegel S, Lesniak MS, Ogretmen B, Dash R, Sarkar D, Broaddus WC, et al. PERK-dependent regulation of ceramide synthase 6 and thioredoxin play a key role in mda-7/IL-24-induced killing of primary human glioblastoma multiforme cells. Cancer Res 2010; 70:1120-9; PMID:20103619; https://doi.org/10.1158/0008-5472.CAN-09-4043
- Walker T, Mitchell C, Park MA, Yacoub A, Rahmani M, Haussinger D, Reinehr R, Voelkel-Johnson C, Fisher PB, Grant S, et al. 17-allylamino-17-demethoxygeldanamycin and MEK1/2 inhibitors kill GI tumor cells via Ca2+-dependent suppression of GRP78/BiP and induction of ceramide and reactive oxygen species. Mol Cancer Ther 2010; 9:1378-95; PMID:20442308; https://doi.org/10.1158/1535-7163.MCT-09-1131
- Separovic D, Breen P, Joseph N, Bielawski J, Pierce JS, VAN Buren E, Gudz TI. Ceramide synthase 6 knockdown suppresses apoptosis after photodynamic therapy in human head and neck squamous carcinoma cells. Anticancer Res 2012; 32:753-60; PMID:22399588
- White-Gilbertson S, Mullen T, Senkal C, Lu P, Ogretmen B, Obeid L, Voelkel-Johnson C. Ceramide synthase 6 modulates TRAIL sensitivity and nuclear translocation of active caspase-3 in colon cancer cells. Oncogene 2009; 28:1132-41; PMID:19137010; https://doi.org/10.1038/onc.2008.468
- Walker T, Mitchell C, Park MA, Yacoub A, Graf M, Rahmani M, Houghton PJ, Voelkel-Johnson C, Grant S, Dent P. Sorafenib and vorinostat kill colon cancer cells by CD95-dependent and -independent mechanisms. Mol Pharmacol 2009; 76:342-55; PMID:19483104; https://doi.org/10.1124/mol.109.056523
- Park MA, Walker T, Martin AP, Allegood J, Vozhilla N, Emdad L, Sarkar D, Rahmani M, Graf M, Yacoub A, et al. MDA-7/IL-24-induced cell killing in malignant renal carcinoma cells occurs by a ceramide/CD95/PERK-dependent mechanism. Mol Cancer Ther 2009; 8:1280-91; PMID:19417161; https://doi.org/10.1158/1535-7163.MCT-09-0073
- Park MA, Mitchell C, Zhang G, Yacoub A, Allegood J, Haussinger D, Reinehr R, Larner A, Spiegel S, Fisher PB, et al. Vorinostat and sorafenib increase CD95 activation in gastrointestinal tumor cells through a Ca(2+)-de novo ceramide-PP2A-reactive oxygen species-dependent signaling pathway. Cancer Res 2010; 70:6313-24; PMID:20631069; https://doi.org/10.1158/0008-5472.CAN-10-0999
- Park MA, Reinehr R, Haussinger D, Voelkel-Johnson C, Ogretmen B, Yacoub A, Grant S, Dent P. Sorafenib activates CD95 and promotes autophagy and cell death via Src family kinases in gastrointestinal tumor cells. Mol Cancer Ther 2010; 9:2220-31; PMID:20682655; https://doi.org/10.1158/1535-7163.MCT-10-0274
- Coant N, Sakamoto W, Mao C, Hannun YA. Ceramidases, roles in sphingolipid metabolism and in health and disease. Adv Biol Regulation 2017; 63:122-31; PMID:27771292; https://doi.org/10.1016/j.jbior.2016.10.002
- Bedia C, Casas J, Andrieu-Abadie N, Fabrias G, Levade T. Acid ceramidase expression modulates the sensitivity of A375 melanoma cells to dacarbazine. J Biol Chem 2011; 286:28200-9; PMID:21700700; https://doi.org/10.1074/jbc.M110.216382
- Tan SF, Liu X, Fox TE, Barth BM, Sharma A, Turner SD, Awwad A, Dewey A, Doi K, Spitzer B, et al. Acid ceramidase is upregulated in AML and represents a novel therapeutic target. Oncotarget 2016; 7:83208-22; PMID:27825124
- Sanger N, Ruckhaberle E, Gyorffy B, Engels K, Heinrich T, Fehm T, Graf A, Holtrich U, Becker S, Karn T. Acid ceramidase is associated with an improved prognosis in both DCIS and invasive breast cancer. Mol Oncol 2014; 9(1):58-67; PMID:25131496; https://doi.org/10.1016/j.molonc.2014.07.016
- Hanker LC, Karn T, Holtrich U, Gatje R, Rody A, Heinrich T, Ruckhäberle E, Engels K. Acid ceramidase (AC)–a key enzyme of sphingolipid metabolism–correlates with better prognosis in epithelial ovarian cancer. Int J Gynecol Pathol 2013; 32:249-57; PMID:23518908; https://doi.org/10.1097/PGP.0b013e3182673982
- Sabbadini RA. Sphingosine-1-phosphate antibodies as potential agents in the treatment of cancer and age-related macular degeneration. Br J Pharmacol 2011; 162:1225-38; PMID:21091645; https://doi.org/10.1111/j.1476-5381.2010.01118.x
- Pitman MR, Costabile M, Pitson SM. Recent advances in the development of sphingosine kinase inhibitors. Cell Signal 2016; 28:1349-63; PMID:27297359; https://doi.org/10.1016/j.cellsig.2016.06.007
- Saied EM, Arenz C. Small molecule inhibitors of ceramidases. Cell Physiol Biochem 2014; 34:197-212; PMID:24977492; https://doi.org/10.1159/000362995
- Szulc ZM, Bielawski J, Gracz H, Gustilo M, Mayroo N, Hannun YA, Obeid LM, Bielawska A. Tailoring structure-function and targeting properties of ceramides by site-specific cationization. Bioorg Med Chem 2006; 14:7083-104; PMID:16919460; https://doi.org/10.1016/j.bmc.2006.07.016
- Zhang L, Wang X, Bullock AJ, Callea M, Shah H, Song J, Moreno K, Visentin B, Deutschman D, Alsop DC, et al. Anti-S1P Antibody as a Novel Therapeutic Strategy for VEGFR TKI-Resistant Renal Cancer. Clin Cancer Res 2015; 21:1925-34; PMID:25589614; https://doi.org/10.1158/1078-0432.CCR-14-2031
- Bai A, Szulc ZM, Bielawski J, Pierce JS, Rembiesa B, Terzieva S, Mao C, Xu R, Wu B, Clarke CJ, et al. Targeting (cellular) lysosomal acid ceramidase by B13: design, synthesis and evaluation of novel DMG-B13 ester prodrugs. Bioorg Med Chem 2014; 22:6933-44; PMID:25456083; https://doi.org/10.1016/j.bmc.2014.10.025
- Korbelik M, Banath J, Zhang W, Saw KM, Szulc ZM, Bielawska A, Separovic D. Interaction of acid ceramidase inhibitor LCL521 with tumor response to photodynamic therapy and photodynamic therapy-generated vaccine. Int J Cancer 2016; 139:1372-8; PMID:27136745; https://doi.org/10.1002/ijc.30171
- French KJ, Zhuang Y, Maines LW, Gao P, Wang W, Beljanski V, Upson JJ, Green CL, Keller SN, Smith CD. Pharmacology and antitumor activity of ABC294640, a selective inhibitor of sphingosine kinase-2. J Pharmacol Exp Ther 2010; 333:129-39; PMID:20061445; https://doi.org/10.1124/jpet.109.163444
- Ju T, Gao D, Fang ZY. Targeting colorectal cancer cells by a novel sphingosine kinase 1 inhibitor PF-543. Biochem Biophys Res Commun 2016; 470:728-34; PMID:26775841; https://doi.org/10.1016/j.bbrc.2016.01.053
- Sharma AK. Sphingo-guanidines and their use as inhibitors of sphingosine kinase (WO2010078247). Expert Opin Ther Pat 2011; 21:807-12; PMID:21457086; https://doi.org/10.1517/13543776.2011.573787
- Chumanevich AA, Poudyal D, Cui X, Davis T, Wood PA, Smith CD, Hofseth LJ. Suppression of colitis-driven colon cancer in mice by a novel small molecule inhibitor of sphingosine kinase. Carcinogenesis 2010; 31:1787-93; PMID:20688834; https://doi.org/10.1093/carcin/bgq158
- Xun C, Chen MB, Qi L, Tie-Ning Z, Peng X, Ning L, Zhi-Xiao C, Li-Wei W. Targeting sphingosine kinase 2 (SphK2) by ABC294640 inhibits colorectal cancer cell growth in vitro and in vivo. J Exp Clin Cancer Res 2015; 34:94; https://doi.org/10.1186/s13046-015-0205-y
- Britten CD, Thomas MB, Garrett-Mayer E, Chin SH, Shirai K, Ogretmen B, Bentz TA, Brisendine A, Anderton K, Cusack SL, et al. A phase I study of ABC294640, A First-in-Class sphingosine kinase-2 inhibitor, in patients with advanced solid tumors. Clin Cancer Res 2017; PMID:28420720; https://doi.org/10.1158/1078-0432.CCR-16-2363
- Lewis CS, Voelkel-Johnson C, Smith CD. Suppression of c-Myc and RRM2 expression in pancreatic cancer cells by the sphingosine kinase-2 inhibitor ABC294640. Oncotarget 2016; 7:60181-92; PMID:27517489
- Venant H, Rahmaniyan M, Jones EE, Lu P, Lilly MB, Garrett-Mayer E, Drake RR, Kraveka JM, Smith CD, Voelkel-Johnson C. The sphingosine kinase 2 inhibitor ABC294640 reduces the growth of prostate cancer cells and results in accumulation of dihydroceramides in vitro and in vivo. Mol Cancer Ther 2015; 14:2744-52; PMID:26494858; https://doi.org/10.1158/1535-7163.MCT-15-0279
- Venkata JK, An N, Stuart R, Costa LJ, Cai H, Coker W, Song JH, Gibbs K, Matson T, Garrett-Mayer E, et al. Inhibition of sphingosine kinase 2 downregulates the expression of c-Myc and Mcl-1 and induces apoptosis in multiple myeloma. Blood 2014; 124:1915-25; PMID:25122609; https://doi.org/10.1182/blood-2014-03-559385
- Hou Q, Jin J, Zhou H, Novgorodov SA, Bielawska A, Szulc ZM, Hannun YA, Obeid LM, Hsu YT. Mitochondrially targeted ceramides preferentially promote autophagy, retard cell growth, and induce apoptosis. J Lipid Res 2011; 52:278-88; PMID:21081756; https://doi.org/10.1194/jlr.M012161
- Sentelle RD, Senkal CE, Jiang W, Ponnusamy S, Gencer S, Selvam SP, Ramshesh VK, Peterson YK, Lemasters JJ, Szulc ZM, et al. Ceramide targets autophagosomes to mitochondria and induces lethal mitophagy. Nat Chem Biol 2012; 8:831-8; PMID:22922758; https://doi.org/10.1038/nchembio.1059
- Dahm F, Bielawska A, Nocito A, Georgiev P, Szulc ZM, Bielawski J, Jochum W, Dindo D, Hannun YA, Clavien PA. Mitochondrially targeted ceramide LCL-30 inhibits colorectal cancer in mice. Br J Cancer 2008; 98:98-105; PMID:18026195; https://doi.org/10.1038/sj.bjc.6604099
- Dindo D, Dahm F, Szulc Z, Bielawska A, Obeid LM, Hannun YA, Graf R, Clavien PA. Cationic long-chain ceramide LCL-30 induces cell death by mitochondrial targeting in SW403 cells. Mol Cancer Ther 2006; 5:1520-9; PMID:16818511; https://doi.org/10.1158/1535-7163.MCT-05-0513
- Barth BM, Cabot MC, Kester M. Ceramide-based therapeutics for the treatment of cancer. Anticancer Agents Med Chem 2011; 11:911-9; PMID:21707481; https://doi.org/10.2174/187152011797655177
- Hankins JL, Doshi UA, Haakenson JK, Young MM, Barth BM, Kester M. The therapeutic potential of nanoscale sphingolipid technologies. Handbook Exp Pharmacol 2013:197-210; PMID:23579457; https://doi.org/10.1007/978-3-7091-1368-4_11
- Kester M, Bassler J, Fox TE, Carter CJ, Davidson JA, Parette MR. Preclinical development of a C6-ceramide NanoLiposome, a novel sphingolipid therapeutic. Biol Chem 2015; 396:737-47; PMID:25838296; https://doi.org/10.1515/hsz-2015-0129
- Lin L, Liu A, Peng Z, Lin HJ, Li PK, Li C, Lin J. STAT3 is necessary for proliferation and survival in colon cancer-initiating cells. Cancer Res 2011; 71:7226-37; PMID:21900397; https://doi.org/10.1158/0008-5472.CAN-10-4660