ABSTRACT
Eosinophilic cystitis is a rare manifestation of hypereosinophilia and a cause of morbidity, including dysuria and hematuria. Although some cases can be attributed to infection or allergy, most cases are assessed to be idiopathic and treated with corticosteroids. However, hypereosinophilia can also be due to actionable clonal molecular alterations in the haematopoietic cells, similar to other myeloproliferative neoplasms. Common mutations associated with eosonophilic syndromes are of platelet-derived growth factor receptor α or β or c-kit, though other pathogenic mutations have been found by next generation sequencing. Determination of a specific mutation may therefore identify clonality and refine treatment of some cases. Here we review the molecular features of eosinophilic disorders. We also describe the use of a liquid biopsy of circulating cell-free DNA in the workup of a case of eosinophilic cystitis in which next generation sequencing of cell-free DNA showed a BRAF I463T mutation. In silico modeling supports the functional impact and potential clinical relevance of BRAF I463T.
Introduction
Molecular alterations are being used as a basis of understanding tumors and deploying appropriately matched targeted therapy. Specific examples include the use of BRAF inhibitors in melanoma harboring BRAF mutationsCitation1 as well as several other cancer types,Citation2 or imatinib for chronic myelogenous leukemia.Citation3 Identification of molecular alterations can also establish the clonal origin of hematologic diseases.Citation4 Currently, most molecular tests are performed on archived tissues or require invasive biopsies that involve risk and cost. Furthermore, some patients may be difficult to biopsy or have inadequate tissue or low numbers of circulating malignant cells. One approach that could be useful in these situations is to interrogate circulating tumor cell-free DNA (ctDNA) shed into the circulation or released when cancer cells die.Citation5
Eosinophilic cystitis is a rare condition in which transmural eosinophilic inflammation of the bladder causes urinary frequency, hematuria, dysuria and suprapubic pain.Citation6 There is no curative treatment of this condition, and most patients are managed with corticosteroids. Although some cases can be associated with bladder infections or allergens, the underlying cause remains unclear.Citation6,Citation7 Here we report the result of a liquid biopsy demonstrating a novel BRAF mutation in ctDNA from a patient with eosinophilic cystitis and show in silico modeling indicating functional impact of this alteration.
Case presentation
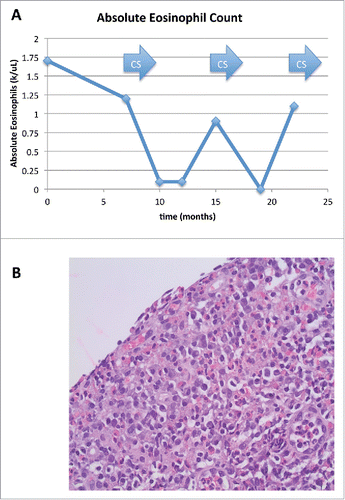
A 65 year-old Caucasian male was evaluated for blood and solid tissue in his urine. He had peripheral eosinophilia (up to 1.7 × 103 eosinophils/uL (normal <0.5 eosinophils/uL)) (). Urine cytology showed numerous eosinophils in a background of heavy inflammation. Cystoscopy showed denuded lamina propria with chronic inflammation and abundant eosinophils (). Treatment with prednisone 1 mg/kg/day by mouth resulted in some improvement, but both peripheral and urinary eosinophilia recurred with tapering, prompting restarting steroids. No potential cause of reactive eosinophilia such as parasitic infection was found, and conventional molecular testing showed no translocations or mutations commonly associated with clonal eosinophilia. cKIT (D816V) mutation, BCR-ABL fusion, T-cell clonality, and rearrangements of platelet-derived growth factor receptor α or β (PDGFRA or PDGFRB) were normal, as was karyotype. To investigate further for any underlying molecular cause, the bladder biopsy specimen was sent for next generation sequencing (Foundation Medicine, Cambridge, MA). However, the material was determined to be insufficient for analysis. Peripheral blood was evaluated by next generation sequencing of ctDNA. (Guardant Health, 54 gene panel; Digital Sequencing™ (CLIA-certified)) https://www.guardanthealth.com/). Next-generation sequencing of ctDNA revealed a BRAF alteration (I463T).
Figure 1. Clinical Data: (1A) Absolute eosinophil counts showing levels above the upper limit of normal. Arrows indicates periods of administration of systemic corticosteroids (CS). (1B) Bladder biopsy with hematoxylin and eosin staining showing eosinophilic infiltrate of the lamina propria of denuded urinary bladder mucosa. Magnification is 400x.
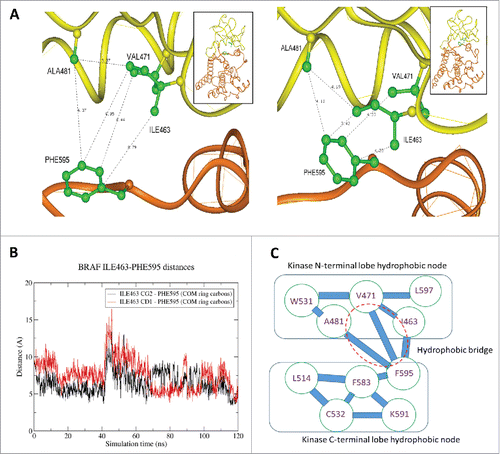
In silico modeling was performed to determine the significance of the BRAF I463T mutation. Based on the crystal structure of the protein (Protein Data Bank (PDB) index 1UWH) we analyzed the network of contacts that keep the conformation in a closed state by adding the residues missing in the crystal structure using the program Homology Insight II software package (Accelrys, San Diego).Citation8,Citation9 After 5,000 iterations of steepest descent minimization, the protein molecules were subjected to molecular dynamics (MD) simulation in a truncated octahedral TIP3P water box containing 17111 H2O molecules and 8 chloride counterions. MD was performed using the GPU-accelerated pmemd.cuda module implemented in the AMBER14 program package,Citation8-11 using AMBER ff14SB parameters. Joung/Cheatham parametersCitation10 were applied for the counterions. We determined that I463 forms a hydrophobic bridge between the N-terminal and C-terminal hydrophobic nodes of BRAF in its inactive closed conformation (). Its substitution to threonine is be predicted to disrupt this bridge, increasing repulsion of the 2 lobes, and increasing the probability of kinase activation. Therefore, we propose that this BRAF mutation is an activating mutation.
Figure 2. In silico analysis: (2A) Close-up of BRAF PHE595 – ILE463 interaction: Left: Initial structure after deletion of BRAF inhibitor. Right: Structure after 117 ns of molecular dynamics (MD) simulations. An initial minimization was succeeded by: (i) 5 picoseconds gradual heating from 0 to 100 K at constant volume with weak restraints on the protein backbone; (ii) 100 picoseconds gradual heating from 100 to 310 K at constant pressure with weak restraints on the protein backbone; and (iii) unrestrained constant pressure simulation at 310 K. Analysis of the simulation trajectory was conducted with C-language Process TRAJectory. (2B) Evolution of the distance between ILE463 (atoms CG2 – black curve and CD1 – red curve) and the center of mass of the side chain of PHE595. During MD the interaction between PHE595 and ILE463 defining a hydrophobic bridge between the upper and lower lobes of the kinase became significant with the distance between these residues evolving from around 7A to 3.4 A. (2C) BRAF kinase hydrophobic stabilization of the inactive conformation. When ILE463 is substituted to TYR, a hydrophobic bridge is disrupted and results in repulsion of neighboring groups. Such repulsion can increase the probability of kinase activation. The distance between these 2 residues varies during MD with one of the 2 terminal sidechain carbons of ILE463 alternating in having the closer contact with PHE595.
Discussion
We present a patient with a diagnosis of eosinophilic cystitis who had recurrent cystitis with infiltration of bladder tissue by eosinophils and peripheral eosinophilia. Eosinophilic cystitis is a rare problem, but one that can result in significant morbidity. It is relatively rare, and literature is primarily based on case reports or small case series. A pooled analysis by van den Ouden reported a median age at diagnosis of 41.6 y (range 5 d to 87 years) and common presenting symptoms including the following: increased urinary frequency, dysuria, gross or microscopic hematuria, suprapubic pain, and urinary retention.Citation12 Eosinophilic cystitis has been associated with chronic vesical injury, bladder neoplasms, interstitial cystitis, recurrent urinary tract infections, parasitic infections, atopic diseases, food allergens, and chronic granulomatous disease, but in some cases. Cases have also been reported in association with idiopathic hypereosinophilic syndrome, such as observed in the present case.Citation13,Citation14 Peripheral eosinophilia is detected in about 40% of cases. Radiologic testing by intravenous urography, ultrasonography, or CT scan often detects bladder wall thickening or solitary tumor-like lesions that can mimic bladder cancer.Citation6,Citation15 Cystoscopy and biopsy is necessary for diagnosis. Histologic findings are of transmural inflammation of the bladder with eosinophils. There is no curative treatment, and most patients are treated supportively with non-steroidal anti-inflammatory agents or steroids.Citation7 Cyclosporine has also been used.Citation16 For cases with severe refractory symptoms, transurethral resection of the bladder lesion can be performed in addition to systemic therapy, but lesions tend to recur in spite of therapy.Citation17
While very little has been established the molecular pothegenesis of eosinophilic cystits, this can also be considered within the general context of hypereosinophilia and hypereosinophilic syndrome (HES). Patients with persistent peripheral eosinophilia (more than 1.5 × 109/L) and eosinophilic infiltration into organs meet criteria for having an HES. Eosinophils release toxic granule products that mediate epithelial cell damage, recruitment of other inflammatory cells, and fibrosis. Commonly involved organs include the skin, lungs, and gastrointestinal tract. Many patients may be asymptomatic with an incidental detection eosinophilia, or have an insidious onset of symptoms, based on the organ involved. Specific symptoms commonly reported at presentation include rash, cough, abdominal pain, vomiting, or diarrhea. However some patients can initially present with potentially life-threatening involvement of the cardiovascular or central nervous system.
Some cases of eosinophilia are reactive to conditions like parasites, allergic or hypersensitivity conditions, vasculitis, or certain malignancies that produce cytokines like IL-3, IL-5, and GM-CSF, and designed as secondary eosinophilia.Citation18 On the other hand, primary eosinophilia (clonal eosinophilia) results from molecular defects of haematopoietic stem cells or myeloid cells that directly causes over-production of eosinophilas. Specific abnormalities of platelet-derived growth factor receptor α or β (PDGFRA or PDGFRB) and fibroblast growth factor receptor 1 (FGFR1) have been identified. These genes are typically mutated via chromosome translocations that result in dysregulated fusion genes.Citation18 A fusion of pericentriolar material 1 (PCM1) and Janus kinase 2 (JAK2) was also identified,Citation19 and likely new molecular abnormalities will be defined.
HES has been subdivided into variants based on etiology and associated conditions, including myeloproliferative variants of HES (M-HES), T-lymphocytic variants (L-HES), familial HES, idiopathic HES, and organ-restricted HES in which a single organ is involved. The 2016 World Health Organization (WHO) classification system included a specific category for Myeloid/lymphoid neoplasms with eosinophilia and specific genetic abnormalities.Citation20 The revised classification includes the following:
| · | PDGFRA – PDGFRA is located on chromosome 4 and is most commonly involved in a fusion with FIP1L1 due to an interstitial deletion on chromosome 4q12, but may be involved in translations with various other fusion partners. The The FIP1L1-PDGFRA fusion results in constitutive tyrosine kinase activity and may account for approximately 10–14% of cases that meet criteria for M-HES. | ||||
| · | PDGFRB – More than 30 fusion partners of PDGFRB that have been characterized. These involve a partner protein that contains dimerization/oligomerization motif(s) that can mimic receptor dimerization and activation in the absence of PDGFRB ligand. | ||||
| · | FGFR1 – FGFR1 is located on chromosome 8p11 and often involved in a translocation with the ZMYM2 gene (zinc finger, MYM2 gene) on chromosome 13q12. | ||||
| · | M-HES may be caused by other mutations or chromosomal rearrangements. M-HES due to PCM1-JAK2 is included as a provisional entity. In this, translocation t(8;9)(p22;q24.1) results in the fusion of PCM1 (pericentriolar material 1) and JAK2 (Janus kinase 2). Additionally, clonal eosinophilia is a common manifestation of D816V KIT-positive systemic mastocytosis. | ||||
In many cases, identification of the driver mutation has therapeutic implications. For patients with PDGFRA/B rearrangements, targeted therapy by the tyrosine-kinase inhibitor imatinib is effective.Citation21,Citation22 Midostaurin has activity in cases of KIT alterations.Citation23 Hypereosinophilia in association with JAK2 mutations has also been reported, with response to ruxolitinib.Citation24-26
However, more than 50% of cases do not have these identifiable alterations and are labeled as idiopathic hypereosinophiliaCitation18 Recently, next generation sequencing has been used to evaluate patients with HE. Wang et al evaluated 51 patients with idiopathic HES.Citation27 They found mutations in 28% of cases, with mutated genes including ASXL1, TET2, EZH2, CBL, SETBP1, and NOTCH1. 14% of patients had a single mutation and 14% of patients had 2 or more gene mutations. The patients with these mutations were found to have clinical and marrow findings consistent with chronic eosinophilic leukemia (CEL), including adverse prognosis and poor overall survival, similar to patients with CEL. This result suggests that NGS may be used in cases of idiopathic HES to identify clonality and establish a diagnosis of CEL, in conjunction with other clinical information. Pardanani et al also evaluated a cohort of 98 patients with idiopathic HES.Citation28 They similarly found mutations in genes that implied clonal myeloid disorders. Eleven percent of patients had known or predicted pathogenic mutations in one gene, including TET2, ASXL1, KIT, IDH2, JAK2, SF3B1, and TP53. Fifteen percent had mutations in TET2, ASXL1, SETBP1, CALR, CEBPA, and CSF3R that were labeled as variants of unknown significance. Interestingly, there were not significant differences in clinical characteristics or prognosis for patients with or without mutations. Differences in overall survival were not statistically significant, and the authors predicted that these particular mutations may not be driver mutations.
In our case, NGS identified a novel point mutation in BRAF. BRAF encodes a serine/threonine protein kinase that is a key regulator of the mitogen activated protein (MAP) kinase pathway.Citation29 Somatic BRAF mutations are among the most commonly altered oncogenes, and are considered as drivers in numerous malignant diseases: melanoma (∼50%), colorectal carcinoma (10%), lung carcinoma (6%), Erdhein-Chester disease (non-Langerhans histiocytosis), and nearly all cases of hairy cell leukemia.Citation30 V600E is the most common BRAF mutation. This mutation is in the activation loop (A-loop) of the BRAF kinase domain and results in constitutive kinase activation by disrupting the inactive e conformation that normally requires interaction between the A-loop and the phosphate-binding loop (P-loop) of the protein. But, other point mutations have been found in cancer cell lines or primary tumor samples (http://cancer.sanger.ac.uk/wgs/gene/analysis?ln = BRAF#histo).Citation29 Many of these are located within the P-loop (residues 464–469); like the V600E mutation, they disrupt the stability of the inactive conformation of BRAF and shift the probability toward kinase-active conformation of BRAF. For example, an I463S variant has been classified as having intermediate activity.Citation31 Hererin, we report an analysis of a novel somatic mutation of the BRAF gene–I463T. Based on our computational modeling, this variant is predicted to result in disruption of a hydrophobic bridge that normally stabilizes the inactive conformation. Therefore, we propose that this BRAF mutation is a new putative driver mutation for this patient's eosinophilic cystitis.
There are potential weaknesses of our study. The patient has not yet received treatment besides steroids, so the effect of a BRAF inhibitor on this alteration is not known. Second, we identified the BRAF mutation in ctDNA, as bladder tissue was not sufficient for testing. Even so, ctDNA assays have demonstrated reliable detection of similar BRAF mutations in the context of melanoma or Erdheim-Chester.Citation32
In conclusion, our study suggests that an activating BRAF I463T mutation was associated with eosinophilic cystitis. Importantly, analysis of ctDNA obtained through “liquid biopsies” can identify potentially important genomic alterations in patients for whom biopsy may be difficult in terms of risk or cost. Further, in silico modeling can help elucidate their functional impact.
Declarations
Dr. Kurzrock has research funds from Foundation Medicine, Guardant health, Genentech, Merck Serono, Pfizer, and Sequenom, is a paid consultant of Sequenom, and has an ownership interest in RScueRx Inc.
Funding
This work was funded in part by National Cancer Institute grant P30 CA016672 (R.K.), Joan and Irwin Jacobs Fund philanthropic fund (R.K.), and the Tower Cancer Research Foundation (M.C.)
References
- Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, O'Dwyer PJ, Lee RJ, Grippo JF, Nolop K, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809-19. doi:10.1056/NEJMoa1002011. PMID:20818844
- Hyman DM, Puzanov I, Subbiah V, Faris JE, Chau I, Blay JY, Wolf J, Raje NS, Diamond EL, Hollebecque A, et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N Engl J Med. 2015;373:726-36. doi:10.1056/NEJMoa1502309. PMID:26287849
- Thiesing JT, Ohno-Jones S, Kolibaba KS, Druker BJ. Efficacy of STI571, an abl tyrosine kinase inhibitor, in conjunction with other antileukemic agents against bcr-abl-positive cells. Blood. 2000;96:3195-9. PMID:11050003
- Kwok B, Hall JM, Witte JS, Xu Y, Reddy P, Lin K, Flamholz R, Dabbas B, Yung A, Al Hafidh J, et al. MDS-associated somatic mutations and clonal hematopoiesis are common in idiopathic cytopenias of undetermined significance. Blood. 2015;126:2355-61. doi:10.1182/blood-2015-08-667063. PMID:26429975
- Janku F, Angenendt P, Tsimberidou AM, Fu S, Naing A, Falchook GS, Hong DS, Holley VR, Cabrilo G, Wheler JJ, et al. Actionable mutations in plasma cell-free DNA in patients with advanced cancers referred for experimental targeted therapies. Oncotarget. 2015;6:12809-21. doi:10.18632/oncotarget.3373. PMID:25980577
- Li G, Cai B, Song H, Yang Z. Clinical and radiological character of eosinophilic cystitis. Int J Clin Exp Med. 2015;8:533-9. PMID:25785027
- Mosholt KS, Dahl C, Azawi NH. Eosinophilic cystitis: Three cases, and a review over 10 years. BMJ Case Rep. 2014;2014. doi:10.1136/bcr-2014-205708. PMID:25312971.
- Gotz AW, Williamson MJ, Xu D, Poole D, Le Grand S, Walker RC. Routine microsecond molecular dynamics simulations with AMBER on GPUs. 1. generalized born. J Chem Theory Comput. 2012;8:1542-55. doi:10.1021/ct200909j. PMID:22582031
- Salomon-Ferrer R, Case DA, Walker RC. An overview of the Amber biomolecular simulation package. WIREs Comput Mol Sci. 2013;3:198-201. doi:10.1002/wcms.1121
- Joung IS, Cheatham TEr. Determination of alkali and halide monovalent ion parameters for use in explicitly solvated biomolecular simulations. J Phys Chem B. 2008;112:9020-41. doi:10.1021/jp8001614. PMID:18593145
- Salomon-Ferrer R, Gotz AW, Poole D, Le Grand S, Walker RC. Routine microsecond molecular dynamics simulations with AMBER on GPUs. 2. Explicit solvent particle mesh ewald. J Chem Theory Comput. 2013;9:3787-888. doi:10.1021/ct400314y
- van den Ouden D. Diagnosis and management of eosinophilic cystitis: A pooled analysis of 135 cases. Eur Urol. 2000;37:386-94. doi:10.1159/000020183. PMID:10765067
- Derakhshan D, Ilkhanipoor H, Derakhshan A. Eosinophilic cystitis and idiopathic hypereosinophilic syndrome in an eight-year-old girl. Saudi J Kidney Dis Transpl. 2014;25:1301-3. doi:10.4103/1319-2442.144272. PMID:25394455
- Jiang P, Wang C, Jin B, Lin Y, Chen S. Eosinophilic cystitis in a patient with hypereosinophila syndrome: A case report. Exp Ther Med. 2014;8:49-51. PMID:24944595
- Werbrouck C, Marrannes J, Verhamme L, Steenkiste E, Laridon E, Van Holsbeeck B. Eosinophilic cystitis mimicking bladder tumor. JBR-BTR. 2014;97:375. PMID:25786303
- Aleem S, Kumar B, Fasano MB, Takacs E, Azar AE. Successful use of cyclosporine as treatment for eosinophilic cystitis: A case report. World Allergy Organ J. 2016;9:22. doi:10.1186/s40413-016-0113-4. PMID:27458500
- Teegavarapu PS, Sahai A, Chandra A, Dasgupta P, Khan MS. Eosinophilic cystitis and its management. Int J Clin Pract. 2005;59:356-60. doi:10.1111/j.1742-1241.2004.00421.x. PMID:15857336
- Gotlib J. World Health Organization-defined eosinophilic disorders: 2014 update on diagnosis, risk stratification, and management. Am J Hematol. 2014;89:325-37. doi:10.1002/ajh.23664. PMID:24577808
- Reiter A, Walz C, Watmore A, Schoch C, Blau I, Schlegelberger B, Berger U, Telford N, Aruliah S, Yin JA, et al. The t(8;9)(p22;p24) is a recurrent abnormality in chronic and acute leukemia that fuses PCM1 to JAK2. Cancer Res. 2005;65:2662-7. doi:10.1158/0008-5472.CAN-04-4263. PMID:15805263
- Bamford S, Dawson E, Forbes S, Clements J, Pettett R, Dogan A, Flanagan A, Teague J, Futreal PA, Stratton MR, et al. The COSMIC (Catalogue of Somatic Mutations in Cancer) database and website. Br J Cancer. 2004;91:355-8. PMID:15188009
- Apperley JF, Gardembas M, Melo JV, Russell-Jones R, Bain BJ, Baxter EJ, Chase A, Chessells JM, Colombat M, Dearden CE, et al. Response to imatinib mesylate in patients with chronic myeloproliferative diseases with rearrangements of the platelet-derived growth factor receptor beta. N Engl J Med. 2002;347:481-7. doi:10.1056/NEJMoa020150. PMID:12181402
- Pardanani A, Reeder T, Porrata LF, Li CY, Tazelaar HD, Baxter EJ, Witzig TE, Cross NC, Tefferi A. Imatinib therapy for hypereosinophilic syndrome and other eosinophilic disorders. Blood. 2003;101:3391-7. doi:10.1182/blood-2002-10-3103. PMID:12506022
- Jawhar M, Schwaab J, Naumann N, Horny HP, Sotlar K, Haferlach T, Metzgeroth G, Fabarius A, Valent P, Hofmann WK, et al. Response and progression on midostaurin in advanced systemic mastocytosis: KIT D816V and other molecular markers. Blood. 2017;130:137-45. doi:10.1182/blood-2017-01-764423. PMID:28424161
- Dasari S, Naha K, Hande M, Vivek G. A novel subtype of myeloproliferative disorder? JAK2V617F-associated hypereosinophilia with hepatic venous thrombosis. BMJ Case Rep. 2013;2013. published online. doi:10.1136/bcr-2013-200087.
- Lierman E, Selleslag D, Smits S, Billiet J, Vandenberghe P. Ruxolitinib inhibits transforming JAK2 fusion proteins in vitro and induces complete cytogenetic remission in t(8;9)(p22;p24)/PCM1-JAK2-positive chronic eosinophilic leukemia. Blood. 2012;120:1529-31. doi:10.1182/blood-2012-06-433821. PMID:22899477
- Rumi E, Milosevic JD, Casetti I, Dambruoso I, Pietra D, Boveri E, Boni M, Bernasconi P, Passamonti F, Kralovics R, et al. Efficacy of ruxolitinib in chronic eosinophilic leukemia associated with a PCM1-JAK2 fusion gene. J Clin Oncol. 2013;31:e269-71. doi:10.1200/JCO.2012.46.4370. PMID:23630205
- Wang SA, Tam W, Tsai AG, Arber DA, Hasserjian RP, Geyer JT, George TI, Czuchlewski DR, Foucar K, Rogers HJ, et al. Targeted next-generation sequencing identifies a subset of idiopathic hypereosinophilic syndrome with features similar to chronic eosinophilic leukemia, not otherwise specified. Modern Pathology. 2016;29:854-64. doi:10.1038/modpathol.2016.75. PMID:27174585
- Pardanani A, Lasho T, Wassie E, Finke C, Zblewski D, Hanson CA, Ketterling RP, Gangat N, Tefferi A. Predictors of survival in WHO-defined hypereosinophilic syndrome and idiopathic hypereosinophilia and the role of next-generation sequencing. Leukemia. 2016;30:1924-6. doi:10.1038/leu.2016.73. PMID:27125206
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949-54. doi:10.1038/nature00766. PMID:12068308
- Tiacci E, Trifonov V, Schiavoni G, Holmes A, Kern W, Martelli MP, Pucciarini A, Bigerna B, Pacini R, Wells VA, et al. BRAF mutations in hairy-cell leukemia. N Engl J Med. 2011;364:2305-15. doi:10.1056/NEJMoa1014209. PMID:21663470
- Wan PT, Garnett MJ, Roe SM, Lee S, Niculescu-Duvaz D, Good VM, Jones CM, Marshall CJ, Springer CJ, Barford D, et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004;116:855-67. doi:10.1016/S0092-8674(04)00215-6. PMID:15035987
- Janku F, Vibat CR, Kosco K, Holley VR, Cabrilo G, Meric-Bernstam F, Stepanek VM, Lin PP, Leppin L, Hassaine L, et al. BRAF V600E mutations in urine and plasma cell-free DNA from patients with Erdheim-Chester disease. Oncotarget. 2014;5:3607-10. doi:10.18632/oncotarget.1964. PMID:25003820
