ABSTRACT
A major recent advance in cancer therapy involves the use of immune checkpoint therapy for tumors with mismatch repair deficiency, as they have a high tumor mutation load and neoantigen burden. Approximately 4% of advanced colorectal cancer harbors a mismatch repair deficiency. When mismatch repair deficiency exists in the germline, there is increased susceptibility to a variety of cancers including colorectal cancer, uterine cancer, urothelial carcinoma, and skin cancer. Herein we report the case of a 62-year-old man with mismatch repair deficient metastatic colorectal adenocarcinoma, urothelial carcinoma and a history of sebaceous carcinomas. As the patient in 2016 was ineligible for clinical trials he received immune checkpoint anti-PD-1 therapy with pembrolizumab (200 mg every 3 weeks), on compassionate use basis, after the failure of second-line treatment. The patient's CEA initially responded to pembrolizumab for 4 months and then kept rising for 5 months before mildly declining again. His treatment was then switched to anti-PD-L1 therapy with atezolizumab as it was approved for urothelial carcinoma at that time, and his CEA declined again. This case raises interesting questions about caring for patients with mismatch repair deficient colorectal cancer, including the role of PD-L1 therapy, sequencing of immunotherapy, relying on CEA trends and determining future therapies after progression on pembrolizumab.
Background
Mismatch-repair-deficient (dMMR) colorectal cancer (CRC) represents ∼15% of all patients with CRC, but only 4% of patients with advanced CRC.Citation1,2 Microsatellite instability (MSI), which refers to increased mutations due to slippage of DNA within tracts of tandemly repetitive DNA motifs (microsatellites), is typically caused by mutation or epigenetic silencing of DNA mismatch repair genes. The suggested approach to evaluate MSI is via PCR of 5 DNA markers or immunohistochemical analysis of DNA mismatch repair enzymes. Immunostaining of the mismatch repair enzymes MLH1, MSH2, MSH6 and PMS2 has been adopted as a universal testing methodology in CRC and is equivalent in levels of sensitivity and specificity to MSI analysis via PCR.Citation3
The microenvironment of dMMR, microsatellite unstable, or MSI-high (MSI-H) colon cancer displays high infiltration with activated CD8+ CTL as well as activated Th1 cells. It has been shown that MSI tumors counterbalance this active Th1/CTL microenvironment by expression of multiple immune checkpoints such as programmed death 1 (PD-1). The binding of PD-L1 or PD-L2 to PD-1 results in inhibition of T-cell immune function and constitutes an immune escape mechanism.Citation4 In May 2015, Le et al. demonstrated the clinical activity of pembrolizumab, a humanized monoclonal anti PD-1 antibody of the IgG4 κ isotype, blocking the interaction between PD-1 and its ligands, PD-L1 and PD-L2, on treatment-refractory progressive mCRC with dMMR. In this study, a subgroup of patients (n = 10) with dMMR CRC had a disease control rate of 90% at week 12, with 50% partial response and 50% stable disease. By contrast, patients with mismatch-repair-proficient tumors did not respond to immunotherapy and pembrolizumab was almost always ineffective.Citation5 On May 23, 2017, the US. Food and Drug Administration granted accelerated approval to pembrolizumab for patients with unresectable or metastatic, MSI-H solid tumors that have progressed following prior treatment or with MSI-H CRC that has progressed following treatment with a fluoropyrimidine, oxaliplatin, and irinotecan.
Here we present a case of a 63 y old man with a history of metastatic colon cancer and urothelial carcinoma with mismatch repair deficiency who experienced good disease control with immunotherapy. We present this case as it raises several interesting and relevant questions in the treatment of mismatch repair deficient colon cancer that remain unanswered.
Case report
A 62 y old man with history of metastatic colon cancer presented to our institution in May 2016 for further care. Eleven years before presentation, in August 2005, he was diagnosed with stage III colon cancer and was found to have 3 synchronous colon cancers on colonoscopy. He underwent a subtotal colectomy on Aug 23, 2005 with pathology revealing 3 malignant lesions (pT2 in the cecum, pT3 in the transverse colon and pT2 in the sigmoid colon). Two of 26 lymph nodes were positive and imaging did not reveal the presence of metastatic lesions. Immunohistochemistry of the tumor revealed absent expression of MSH-2 and MSH-6. He received adjuvant FOLFOX (5-Fluorouracil, Leucovorin and Oxaliplatin) therapy on a clinical trial in 2005.
Unfortunately, in April 2015, he developed new liver lesions consistent with metastatic colon adenocarcinoma (). The liver lesions were not resectable. Further testing of the liver biopsy tissue did not reveal presence of KRAS, NRAS or BRAF mutations. He was treated with FOLFIRI (5-Fluorouracil, Leucovorin, Irinotecan) with bevacizumab from April 2015 to December 2015, with therapy discontinuation due to disease progression in the liver. He was then treated with Irinotecan and Cetuximab from December 2015 to April 2016, but therapy was discontinued due to disease progression in the liver and lymph nodes, as well as new hydroureteronephrosis.
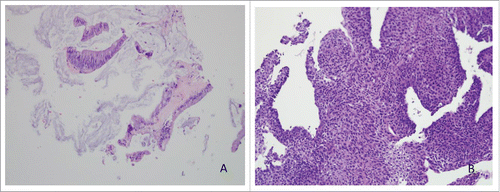
Figure 1. (A) Metastatic well-differentiated mucinous adenocarcinoma in liver, H&E, 20x. (B) High grade urothelial carcinoma, H&E, 20x (cell block section, cytology).
To evaluate the hydronephrosis, he underwent bilateral retrograde pyelograms which revealed bilateral large mid-ureteral filling defects suggestive of bilateral upper tract urothelial carcinoma. He had bilateral ureteral stent placements. Urine cytology from right ureter confirmed urothelial carcinoma with absence of MSH-2 and MSH-6 expression by IHC on cellblock material ( and ).
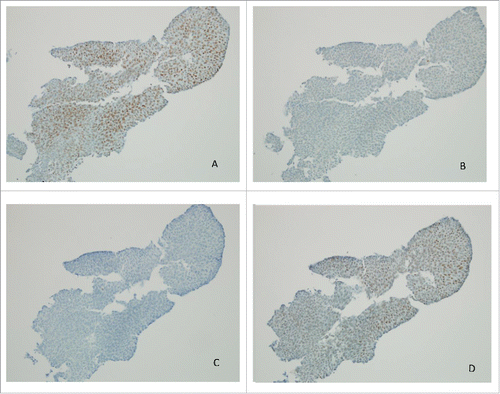
Figure 2. Immunohistochemistry of MLH-1 (A), MSH-2 (B), MSH-6 (C) and PMS2 (D) in urothelial carcinoma, 20x (cell block section, cytology) showing loss of MSH-2 and MSH-6.
When the patient presented to our clinic in May 2016, he had grade 1 neuropathy from prior oxaliplatin and intermittent hematuria. He also provided additional history of multiple sebaceous cancers, first diagnosed on the nose around age 45, for which he underwent multiple surgical procedures. The patient was an ongoing smoker with a 20 pack year smoking history. He had a strong family history of cancers. His father passed away at age 30 from some cancer that he was not aware of and his paternal cousin was diagnosed with colon cancer in his 40s.
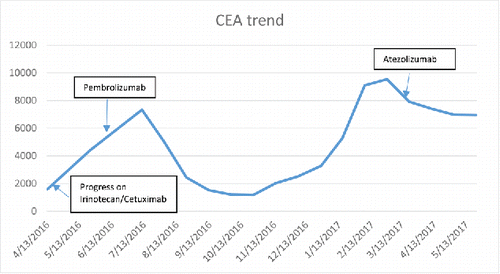
Given the presence of mismatch repair deficiency in the primary colon cancer, he was started on pembrolizumab IV 200mg q3 weeks in June 2016 on compassionate use basis, and continued this therapy until February 2017. Of note, at the time he started immune checkpoint therapy he was ineligible for participation in clinical trials due to the presence of multiple tumors. His CEA declined until October 2017 and was consistent with partial response on his PET/CT scan ( and ). However, from November 2016 to February 2017 the CEA began to rise and this was accompanied by increased FDG+ activity in the liver lesions based on PET/CT scan from March 2017 (). The scans also showed persistent activity along the ureters bilaterally.
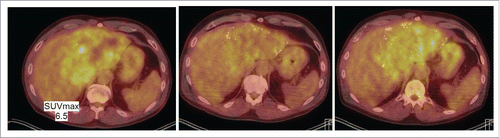
Figure 4. PET/CT scan image of liver lesions (left to right) from June 2016, January 2017 and March 2017.
As the patient continued to have intermittent hematuria, he received repeat cystoscopy in March 2017, which revealed the presence of high grade T1 urothelial cancer in the bladder as well as the ureteral neoplasms known before. Given his ongoing hematuria, the persistent urothelial carcinoma, worsening of the liver lesions on the PET scan and the recent approval of the anti-PD-L1 therapy atezolizumab for advanced urothelial carcinoma, his therapy was switched from pembrolizumab to atezolizumab in March 2017. Of note, his rising CEA had started to slowly decline before switching the atezolizumab (see ). As of June, 2017, the patient continues therapy with atezolizumab with declining CEA.
Due to mismatch repair deficiency, a family history of cancers and young age at diagnosis of colon cancer, the patient underwent germ-line testing for Lynch syndrome thrice, originally at a different institution, and the testing was inconclusive. Genetic testing results were repeated at our institution and revealed the presence of a pathogenic mutation called IVS1+2T>G in the MSH2 gene. This result was considered positive for a deleterious mutation and is consistent with a diagnosis of MSH-2 related Lynch syndrome.
Discussion
Lynch Syndrome (LS) is an autosomal-dominant disease caused by a mono-allelic germ-line mutation in a DNA mismatch repair (MLH1, MSH2 , MSH6 , PMS2) or EPCAM genes, and is the most common cause of inherited CRC.Citation6 The tumor DNA of LS patients is characterized by microsatellite instability (microsatellite instability–high [MSI-H], or, by convention, MSI) and clinically LS individuals experience early onset of visceral tumors, mainly colorectal cancer (CRC) and high frequency of synchronous and metachronous malignancies. Other tumors seen in LS include uterine cancer, ovarian cancer urothelial cancer, brain cancer, or skin cancer among others.
Our patient's diagnosis of MSI-H colon and urothelial carcinoma, history of multiple sebaceous neoplasms and a strong family history of cancers makes diagnosis of Muir-Torre syndrome (MTS) a likely diagnosis. MTS is a variant of LS, clinically characterized by the genetic susceptibility to the occurrence of sebaceous tumors (sebaceous adenomas and carcinomas, basal cell carcinomas with sebaceous differentiation and/or keratoacanthomas) associated with visceral malignancies. Our patient's initial mutation analysis was reported as inconclusive as only some of the patient's blood and saliva cells contained the MSH-2 mutation, but most of the cells did not. Because the mutant allele frequency was not at the expected ratio, the laboratory labeled the result as inconclusive. This type of result is now referred to as a potentially mosaic test result and can be difficult to interpret in practice. In this case, given the clinical presentation and history of the patient and because the mutation was identified in the MSH2 gene, we believe that the mutation was pathogenic and was causative of Lynch/Muir-Torre syndrome.
Several features of this case raise interesting and important questions. First, it is unclear why the patient's CEA initially declined on pembrolizumab therapy from June 2016 to October 2016, then started rising between November 2016 to February 2017 and then declined slightly in March 2017 before atezolizumab therapy was initiated. CEA is a biomarker that is routinely used in practice to monitor response to chemotherapy in patients with metastatic colon cancer. Both physicians caring for the patient were apprehensive about continuing pembrolizumab while the patient's CEA was rising, and with evidence for increased FGD-avidity in March 2017 in the liver metastases on PET/CT scan.
We switched the patient from pembrolizumab to atezolizumab in March 2017 given the FDA approval of the latter in metastatic urothelial carcinoma in the second line setting.Citation7 Of note, pembrolizumab was not FDA approved for metastatic urothelial carcinoma in March 2017. Atezolizumab with MEK inhibitor cobimetinib is also being studied in MSI-stable colorectal cancers, with promising results.Citation8 Should our patient have progression on atezolizumab, we will reserve the option of switching him back to pembrolizumab given prior response. However, in this patient with dMMR colon cancer, it is unclear whether a PD-1 or PD-L1 inhibitor is the preferred therapy. Similarly, sequencing immunotherapies has not been studied, nor has the approach using combination immunotherapies in the front-line or second-line setting been reported. Answers to these questions would be helpful for the management of our patient, should his disease progress in the future. Furthermore, our patient would not likely be considered eligible for most available clinical trials due to the presence of multiple separate malignancies.
In addition to immunotherapies, there is some evidence that targeted therapy may have a future role in the treatment of dMMR colorectal cancers. We recently profiled dMMR CRC for potentially actionable gene mutations. Of 1104 CRC from a large cohort (COSMIC) in our study, we found that MSH2/MLH-1 mutant colorectal cancers show higher mutation rates in BRCA2 as compared with non-MSH2/MHL1 mutant tumors (38% vs 6%, P<0.0001).Citation9 Additionally, EGFR was mutated in 45.5% of the MSH2/MLH1-mutant and 6.5% of the non-MSH2/MLH1-mutant tumors (P<0.0001) and 15% of the EGFR mutations were actionable. These findings pose additional questions regarding the clinical relevance of therapeutic targeting of BRCA2 vulnerabilities, EGFR mutations or other identified drivers in MSH2/MLH1 colorectal cancer, including the possibility of combining targeted therapy with immune-based therapeutics for selected patients.
Conclusion
Here we present the course of treatment in an interesting and challenging case of a patient with MSI-H metastatic colon cancer, urothelial carcinoma, and history of sebaceous carcinomas treated with immunotherapy. The patient likely harbors a mosaic germ-line MSH2 mutation and has the Lynch Syndrome variant Muir-Torre Syndrome. We reflect on the various clinical and laboratory findings as the patient was treated with sequential immune checkpoint blockade with pembrolizumab and subsequently with atezolizumab. We discuss some dilemmas that were faced while managing this patient, and raise several questions that remain to be answered in this field which begs further clinical investigation. Some ongoing clinical trials are addressing the role of immune checkpoint therapy in MSI-high colorectal cancer in the frontline setting. Other unresolved questions from our case include the prioritization of anti-PD1 versus anti-PD-L1, the potential for various combinations of immune checkpoint blockade (including anti-PD1, anti-PD-L1 or anti-CTLA4), and the potential to combine immune-based therapies with targeted therapies based on molecular profiling of mismatch repair deficient tumors.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Koopman M, Kortman GA, Mekenkamp L, Ligtenberg MJ, Hoogerbrugge N, Antonini NF, Punt CJ, van Krieken JH. Deficient mismatch repair system in patients with sporadic advanced colorectal cancer. Br J Cancer. 2009;100(2):266-73; doi:10.1038/sj.bjc.6604867. PMID:19165197.
- Goldstein J, Tran B, Ensor J, Gibbs P, Wong HL, Wong SF, Vilar E, Tie J, Broaddus R, Kopetz S, et al. Multicenter retrospective analysis of metastatic colorectal cancer (CRC) with high-level microsatellite instability (MSI-H). Ann Oncol. 2014;25(5):1032-8; doi:10.1093/annonc/mdu100. PMID:24585723.
- Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology. 2010;138(6):2073-2087. e3. doi:10.1053/j.gastro.2009.12.064. PMID:20420947.
- Llosa NJ, Cruise M, Tam A, Wicks EC, Hechenbleikner EM, Taube JM, Blosser RL, Fan H, Wang H, Luber BS, et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 2015; 5(1):43-51; doi:10.1158/2159-8290.CD-14-0863. PMID:25358689.
- Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372(26):2509-20; doi:10.1056/NEJMoa1500596. PMID:26028255.
- Boland CR. Evolution of the nomenclature for the hereditary colorectal cancer syndromes. Fam Cancer. 2005;4(3):211-8; doi:10.1007/s10689-004-4489-x. PMID:16136380.
- Ning YM, Suzman D, Maher VE, Zhang L, Tang S, Ricks T, Palmby T, Fu W, Liu Q, Goldberg KB, et al. FDA Approval Summary: Atezolizumab for the Treatment of Patients with Progressive Advanced Urothelial Carcinoma after Platinum-Containing Chemotherapy. Oncologist. 2017;22(6):743-49; doi:10.1634/theoncologist.2017-0087. PMID:28424325.
- Bendell J, Kim T, Goh B. Clinical activity and safety of cobimetinib (cobi) and atezolizumab in colorectal cancer (CRC). J Clin Oncol. 2016;34(suppl; abstr 3502).
- Deihimi S, Lev A, Slifker M, Shagisultanova E, Xu Q, Jung K, Vijayvergia N, Ross EA, Xiu J, Swensen J, et al. BRCA2, EGFR, and NTRK mutations in mismatch repair-deficient colorectal cancers with MSH2 or MLH1 mutations. Oncotarget. 2017;8(25):39945-62; doi:10.18632/oncotarget.18098. PMID:28591715.