ABSTRACT
Background: Pulmonary sclerosing pneumocytoma (PSP) typically presents solitary and peripheral mass, while only rarely cases display unusual multiple lesions. We reported a extremely rare case of PSP with diffusely-scattered nodules in the right lung.
Case presentation: Diffusely round-shaped nodular shadows in the right lung were found by CT scan in a 31-year-old Chinese woman. The patient undergone the right pneumnectomy. Grossly, numerous small nodules, up to 2.5 cm in greatest dimension were identified in the upper, middle and lower lobes of the right lung. Histologically, the tumor presented the typical features of PSP, with a variable proportion of solid, sclerotic and papillary patterns. Immunohistochemical staining further revealed that cuboidal surface epithelial cells were positive for TTF-1, EMA, AE1/3 and vimentin (partially), and round or polygonal cells expressed TTF-1, vimentin, EMA (weakly), synaptophysin (partially), progesterone receptor (partially), and estrogen receptor (scatteredly). The patient has been followed up for 83 months after surgery by annual chest CT and no new lesions are detected in her left lung and other organs. The whole-exome sequencing identified 15 somatic mutations genes (MEGF6, DNAH5, AKT1, GPRIN2, PIK3AP1, FBXO40, HERC1, VPS16, MORN1, ZNF474, CTNNB1, ZNF251, TSC1, ATM, KDR). Pathway analysis showed possible pathways like the components of CTNNB1, AKT1, and TSC1 mutations in the PI3K/AKT signalings and AKT1, KDR and ATM in VEGF signaling pathway and AKT1 activation seemed closely related with these pathways.
Conclusion: According to our and previous data, PSP with diffuse or multiple lesions is very rare, and the patients are most commonly seen in women in Asian countries. The misdiagnosis rate by clinical and intraoperative frozen-section assessment is high because of the multiple nodules in the lung and its confusing histological features. Long time follow up indicates surgical resection should not be considered as the preferred strategy for treating multiple PSP in the intralobar sites. AKT1 activation may contribute to the development of PSP while the pathogenesis of diffuse or multiple PSP still needs to be further analyzed.
Background
Pulmonary sclerosing pneumocytoma (PSP), also known as sclerosing hemangioma, is a slow-growing tumor, and behaves in a clinically benign fashion. No recurrence or disease-related deaths have been reported. Most tumors are solitary and peripheral, only rare cases display unusual presentations, such as regional lymph node metastases,Citation1-3 the progressive dissemination of pleuraCitation4 and multiple lesions.Citation1,Citation4-17 This report described a unique case of PSP with diffusely-scattered nodules in the right lung, but spared the left lung within a seven-year follow-up in a 31 year-old woman.
Case presentation
A 31-year-old woman was admitted to Drum Tower hospital with abnormal shadows of multiple nodules in the right lung detected by chest X-ray at an annual medical examination 5 months ago. On examination at our center, the patient was afebrile and general physical examination was unremarkable. Laboratory data and tumor markers were all within normal ranges. Chest X-ray showed multiple nodules in the right lung and computed tomography (CT) demonstrated a number of round-shaped nodular shadows in the upper, middle and lower lobes of the right lung, up to 2.5 cm in greatest dimension ( and ). As metastatic lung tumors were highly suspected, wedge resection of the right lung was performed for a frozen section evaluation.
Figure 1. Chest X-ray and CT (CT) demonstrated diffusely round-shaped nodules in the upper, middle and lower lobes of the right lung (A and B). Grossly, multiple demarcated nodules with a maximum diameter up to 2.5 cm within the lung parenchyma (C) and some nodules located just beneath the visceral pleura (C and D).
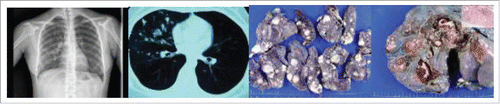
Frozen section taken from one of the nodules showed that the tumor was composed of low grade neoplastic cells with low mitotic activity, forming solid and papillary architectures. Based on these findings, intraoperative pathology of the lung tumor showed low grade malignant potential and right pneumonectomy was performed. The upper, middle and lower lobes were 12 × 9 × 3cm, 9 × 6 × 3cm and 4.5 × 2.5 × 1.5cm, respectively. There were numerous well-circumscribed nodules with variable sizes, up to 2.5 cm in greatest dimension within the lung parenchyma (), some of which were located just beneath the visceral pleura (). In the gross specimen, the cut surfaces of these tumor nodules appeared firm and grayish-white () with foci of calcifications.
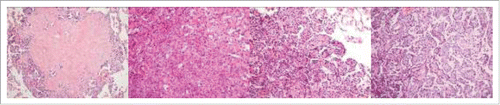
Microscopically, micronodules were found, and the tumors were well-circumscribed from the surrounding lung parenchyma but not encapsulated, and showed the typical histological features of PSP (), with a variable proportion of sclerotic (), solid ( and ) and papillary ( and ) patterns, with rarely hemorrhagic cellular patterns. In minute nodules, the tumor mainly presented solid pattern or sclerotic stroma with peripheral papillary architectures. The tumor with the solid pattern was composed of a sheet-like proliferation of round to polygonal cells with pale cytoplasm. The tumor with the papillary pattern was composed of cuboidal surface cells and the round tumor cells presented a solid proliferation beneath the surface cells. In the mostly hyalinized stroma, nests of small polygonal cells with pale cytoplasm and scattered foam-like macrophages were observed. Some tumor nodules were located just beneath the visceral pleura ().
Figure 2. The tumor showed the typical histological features of PSP, with varied proportions of sclerotic (A), solid (B and C) and papillary (C and D) patterns.
Immunohistochemically, cuboidal surface cells expressed thyroid transcription factor-1 (TTF-1) (), EMA (), Cytokeratin AE1/3 () and vimentin (partially), round or polygonal cells expressed TTF-1 (), vimentin, EMA (weakly) (), progesterone receptor (PR) (partially) (), synaptophysin (partially), estrogen receptor (ER) (scatteredly), but chromogranin, CD56, CD31 and CD34 were negative in both types of tumor cells (). Based on the histological and immunohistochemical findings above, final diagnosis of diffuse PSP was made. The patient remained alive without any evidence of recurrence or metastasis for 83 months after the right lung resection, and no new nodules were found in her left lung and other organs.
Figure 3. Two populations of cells in PSP were present: solid growing polygonal round cells with pale cytoplasm and papillary structures covered with cuboidal surface cells. Cuboidal surface cells expressed TTF-1 (A), EMA (B) and AE1/3 (C), and round or polygonal cells expressed TTF-1 (A), EMA (weakly) (B), PR (partially) (D).
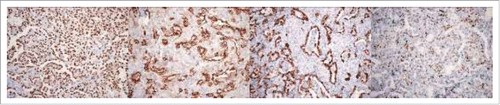
Table 1. Immunohistochemical staining pattern of PSP.
To comprehensively elucidate the genetic alternations in this rare case, whole-exome sequencing was performed with the formalin-fixed paraffin-embedded tissue (FFPE, which fixed seven years ago) of PSP and the matched normal sample of peripheral blood. The mean coverage of the sequencing depth were 125X for PSP and 60X for matched normal sample, with an average of 96% and 90%of bases covered by at least 20 reads in each sample, respectively. Somatic mutations were identified using MuTectCitation18 for point mutations and indels. The ANNOVAR package was used to select somatic mutations located in the exonic sequences and to predict their functional consequences.Citation19 To obtain reliable and robust mutation calling, the following somatic variants were eliminated: (i) read depth fewer than 10 in either of the samples; (ii) polymorphisms referenced in either 1000 Genomes Project, Exome Aggregation Consortium, or Exome Sequencing Project with a minor allele frequency greater than 1%; and (iii) variants with variant allele fraction(VAF) between 45% and 55% in a copy-neutral region. Based on the above criteria, somatic mutations were identified in 15 genes (MEGF6, DNAH5, AKT1, GPRIN2, PIK3AP1, FBXO40, HERC1,VPS16, MORN1, ZNF474, CTNNB1, ZNF251, TSC1, ATM, KDR), which were overlapped with both the cancer gene census and cancer drivers database ().
Table 2. Somatic mutations in PSP.
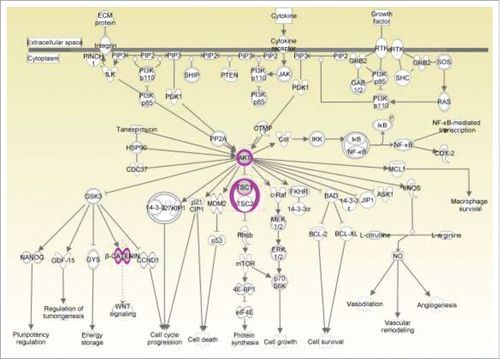
To further gain insights into the role of genomic alterations underlying PSP development, we analyzed the genome data through pathway analysis using the Kyoto Encyclopedia of Genes and Genomes (KEGG) database. Mutated genes in PSP were significantly enriched in pathways related to cell survival, proliferation, growth, and angiogenesis activities (). Mutations in multiple components of the PI3K/AKT signaling, including CTNNB1, AKT1, and TSC1 were identified and several gene alterations such as AKT1, KDR, ATM, were also shown to be related to the VEGF signaling pathway (). Of note, AKT1 activation was closely connected to these pathways ().
Table 3. Top 20 important pathways in PSP.
Discussion
PSP is an uncommon neoplasm, and its origin remains controversial but the histological features are distinctive. The majority of patients with PSP were presented with a solitary nodule, whereas multiple lesions have been reported in 4% of all cases in a Japanese cohortCitation20 and 3.6% in a Chinese group.Citation21 To our knowledge, only 17 cases of multiple PSP in lung have been reported in the literaturesCitation1,Citation4-17 including the present case, almost of all patients (14/15) were female adults in the age of 16 to 73 years old and a majority of the reported cases (16/17) were from Asia, especially China, Japan and India. In the reported cases, there were two nodules in 3 cases, three in 3 cases, four in 1 case and more than four in 10 cases. The lesions were located at bilateral, right and left lungs in 8, 6 and 3 cases, respectively, and the maximum diameters of the nodules were no more than 5 cm in all the 17 cases (). Lung metastasis was usually considered as the favorable differential diagnosis against the patients with multiple pulmonary nodules of PSP. A systemic workup was always pursued but the definite clinical diagnosis was failed. PSP was usually an incidental finding by chest X-ray because the patients were often asymptomatic (), even with multiple pulmonary nodules, as described in our case. The following chest CT usually revealed well-defined and round-shaped multiple pulmonary small nodules. Only in one reported case, the patient complained of an oppressive feeling in her chest because of the enlarged tumor compressing the surrounding tissues.Citation13 The physical examination was also asymptomatic, and the serum levels of some tumor markers, such as CEA, SLX, NSE, Pro-GRP, SCC, Cyfra21-1, CA19-9, AFP, CA125 and CA15-3, were normal.Citation4,Citation6,Citation12 All cases of multiple PSP were reported to be localized within the lung except one exhibiting pleural dissemination.Citation4
Table 4. The reported cases of multiple PSP.
Histologically, being the same as a solitary lesion, multiple PSP was also mainly composed of variable proportions of four patterns and two types of cells. In typical cases, there was a mixture of papillary, solid, sclerotic and hemorrhagic components (). In these components, there were two populations of cells present, which were solid growing polygonal round cells with pale cytoplasm and cuboidal surface cells covering papillary structures, respectively. In the present case, the tumor predominantly displayed solid, sclerotic and papillary patterns, but rarely had hemorrhagic component. Because the patient exhibited diffuse pulmonary nodules and the tumors had the histological features of solid and papillary patterns, it was very difficult to establish the definitive diagnosis. The differential diagnosis mainly included primary or metastatic well-differentiated adenocarcinomas or carcinoid tumors. The small biopsy or fine needle aspiration can be performed but the diagnosis may not be rendered due to the limitation of sampling and lack of architectures. Intraoperative frozen-section might provide more diagnostic information and guild the operative procedure. Immunohistochemically, both surface-lining cuboidal and pale polygonal cells in the present case were positive for TTF-1, EMA and vimentin. However, only surface-lining cuboidal cells stained positive for AE1/3, whereas only pale polygonal cells were diffusely immunoreactive against vimentin, with ER, PR and synaptophysin partially or scatteredly stained.
Genetically, 15 somatic mutations (MEGF6, DNAH5, AKT1, GPRIN2, PIK3AP1, FBXO40, HERC1, VPS16, MORN1, ZNF474, CTNNB1,ZNF251, TSC1, ATM, KDR) were identified and pathway analysis showed multiple components of CTNNB1, AKT1, and TSC1 mutations in the PI3K/AKT signaling pathway. Notably, AKT1 activation was closely connected to the PI3K/AKT and VEGF pathways and might play an important role (). AKT1 encodes a serine/threonine kinase that regulates many cellular processes, such as proliferation, survival, and growth.Citation23 AKT1 E17K is localized to the pleckstrin homology domain, which is crucial for membrane localization and downstream activation of AKT1.Citation24 This alteration is the most frequently reported AKT1 mutation and has been found in multiple solid tumor types including breast, skin, urinary tract, and endometrial cancers.Citation25 The frequency of AKT1 mutation in cancer is relatively low while breast cancer having the highest frequency(4-8%).Citation26,Citation27 In addition, CTNNB1 and beta-catenin, are members of the WNT signaling pathway and component of cadherin-based adherens junctions. Imbalance of beta-catenin has been implicated in cancer progression and metastasis.Citation28 Multiple solid tumor types such as soft tissue, pituitary, liver, endometrium cancers have been reported to carry CTNNB1 mutations. Remarkably, AKT1 and CTNNB1 are recurrent somatic genes that have been previously reported in pulmonary sclerosing hemangiomas (31 of 68, 45.6%, AKT1; 23 of 68, 33.8%, p.E17K; 3 of 68, 4.4%, CTNNB1), implicating that mutations in these genes may act as important tumor promoting event in PSP.Citation29 Interestingly, several gene alterations such as AKT1, KDR and ATM, are also related to the VEGF signaling pathway that activates angiogenesis, suggesting their possible roles in the vascular phenotype of PSP. Moreover, AKT1 has been engaged as a target in ongoing clinical trials,Citation30 suggesting that AKT1 inhibitors may have potential therapeutic value in PSP.
According to the WHO Classification of pulmonary tumors, current consensus to PSP favored a benign or very low grade neoplasm arising from primitive respiratory epithelium. As to the cases presented multiple lesions located at lung, all patients remained alive, well and no evidence of recurrence or metastasis for 1 to 10 years after surgical resection, or the tumor nodules remained unchanged after biopsy. The present case did not display any evidence of occurrence or metastasis either for 83 months after the resection of right lung. However, there was still one reported progressive dissemination case, which showed irregular thickness of the interlobular pleura demonstrated on CT 3 years postoperatively.Citation4 Thus, though PSP presented multiple lesions behaved as a benign condition, serious follow-up should be conducted for some cases with pleural dissemination. Additionally, it was very interesting and worthy of further study to explore why this benign or very low grade neoplasm involved unilateral lung lobes in some cases but bilateral lung lobes in other cases.
Conclusion
PSP with multiple lesions is extremely rare, and the patients are most commonly women in Asian countries, such as China and Japan. The misdiagnosis rate by clinical and intraoperative frozen-section assessment is high because the multiple nodules in the lung and its histological features could mimic well-differentiated adenocarcinoma or carcinoids. According to the data of long time follow up, it seems that surgical resection should not be considered as the preferred strategy for treating multiple PSP in the intralobar sites, because of no evidence of recurrence, metastasis, enlargement of tumor nodules or respiratory dysfunction in almost all reported cases, but further follow-up is still necessary based on the limited reported cases. Somatic mutations sequencing and pathway analysis conclude that AKT1 activation may contribute to the development of PSP while the pathogenesis of diffuse or multiple PSP still needs to be further analyzed.
Consent
Written informed consent was obtained from the patient and her family for publication of this Case Report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
| Abbreviations | ||
| PSP | = | pulmonary sclerosing pneumocytoma |
| CT | = | computed tomography |
| TTF-1 | = | thyroid transcription factor-1 |
| PR | = | progesterone receptor |
| ER | = | estrogen receptor |
References
- Kim GY, Kim J, Choi YS, Kim HJ, Ahn G, Han J. Sixteen Cases of Sclerosing Hemangioma of the Lung Including Unusual Presentations. Journal of Korean Medical Science. 2004;19:352-8. doi:10.3346/jkms.2004.19.3.352. PMID:15201499
- Miyagawa-Hayashino A, Tazelaar HD, Langel DJ, Colby TV. Pulmonary sclerosing hemangioma with lymph node metastases: report of 4 cases. Archives of pathology & laboratory medicine. 2003;127:321-5; doi:10.1155/2003/534147
- Chan NG, Melega DE, Inculet RI, Shepherd JG. Pulmonary sclerosing hemangioma with lymph node metastases. Canadian Respiratory Journal Journal of the Canadian Thoracic Society. 2003;10:391-2. doi:10.1155/2003/534147. PMID:14571291
- Suzuki H, Saitoh Y, Koh E, Hoshino H, Kase D, Kasei Y, Azuhata Y, Kishi H, Hiroshima K, Sekine Y. Pulmonary sclerosing hemangioma with pleural dissemination: report of a case. Surgery today. 2011;41:258-61. doi:10.1007/s00595-009-4220-5. PMID:21264765
- Chen B, Gao J, Chen H, Cao Y, He X, Zhang W, Luo M, Zhang S, Li W. Pulmonary sclerosing hemangioma: a unique epithelial neoplasm of the lung (report of 26 cases). World journal of surgical oncology. 2013;11:85. doi:10.1186/1477-7819-11-85
- Hanaoka J, Ohuchi M, Inoue S, Sawai S, Tezuka N, Fujino S. Bilateral multiple pulmonary sclerosing hemangioma. The Japanese Journal of Thoracic and Cardiovascular Surgery. 2005;53:157-61. doi:10.1007/s11748-005-0024-8. PMID:1310458
- He C, Fang H, Liu Y, Huang X, Zhen W, Ren L. Pulmonary sclerosing hemangioma: report of two cases. World journal of surgical oncology. 2012;10:182. doi:10.1186/1477-7819-10-182. PMID:22943472
- Hishida T, Yoshida J, Nishimura M, Ishili G, Nishiwaki Y, Nagai K. Multiple sclerosing hemangiomas with a 10-year history. Japanese journal of clinical oncology. 2005;35:37-9. doi:10.1093/jjco/hyi002. PMID:15681603
- Ito K, Sakagami T, Abe T, Tsutsui N, Nakajima H, Haraguchi M. A case of multiple sclerosing hemangiomas of both lungs. Nihon Kokyuki Gakkai zasshi= the journal of the Japanese Respiratory Society. 2006;44:848-52. PMID:17144585
- Joshi K, Shankar SK, Gopinath N, Kumar R, Chopra P. Multiple sclerosing haemangiomas of the lung. Postgraduate medical journal. 1980;56:50-3. doi:10.1136/pgmj.56.651.50. PMID:6247707
- Lee ST, Lee YC, Hsu CY, Lin CC. Bilateral multiple sclerosing hemangiomas of the lung. CHEST Journal. 1992 101:572-3. doi:10.1378/chest.101.2.572. PMID:1310458
- Maeda R, IN, Miura H, Tokuyasu H, Kawasaki Y, Yamamoto K. Bilateral multiple sclerosing hemangiomas of the lung. General thoracic and cardiovascular surgery. 2009;57:667-70. PMID:15828298
- Maezato K, Hitomi S, Kuwabara M. A case of multiple sclerosing hemangiomas of the lung and a review of the literature in Japan. Nihon Kyōbu Shikkan Gakkai Zasshi. 1989;27:230-3. PMID:2545966
- Noguchi M, Kodama T, Morinaga S, Shimosatio Y, Saito T, Tsuboi E. Multiple sclerosing hemangiomas of the lung. The American journal of surgical pathology. 1986;10:429-35. doi:10.1097/00000478-198606000-00008. PMID:3717498
- Pedro Boleo-Tome J, Matos C, Nogueira F, Maya M, Sena LJ. A rare case of multiple sclerosing hemangiomas of the lung. Revista portuguesa de pneumologia. 2007;14:291-4. doi:10.1016/S0873-2159(15)30237-3. PMID:18363024
- Purandare NC, Dua SG, Shah S, Sharma AR, Suryawanshi PV, Rangarajan V. Multiple FDG-avid sclerosing hemangiomas mimicking pulmonary metastases in a case of soft tissue sarcoma. Cancer Imaging. 2010;10:169-72. doi:10.1102/1470-7330.2010.0024. PMID:20675249
- Soumil VJ, Navin B, Sangeeta D, Na J, Sharma S, Deshpande R. Multiple sclerosing hemangiomas of the lung. Asian Cardiovascular and Thoracic Annals. 2004;12:357-9. doi:10.1177/021849230401200416. PMID:15585708
- Cibulskis K, Lawrence MS, Carter SL, Sivachenko A, Jaffe D, Sougnez C, Gabriel S, Meyerson M, Lander ES, Getz G. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nature Biotechnology. 2013;31:213-9. doi:10.1038/nbt.2514. PMID:23396013
- Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38:e164. doi:10.1093/nar/gkq603. PMID:20601685
- Iyoda A, Hiroshima K, Shiba M, Haga Y, Moriya Y, Sekine Y, Shibuya K, Iizasa T, Fujisawa T. Clinicopathological analysis of pulmonary sclerosing hemangioma. The Annals of thoracic surgery. 2004;78:1928-31. doi:10.1016/j.athoracsur.2004.05.069. PMID:15561002
- Xie D, Jiang GN, Chen XF, Xu ZF, Ding JA. Surgery treatment for pulmonary sclerosing hemangioma. Zhonghua wai ke za zhi [Chinese journal of surgery]. 2012;50:120-3. PMID:22490348
- Chen B, Gao J, Chen H, Cao Y, He X, Zhang W, Luo M, Zhang S, Li W. Pulmonary sclerosing hemangioma: a unique epithelial neoplasm of the lung (report of 26 cases). World J Surg Oncol. 2013;11:85.
- Franke TF. PI3K/Akt: getting it right matters. Oncogene. 2008;27:6473-88. doi:10.1038/onc.2008.313. PMID:18955974
- Mahadevan D, Powis G, Mash EA, George B, Gokhale VM, Zhang S, Shakalya K, Du-Cuny L, Berggren M, Ali MA, et al. Discovery of a novel class of AKT pleckstrin homology domain inhibitors. Molecular cancer therapeutics. 2008;7:2621-32. doi:10.1158/1535-7163.MCT-07-2276. PMID:18790745
- Kim MS, Jeong EG, Yoo NJ, Lee SH. Mutational analysis of oncogenic AKT E17K mutation in common solid cancers and acute leukaemias. British journal of cancer. 2008;98:1533-5. doi:10.1038/sj.bjc.6604212. PMID:18392055
- Parikh C, Janakiraman V, Wu WI, Foo CK. Kljavin NM, Chaudhuri S, Stawiski E, Lee B, Lin J, Li H. Disruption of PH-kinase domain interactions leads to oncogenic activation of AKT in human cancers. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:19368-73. doi:10.1073/pnas.1204384109. PMID:23134728
- Banerj S, Cibulskis K, Rangelescareno C, Brown KK, Carter, S. L, Frederick AM, Lawrence MS, Sivachenko AY, Sougnez C, Zou L. Sequence analysis of mutations and translocations across breast cancer subtypes. Nature. 2012;486:405-9. doi:10.1038/nature11154. PMID:22722202
- Valenta T, Hausmann G, Basler K. The many faces and functions of β-catenin. Embo Journal. 2012;31:2714-36. doi:10.1038/emboj.2012.150. PMID:22617422
- Jung SH, Kim MS, Lee SH, Park HC, Choi HJ, Maeng L, Min KO, Kim J, Park TI, Shin OR, et al. Whole-exome sequencing identifies recurrent AKT1 mutations in sclerosing hemangioma of lung. Proc Natl Acad Sci U S A. 2016;113:10672-7. doi:10.1073/pnas.1606946113. PMID:27601661
- G T. First-in-man clinical trial of the oral pan-AKT inhibitor MK-206 in patients with advanced solid tumors. Journal of Clinical Oncology Official Journal of the American Society of Clinical Oncology. 2011;29:4688-95. doi:10.1200/JCO.2011.35.5263. PMID:22025163