ABSTRACT
Aggressive fibromatosis (AF) or desmoid tumors is an aggressive fibroblastic proliferation which is locally invasive but can not metastasize. The treatment of AF is challenging. Surgery was the main treatment modality for AF in the past, other strategies including radiotherapy, systemic therapies and wait-and-see policy. The use of non-steroidal anti-inflammatory drugs (NSAIDs) and targeted therapies has demonstrated good results. In the case report, a 39-year-old man presented with progressive chest wall pain. Computed tomography (CT) showed an approximately 46× 13 mm soft-tissue mass between the inside of the fifth and sixth rib on the right side. The entire mass was excised and an AF was confirmed based on histopathology. Four months after surgery, magnetic resonance imaging (MRI) showed a soft-tissue mass in surgical areas and biopsy confirmed local recurrence. Therefore, Tomotherapy was administered. However, two months later, an (18)F-fluorodeoxyglucose (FDG) Positron Emission Tomography combined with CT (PET-CT) revealed the presence of an FDG-avid mass in the area of local recurrence. Genetic testing reported the presence of a p.T41A mutations on the CTNNB1 gene, which predicted that he is sensitive to the COX-2 inhibitor celecoxib. The tumor regressed rapidly after the application of celecoxib. Within the 20-month follow-up period, the patient showed remarkable regression without any signs and symptoms. Our case report provides further evidence for the efficacy of celecoxib in AF with CTNNB1 gene mutations. To our knowledge, this is the first report of AF treated with celecoxib under the guidance of the genetic testing. However, further studies are required.
Introduction
Aggressive fibromatosis (AF), also known as desmoid tumor, is aggressive, destructive, and infiltrative soft tissue neoplasms. It has a high risk of recurrence, but lacks the ability to metastasize. AF accounts for 3% of all soft-tissue malignancies with an incidence of 2.4–4.3 new patients per million population per year.Citation1 Overall AF is present in people between 15–60 years old, and in more than twice as many women as men.Citation2 There are two different types. The first type is mostly sporadic. However, at least 7.5% of AF is associated with the other type, familial adenomatous polyposis (FAP), equating to an 800-fold risk compared to the general population.Citation3,4 AF is classified into intra-abdominal, extra-abdominal and abdominal wall according to sites. Extra-abdominal fibromatosis occurs in the limb, the head and neck or the trunk, while intra-abdominal fibromatosis involves the pelvis, retroperitoneum, mesentery and the intestinal wall.Citation5,6 Historically, surgical resection was the first-line therapy for AF. However, depending on margins, recurrence rates of up to 27%-54% are reported.Citation7 Recently, due to the spontaneous regression of AF without treatment and high recurrence rate, “watch and wait” policy has been proposed. Radiotherapy has been used either as an adjunct to surgery or in isolation. It improves local control rate regardless of negative or positive margins. The complications include tissue fibrosis and radiation-induced neoplasms, which are associated with total doses>56Gy.Citation8 Several agents have been used successfully including hormone therapy, NSAIDs and tyrosine kinase inhibitors. Here, CTNNB1 gene mutations are present as shown in the report of the patient with chest wall AF. The tumor showed remarkable regression after celecoxib therapy.
Case report
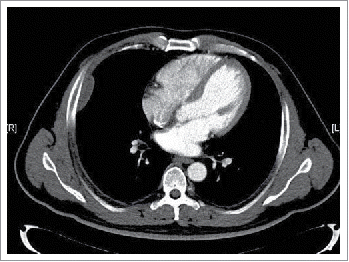
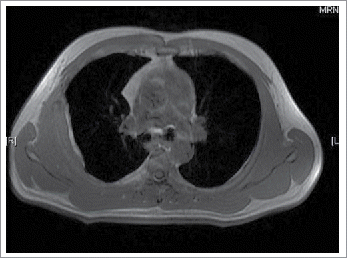
A 39-year-old man first presented to our outpatient clinic with chest wall pain in June 2014. Upon physical examination, an irregularly mass (approximately 5× 1 cm) was noted in the right rib. An enhanced computed tomography (CT) showed an approximately 46× 13 mm soft-tissue mass between the inside of the fifth and sixth rib on the right side, and the mass was enhanced slightly with periosteal reaction in adjacent ribs (). He neither has FAP nor any family history. Given the patient,s strong desire, the surgery was performed on July 16, 2014. During the operation, we detected that the lesion was an approximately 5× 5.5 cm soft-tissue mass, grey and toughening. It did not involve the rib but the surrounding skeletal muscles through macroscopical observation. Examination of frozen issue section found tumor cells, and the pathologic analysis revealed spindle-cellular tumors. The surgery was successful with no further treatment. CT or MRI was rechecked regularly after operation. Gradually, the patient felt pain on the chest wall once more. MRI showed obvious thickened soft tissue lesion on the chest wall with low signal intensity on T1 weighted images, and high signal intensity on T2 in November 2014 (). CT-guided biopsy indicated local recurrence.18 F-FDG PET/CT images showed the SUVmax of soft tissue on the chest wall was 4.5 on January 29, 2015. Tomotherapy had been given at doses of 60 Gy (2Gy/fraction) in February 2015. However, a follow-up 18F-FDG PET/CT demonstrated intense18 F-FDG uptake in a spindle soft tissue mass with an SUVmax of 4.3 on April 28, 2015 (). For personalized therapy, genetic testing was performed in BGI on Jane 24, 2015. 508 cancer-related genes on tumor tissue DNA and peripheral blood DNA were detected through an Illumina Hiseq 2500 platform. The presence of a p.T41A mutations on the CTNNB1 gene was detected, which indicated his sensitivity to the celecoxib, the COX-2 inhibitor, based on targeted therapies and carboplatin-gemcitabine chemotherapy (). After a 5-month treatment with Celecoxib (200 mg/day), the CT scan showed tumor regression (). Until now, the patient showed remarkable regression without any signs or symptoms.
Figure 3. Tumor remarkable shrinkage was confirmed. PET-CT before the commencement of celecoxib on April 2014 A. CT scan on November 2015 B. and on October 2016 C. showed that tumor was smaller after using celecoxib.
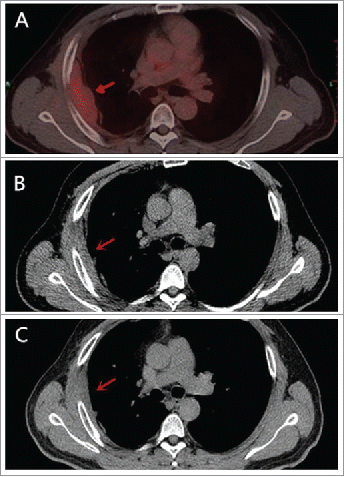
Table 1. Result of gene mutation. CTNNB1 gene codes β-catenin, a kind of adhesive connections protein. ARID1B gene could code protein, playing an important role in cell cycle activation. The protein coded by SMC3 gene could be the kernel protein or secretory protein in specific cells. MSH5 gene codes mutS protein family, participating in DNA mismatch repair and meiotic recombination.
Discussion
Sporadic AF is characterized by somatic mutations in the β-catenin genes (CTNNB1) or adenomatous polyposis coli (APC) gene. Several studies demonstrate that around 85% of sporadic desmoid tumors harbor mutations in exon 3 of CTNNB1 gene, and three kinds of CTNNB1 status, 41A (59%), 45F (33%) and 45P(8%), are found. Citation9 Studies indicate the presence of mutation 45F obviously increases the risk of recurrence in comparison with the other CTNNB1 mutations or AF without 45F mutation.Citation10 By contrast, FAP-associated AF is caused by germline APC mutations.Citation11–13 Both CTNNB1 and APC are the parts of the WNT signaling pathway, which promotes the degradation of β-catenin. In the Wingless/Wnt signaling pathway, a protein named Dishevelled (Dvl) binds to the cell-surface receptor after Wnt binding. A multiprotein complex consisting of APC, glycogen synthase kinase (GSK) 3β and Axin is formed and then combined with β-catenin, which is phosphorylated by GSK-3β and then degraded by the proteasome. However, if mutated, either with the occurrence of CTNNB1 or APC, the multiprotein complex could not be generated, leading to β-catenin protein stabilisation. As a result, β-catenin accumulates in the cytoplasm and subsequently translocates to the nucleus, where it binds to transcription factors of the Tcf-LEF family, through which transcription of target genes including c-JUN, c-MYC, Nr-CAM, MMP7, cyclin D1 and COX-2 is triggered.Citation9,11,14 Cyclooxygenase-2(COX-2) is an enzyme which could adjust the synthesis of prostaglandins and plays important roles in lots of biological processes such as immune function regulation. However, overexpression of COX-2 results in increasing the expression of platelet-derived growth factors (PDGF), which contributes to tumorigenesis by stimulating angiogenesis and invasiveness. In addition, COX-2 has also been found to promote apoptosis resistance e.g. by altering the relative levels of survivin and of pro- and anti-apoptotic proteins of the Bcl-2 family. COX-2 expression is elevated in several tumors, including AF. Celecoxib (Celebrex), an unique NSAIDs/coxibs, is able to potently induce anti-tumor responses by both COX-2 dependent and independent mechanisms. Celecoxib would interfere with prostaglandin(PG)-mediated up regulation of anti-apoptotic proteins by inhibiting COX-2. However, the pro-apoptotic effects do not critically rely on COX-2 inhibition.Citation15 Celecoxib could induce cell death independently from COX-2 mainly by activation of an intrinsic, mitochondria-dependent apoptosis pathway. And it is suggested that celecoxib is able to suppress survivin expression, inhibit the activity of sarcoplasmic/endoplasmic reticulum calcium ATPase (SERCA), block the activity of cyclin-dependent kinases (CDKs), downregulate the activity of 3-phosphoinositide-dependent protein kinase-1 (PDK1), a critical upstream regulator of protein kinase B (PKB/Akt) and reduce beta-catenin signaling in the absence of COX-2.Citation16, 17 In particular, the Wnt/β-catenin pathway is a classical pathway that has been suggested to be a COX-2-independent target for Celecoxib.Citation18,19 It has been reported that Celecoxib could induce dephosphorylation of substrates for the RTKs c-Met, leading to decreased downstream signaling. The decrease in c-Met activation is accompanied with an increase in (GSK)3β kinase activity, resulting in a rapid increase in phosphorylation of β-catenin. Hence, celecoxib makes a decrease of TCF-β-catenin–dependent transcription although the biochemical basis for these effects has not been elucidated clearly.Citation20–22
Considering the unpredictable natural history, the therapy remains enigmatic. The principle treatment has been considered for surgeries with or without radiation. Due to its biological behavior, therapeutic strategy has been shifted and watchful waiting may become an appropriate management.Citation23 Studies indicate that AF expresses nuclear estrogen receptor-β, which provides a biological basis for hormone therapy, but the response rate to anti-hormonal therapies is low.Citation24 Individualized therapy is required, the aims of which include reducing local recurrence rates and function loss. As genomic testing is widely available, the role of mutations has been studied, whereas agents that have CTNNB1 as a molecular target need to be further evaluatedCitation2. Targeted therapies including imatinib and Sorafenib (Tyrosine Kinase Inhibitors) have also been investigated. Prospective phase II clinical trial suggests that imatinib is active in AF,Citation25 and a randomised, double-blind, placebo-controlled phase III clinical trial of Sorafenib is ongoing.
In the present case, local recurrence occurs repeatedly despite operation and radiotherapy. Therefore, the genomic analysis is utilized to direct clinicians in selection treatment drugs. The patient showed remarkable regression after the application of celecoxib according to genetic testing. At the end of 20-monthfollow-up, the patient showed no tumor deterioration and remained asymptomatic.
A Pilot Study reported that 19 (95%) of the 20 patients with Extra-Abdominal Desmoid Tumors had a final status of SD or got better with the use of Meloxicam, a Cyclooxygenase-2 inhibitorCitation26. Yu-Chieh Wang et al reported that a patient who received celecoxib achieved complete remission.Citation27 Another case that was successfully treated with etodolac, another COX-2 inhibitor, was previously reported by Tanaka et al.Citation28 However, above cases used the COX-2 inhibitor without genetic testing.
In conclusion, as shown in our case report, an AF recurrence was successfully treated with celecoxib under the guidance of the genetic testing, which provides further evidence for NSAID. Multicenter randomized controlled trials are required in the future.
Conflict of interests
There is no conflict of interest.
References
- Shelekhova KV. [Changes in the WHO classification of soft tissue tumors]. Arkh Patol. 2015;77:48–54. doi:10.17116/patol201577148.
- Kasper B, Strobel P, Hohenberger P. Desmoid tumors: clinical features and treatment options for advanced disease. Oncologist. 2011;16:682–93. doi:10.1634/theoncologist.2010-0281.
- Nieuwenhuis MH, Casparie M, Mathus-Vliegen LM, Dekkers OM, Hogendoorn PC, Vasen HF. A nation-wide study comparing sporadic and familial adenomatous polyposis-related desmoid-type fibromatoses. Int J Cancer. 2011;129:256–61. doi:10.1002/ijc.25664.
- Seow-Choen F. The management of desmoids in patients with familial adenomatous polyposis (FAP). Acta Chir Iugosl. 2008;55:83–7. doi:10.2298/ACI0803083S.
- Merchant NB, Lewis JJ, Woodruff JM, Leung DH, Brennan MF. Extremity and trunk desmoid tumors: a multifactorial analysis of outcome. Cancer. 1999;86:2045–52. doi:10.1002/(SICI)1097-0142(19991115)86:10<2045::AID-CNCR23>3.0.CO;2-F.
- Levy AD, Rimola J, Mehrotra AK, Sobin LH. From the archives of the AFIP: benign fibrous tumors and tumorlike lesions of the mesentery: radiologic-pathologic correlation. Radiographics: a review publication of the Radiological Society of North America, Inc 2006;26:245–64. doi:10.1148/rg.261055151.
- Ballo MT, Zagars GK, Pollack A, Pisters PW, Pollack RA. Desmoid tumor: prognostic factors and outcome after surgery, radiation therapy, or combined surgery and radiation therapy. J clin oncol: official journal of the American Society of Clinical Oncology. 1999;17:158–67. doi:10.1200/JCO.1999.17.1.158.
- Nuyttens JJ, Rust PF, Thomas CR, Turrisi AT. Surgery versus radiation therapy for patients with aggressive fibromatosis or desmoid tumors – A comparative review of 22 articles. Cancer. 2000;88:1517–23. doi:10.1002/(SICI)1097-0142(20000401)88:7<1517::AID-CNCR3>3.0.CO;2-9.
- Eastley N, McCulloch T, Esler C, Hennig I, Fairbairn J, Gronchi A, Ashford R. Extra-abdominal desmoid fibromatosis: A review of management, current guidance and unanswered questions. Eur J Surg Oncol: the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2016;42:1071–83. doi:10.1016/j.ejso.2016.02.012.
- van Broekhoven DL, Verhoef C, Grunhagen DJ, van Gorp JM, den Bakker MA, Hinrichs JW, de Voijs CM, van Dalen T. Prognostic value of CTNNB1 gene mutation in primary sporadic aggressive fibromatosis. Ann Surg Oncol. 2015;22:1464–70. doi:10.1245/s10434-014-4156-x.
- Lips DJ, Barker N, Clevers H, Hennipman A. The role of APC and beta-catenin in the aetiology of aggressive fibromatosis (desmoid tumors). Ejso-Eur J Surg Onc. 2009;35:3–10. doi:10.1016/j.ejso.2008.07.003.
- Tejpar S, Nollet F, Li C, Wunder JS, Michils G, dal Cin P, Van Cutsem E, Bapat B, van Roy F, Cassiman JJ, et al. Predominance of beta-catenin mutations and beta-catenin dysregulation in sporadic aggressive fibromatosis (desmoid tumor). Oncogene. 1999;18:6615–20. doi:10.1038/sj.onc.1203041.
- Cheon SS, Cheah AY, Turley S, Nadesan P, Poon R, Clevers H, Alman BA. beta-Catenin stabilization dysregulates mesenchymal cell proliferation, motility, and invasiveness and causes aggressive fibromatosis and hyperplastic cutaneous wounds. Proc Nati Acad Sci U S A. 2002;99:6973–8. doi:10.1073/pnas.102657399.
- Signoroni S, Frattini M, Negri T, Pastore E, Tamborini E, Casieri P, Orsenigo M, Da Riva L, Radice P, Sala P, et al. Cyclooxygenase-2 and platelet-derived growth factor receptors as potential targets in treating aggressive fibromatosis. Clin Cancer Res: an official journal of the American Association for Cancer Research. 2007;13:5034–40. doi:10.1158/1078-0432.CCR-07-0336.
- Schonthal AH. Antitumor properties of dimethyl-celecoxib, a derivative of celecoxib that does not inhibit cyclooxygenase-2: implications for glioma therapy. Neurosurg Focus. 2006;20:E21. doi:10.3171/foc.2006.20.4.14.
- Jendrossek V. Targeting apoptosis pathways by Celecoxib in cancer. Cancer Lett. 2013;332:313–24. doi:10.1016/j.canlet.2011.01.012.
- Schonthal AH. Exploiting cyclooxygenase-(in)dependent properties of COX-2 inhibitors for malignant glioma therapy. Anti-cancer agents medi chem. 2010;10:450–61. doi:10.2174/1871520611009060450.
- Xia JJ, Pei LB, Zhuang JP, Ji Y, Xu GP, Zhang ZP, Li N, Yan JL. Celecoxib inhibits beta-catenin-dependent survival of the human osteosarcoma MG-63 cell line. J Int Med Res. 2010;38:1294–304. doi:10.1177/147323001003800411.
- Boon EM, Keller JJ, Wormhoudt TA, Giardiello FM, Offerhaus GJ, van der Neut R, Pals ST. Sulindac targets nuclear beta-catenin accumulation and Wnt signalling in adenomas of patients with familial adenomatous polyposis and in human colorectal cancer cell lines. Br J Cancer. 2004;90:224–9. doi:10.1038/sj.bjc.6601505.
- Poon R, Smits R, Li C, Jagmohan-Changur S, Kong M, Cheon S, Yu C, Fodde R, Alman BA. Cyclooxygenase-two (COX-2) modulates proliferation in aggressive fibromatosis (desmoid tumor). Oncogene. 2001;20:451–60. doi:10.1038/sj.onc.1204107.
- Emori M, Kaya M, Mitsuhashi T, Asanuma H, Yamashita T. Desmoid tumor-associated pain is dependent on mast cell expression of cyclooxygenase-2. Diagn Pathol. 2014;9:14. doi:10.1186/1746-1596-9-14.
- Tuynman JB, Vermeulen L, Boon EM, Kemper K, Zwinderman AH, Peppelenbosch MP, Richel DJ. Cyclooxygenase-2 inhibition inhibits c-Met kinase activity and Wnt activity in colon cancer. Cancer Res. 2008;68:1213–20. doi:10.1158/0008-5472.CAN-07-5172.
- Otero S, Moskovic EC, Strauss DC, Benson C, Miah AB, Thway K, Messiou C. Desmoid-type fibromatosis. Clin Radiol. 2015;70:1038–45. doi:10.1016/j.crad.2015.04.015.
- Deyrup AT, Tretiakova M, Montag AG. Estrogen receptor-beta expression in extraabdominal fibromatoses: an analysis of 40 cases. Cancer. 2006;106:208–13. doi:10.1002/cncr.21553.
- Penel N, Le Cesne A, Bui BN, Perol D, Brain EG, Ray-Coquard I, Guillemet C, Chevreau C, Cupissol D, Chabaud S, et al. Imatinib for progressive and recurrent aggressive fibromatosis (desmoid tumors): an FNCLCC/French Sarcoma Group phase II trial with a long-term follow-up. Ann Oncol: official journal of the European Society for Medical Oncology. 2011;22:452–7. doi:10.1093/annonc/mdq341.
- Nishida Y, Tsukushi S, Shido Y, Wasa J, Ishiguro N, Yamada Y. Successful treatment with meloxicam, a cyclooxygenase-2 inhibitor, of patients with extra-abdominal desmoid tumors: a pilot study. J Clin Oncol: official journal of the American Society of Clinical Oncology. 2010;28:e107−9. doi:10.1200/JCO.2009.25.5950.
- Wang YC, Wong JU. Complete remission of pancreatic head desmoid tumor treated by COX-2 inhibitor-a case report. World J Surg Oncol. 2016;14:190. doi:10.1186/s12957-016-0944-z.
- Tanaka K, Yoshikawa R, Yanagi H, Gega M, Fujiwara Y, Hashimoto-Tamaoki T, Hirota S, Tsujimura T, Tomita N. Regression of sporadic intra-abdominal desmoid tumour following administration of non-steroidal anti-inflammatory drug. World J Surg Oncol. 2008;6:17. doi:10.1186/1477-7819-6-17.