ABSTRACT
Vasohibin-1 is an intrinsic angiogenesis inhibitor, and is expressed in endothelial cells via induction by pro-angiogenesis factors. It is known to inhibit several processes of angiogenesis, with different mechanisms from extrinsic angiogenesis inhibitors. Vasohibin-2 is mainly expressed by mononuclear cells which have been mobilized from bone marrow. It not only promotes angiogenesis, but also modulates the releases of FGF-2 and VEGF, which are the two major inducers for vasohibin1. Hypoxic environment induces the expression of hypoxia-inducible Factor 1α with a result of VEGF release nearly in all tumor cell lines and tissues. However, it has been observed that hypoxia reduces the inducible effects of VEGF on vasohibin, which indicates that a complicated mechanism exists in the angiogenesis. Vasohibin and its family members play important roles in both the physiological and pathological procedures, in contrary but complementary patterns. Furthermore, human aortic smooth muscle cells and fibroblast have also been detected to express vasohibin on a moderate to weak scale range. Recently, the results of an increasing number of studies in vivo have shown that vasohibin can also be detected in several cancers, and is associated with micro-vessel densities, histology grades, invasions, poor clinical features, metastasis, and dissemination in abdominal cavities, as well as EMT. In more recent reports, it has been confirmed that, along with being angiogenesis regulators, a variety of other roles have been associated with this family. The focus of this study was the upstream regulatory mechanisms of vasohibin expressions, and their role in regard to the downstream target proteins of vasohibin, especially in carcinoma. Vasohibin is considered to be an original angiogenesis inhibitor, and has a much broader significance in pathological processes. It can be taken as an independent prognostic factor, as well as a potential strategy for cancer therapy programs.
Introduction
Angiogenesis is modulated by a balance between two groups: the pro-angiogenic factors and the angiogenesis inhibitors. The former group promotes angiogenesis or regulates vascular stability, and includes VEGF family members, angiopoietin family members, fibroblast growth factor (FGF) family members, and so on. The latter group has already been reported in previous studies, including angiostatin, endostatin, thrombospondins, angiopoietin-1, and transforming growth factor-β. The majority of the angiogenesis inhibitors are extrinsic to the vasculature. However, endothelial cells (ECs) have been found to produce intrinsic angiogenesis inhibitors via induction by vascular endothelial growth factors (VEGF), and are referred to as Vasohibin-1 (VASH1). VASH1 has been found to inhibit the migration and proliferation of ECs in cultures, and exhibits a feedback anti-angiogenic activity in vivo.Citation1,2 More recently, VASH1 has been found to be down-regulated in the endothelial cells which are known to be associated with aging.Citation3
Splicing products of the vasohibins
The human VASH1 gene is located on chromosome 14q24.3, and its 44 kDa protein is post-translationally processed into several truncated forms.Citation2 Also, the primary splicing product of VASH1 has been referred to as VASH1A, and contains eight exons composed of 365 amino acids. Meanwhile, an alternative splicing product which lacks exons 6–8 was named VASH1B,Citation4,5 and is composed of 204 amino acids. It is mainly presented in the human umbilical vein endothelial cell (HUVEC) (). Both of these isoforms, VASH1A and VASH1B, share the same 176 amino acids harboring a putative nuclear localization sequence (NLS) in their N terminus. However, they completely differ within the C-terminal protein region. Both the VASH1A and VASH1B have at least two proteolytic cleavage sites in each terminus. The biochemical and functional analyses of VASH1 have revealed that some basic residue at the C-terminus are important for heparin binding and anti-angiogenic activities. The structural analyses have revealed that the VASH1 protein neither contains a classical signal sequence typical of secreted proteins,Citation6 nor colocalizes with the endoplasmic reticulum (ER) marker calnexin. These findings imply that an unconventional protein secretion (UPS) pathway exists in the vasohibin secretion. In recent studies, the SVBP (small vasohibin-binding protein) was observed to bind with vasohibin within cells, and facilitated the secretion of the VASH.Citation7 A more recent study identified that the VASH191-180 and VASH280-169 regions were essential domain architectures (VASH-PS) of the VASH, and regulated the cytosolic punctate structure formation in the absence of SVBP. This study further reported that the SVBP formed a complex with VASH1 through VASH1274-282 (SIa), VASH1139-144 (SIb), and VASH1133-137 (SIc), which led to the dispersion in the cytosol, and the extracellular release of the VASH1. The amino acid sequences of VASH-SIa and VASH-PS, which contain SIb and SIc, are highly conserved among the VASH family members in vertebrates.Citation8 These findings suggested that SVBP-dependent UPS may be common within the VASH family.
Figure 1. The VASH1 gene is located on chromosome14q24.3 and contains eight exons with 5589 bp. The primary product of VASH1 was named VASH1A, and contains 365 amino and 44 kDa. However, only 42 kDa have been detected and exhibit antiangionenic activities. VASH1B contains five exons lacking exon 6–8, with 1459 bp and 204 aa. The VASH2 gene is located on 1q32.3, and contains 1–11 exons. The major transcript contains exons 1/2/4/5/6/8/9/11, with 3589 bp and 355 aa
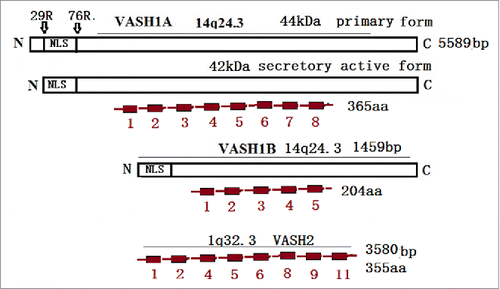
Another gene homologous to VASH1 was also found through a DNA sequence search of genomic databases, and was named Vasohibin-2 (VASH2).Citation9 The gene for human VASH2 is located on chromosome 1q32.3,10 and its protein is composed of 355 amino acid residues (). The overall homology between human VASH1 and VASH2 is 52.5% at the amino acid level. Moreover, any known functional motifs of VASH1 have also been found in the primary structure of VASH2. It has been determined that VASH1 and VASH2 are highly conserved among species.Citation11 VASH2 is mainly expressed in the mononuclear cells mobilized from bone marrow in order to stimulate angiogenesis.Citation12
Recently, VASH proteins were confirmed to be members of the transglutaminase-like cysteine protease super-family, which all possess a non-canonical Cys-His-Ser catalytic triad, and are most likely activated in a calcium-dependent activation mechanism.Citation13
Vasohibins expressions and their regulation
Primitively, VASH1 is predominantly expressed in ECs in vitro, and its mRNA expression is induced by stimulations with angiogenic factors, such as the VEGF/VEGFR2 pathway, and FGF-2 via PKC-δ pathway activationCitation4. The VEGF-induced expressions of vasohibin in ECs can be reduced by some inflammatory cytokines, such as TNFα, interleukin (IL)-1β, and interferon (IFN)γ.Citation1,4 Although hypoxic conditions induce VEGF release via HIF-1 activation. it has been observed to inhibit the VEGF-stimulated vasohibin mRNA expressions, along with protein synthesis in ECs. However, it has not been found to affect the basal expressions of vasohibinCitation4 (), which suggests that a much more complicated mechanism exist under than hypoxic conditions when compared with normoxic conditions. The results of a study regarding the relationship between the HIF-1α and VASH1 in human umbilical vein endothelial cells (HUVECs) showed that H2O2-treatments impaired VEGF induced vasohibin expressions, as well as HUVECs growth. Also, the results of another study confirmed that vasohibin elevated prolyl hydroxylase (PHD), which regulates the prolyl hydroxylation of HIF-1α, and resulted in ubiquitin-mediated proteasomal degradationCitation14 (). The results of this study potentially provide evidence for a triangular relationship among vasohibin, HIF-1, and VEGF in the modulation of angiogenesis. VASH1 protein has been found to be extensively presented in the ECs of developing humans and mice, as well as chicken embryos. However, it has been found to fade in post-neonates,Citation9,15 implies that it has a vital role in the development of vessels. The accumulated information indicates that the range of VASH1 expressions is more extensive than the original concept had presented. For example, human aortic smooth muscle cells have shown weak expressions of vasohibin, and have been induced to modest expressions by platelet derived growth factors. Also, fibroblast cells have been observed to express very low levels of vasohibin. However, these were found to be unresponsive to FGF-2 stimulation. Furthermore, weak expressions of vasohibin mRNA have been detected in brain, heart, and even kidney cells, as well as in the keratinocytes of adult humans.Citation1 Also, VASH1 protein was detected in propagated monocyte-derived macrophages, and cultivated cardiac myocytes in vitro,Citation5 as well as in freshly prepared periphery blood mononuclear cells samples, and the striated muscles of adult rats.Citation16 These findings suggested the existence of a relatively extensive expression profile for the VASH1 protein.
Figure 2. The VASH1 is expressed in the termination zone of vessels in order to stop angiogenesis. TNFα, TL-1β, and IFNγ stop the role of VEGF induction. The VASH2 is mainly presented at the sprout zone of vessels in order to promote angiogenesis. Under hypoxic conditions, the VEGF is up-regulated by HIF-1α. Also, VASH1 increases the PHD levels, which promotes the degradation of HIF-1α
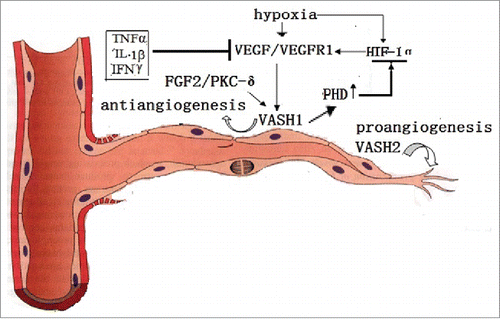
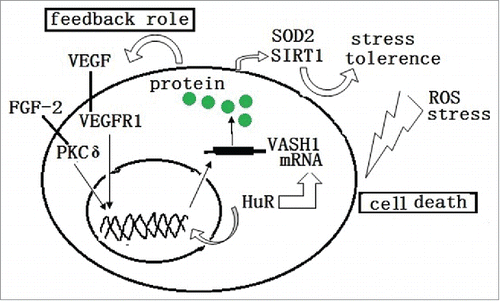
Figure 3. ROS and stress induces the death or damage of endothelial cells. The endogenous VASH1 improves the stress tolerance of endothelial cells to ROS and stress by up-regulating the expression of SOD2 and SIRT1. Also, HuR modulates both the transcription and post transcription of the VASH1
The endogenous expressions of VASH2 in cultured ECs have been observed to be very low, and independent of VEGF induction. However, they have been found to be highly presented in bone marrow-derived mononuclear cells.Citation16 Similar to VASH1, VASH2 proteins have been comparably detected in the ECs of the developing organs of embryos,Citation9 and were observed to be diffusely expressed in the ECs of embryonic organs during mid-gestation. After that time point, they were determined to become faint. However, they persisted to a certain extent from the late-gestation to neonate stages,Citation9,12 which is consistent with the increased demand of angiogenesis before the neonate stage, and the decreased demands after birth.
Role of vasohibins in pathological processes
Purified VASH1 proteins have been found to inhibit the migration and network formation of HUVECs in vitro,Citation12,18 and also exhibit anti-lymphangiogenic activities.Citation19 Moreover, endogenous VASH1A has been found to display anti-angiogenesis effects. However, it has been observed that recombinant VASH1A proteins did not inhibit cell proliferation, tube formation, or vessel growth in chick chorioallantoic membrane (CAM) assay in vivo, or significantly decrease the migration of human endothelial cells in scratch assays in vitro. In contrast, VASH1B proteins have been determined to inhibit cell growth of both endothelial colony forming cells from peripheral blood, and HUVECs and cell migration in scratch assays, as well as tube formations in matrigel assays, and blood vessel formation in CAM assays. Furthermore, the adenoviral overexpression of VASH1B, but not VASH1A, has resulted in inhibitions of endothelial cell growth, migration, and capillary formation. These findings imply that there is a probability that VASH1B may be an active form of VASH1A after the splicing of exons 6–8. Interestingly, the overexpression of VASH1A and VASH1B was found to induce apoptosis in proliferating human fibroblasts. However, they did not affect the cell growth of keratinocytes.Citation5
Human VASH2 takes 52.5% overall homology to VASH1 at the amino acid level, and contains all the known functional motifs of VASH1 in the primary structure. VASH2 regulates angiogenesis in a contradictory way from VASH1. VASH1 is mainly expressed in ECs in the termination zone which halts angiogenesis, and it has been observed that VASH1 (-/-) mice contained numerous immature micro vessels in the area where the angiogenesis should have been terminated.Citation12 VASH2 is mainly expressed in the front of the sprouting zone and stimulates angiogenesis.Citation12 It has also been observed that the fetal vascular areas were significantly decreased in VASH2 (-/-) mice, which indicated that both VASH1 and VASH2 had played roles in a contradictory but a complimentary manner. Previous studies in placenta found that VASH1 was expressed in ECs. Whereas, the VASH2 was selectively localized in the trophoblasts of the placenta,Citation19 which demonstrated the different roles of both vasohibins. These results correlated well with the anti-angiogenic function of VASH1 as an autocrine factor, and the pro-angiogenic function of VASH2 as a paracrine factor.Citation20 However, there some other studies have shown that exogenous VASH2 exhibited anti-angiogenic activities in the corneas of mice,Citation18 and also inhibited network formations by HUVECs with a potency equivalent to VASH1.9
It has been determined that angiogenesis inhibitors generally induce EC death and vascular regression. However, it has been found that VASH1 not only inhibits angiogenesis, but also enhances the maintenance of ECs by strengthening the resistance against stresses via up-regulating the expression of superoxide Dismutase 2, which is an enzyme known to quench reactive oxygen species (ROS), and the synthesis of Sirtuin 1 (SIRT1), which is an anti-aging protein. These functions improve stress tolerances,Citation21 which implies that a differential mechanism of angiogenesis inhibition exists from the others ().
Vasohibins associated with carcinomas
The angiogenesis modulation of VASH is involved in several pathological processes, such as atherosclerosis,Citation22 age-dependent macular degeneration (AMD),Citation23 and diabetic retinopathy,Citation24 as well as several forms of cancer.Citation18,25–27 The dysregulation of angiogenesis is one of the hallmarks of cancer. Tumor vessels are histopathologically different from normal vessels, and are characterized by irregular diameters, abnormal branches, loosely interconnected endothelial cells, abnormal pericytes, and structural abnormalities in the basement membranes. Angiogenesis is a fundamental step in the transition of tumors from benign to malignant, and further supports its growth, invasion, and metastasis. The results of a recent study regarding an animal model for spontaneous adenomatous polyposis and subsequent adenocarcinoma, which is a process termed as the adenoma-carcinoma sequence,Citation28 showed that the morphological changes in the blood vessels occurred earlier than the malignant changes in the epithelium of the intestinal lesions. These findings suggested that angiogenesis patterns may play a critical role in the development and growth of benign tumors during multi-step carcinogenesis.Citation29
As a critical angiogenesis regulator, VASH1 has also been involved in tumor angiogenesis inhibition, and the prevention of tumor growth and metastasis in animal tumor models with lung carcinomaCitation18,19 and hepatocellular carcinoma.Citation30 Furthermore, a negative correlation of stromal VASH1 levels with tumor sizes, advanced clinical stages, and distant metastases, has been confirmed in colon cancer patients.Citation31 Moreover, it is known that cancer cell-derived VASH1 can directly inhibit cell growth, adhesion, and migration in vitro. It can also control tumorigenesis and metastasis in vivo,Citation31 which corresponds with EZH2, a member of the polycomb group (PcG) proteins, and the silenced VASH1 expression in ovarian cancers has been determined to worsen the prognosis.Citation33 In addition, the VASH1-A expressions in tumor cells were found to significantly induce cell senescence. Also, the VASH1-B expressions dramatically induced apoptosis in colon cancer cells,Citation31 which was consistent with the inhibition of DNA synthesis, and induced apoptosis of endothelial cells by VASH1-B.Citation5 The results of the aforementioned studies strongly indicated that VASH1 plays a complicated inhibitory role in malignant tumor behavior.
However, there have been some contradictory reports, such as the VASH1 positive ratio being a measure of the neovascularization in breast tissue, and a strong predictor of accentuated aggressive behaviors and microvascular invasions,Citation34 as well as influencing the response to adjuvant chemotherapy, such as tamoxifen.Citation27 The expressions of VASH1 in hepatocellular carcinomas were found to exhibit increases after curative surgery, and it has been suggested that they are closely related to micro vessel densities and poor clinical outcomes. These findings have implied that up-regulated VASH1 levels may be an independent prognostic indicator of ‘shorter disease-free survival’, and ‘overall survival’.Citation35 In practical applications, the VASH1 expressions can be taken as a novel prognostic marker in several cancers, including breast cancer,Citation26 renal cell carcinomaCitation36 lung cancer,Citation37 upper urinary tract urothelial carcinoma,Citation38 and hepatocellular carcinomaCitation35,39 according to the retrospective analyses of the relationships between the clinicopathological features and the VASH1 expressions of cancer patients.
VASH2 is mainly expressed in the sprouting zone front of ECs in order to stimulate angiogenesis,Citation12 and is also expressed in some cancers with a major role of promoting angiogenesis, malignant transformations, and metastasis. For example, VASH2 expressions in certain ovarian cancers were found to promote tumor growth and peritoneal dissemination by stimulating tumor angiogenesis.Citation17 Furthermore, in hepatocellular carcinoma cells (HCCs) and tissues, these expressions were determined to accelerate HCC angiogenesis and malignant transformationsCitation37. Finally, in pancreatic ductal adenocarcinoma, these expressions enhanced tumor progression, and were found to be associated with poor clinical outcomes.Citation41 The knockdown of VASH2 significantly attenuated tumor growth, as well as the peritoneal dissemination of ovarian cancers,Citation17 and suppressed tumor growth by inhibiting angiogenesis in endometrial cancer cells.Citation42 VASH2 was also expressed by the late stage adenoma of colon and spontaneous adenocarcinoma cells around tumor vessels in ApcMin/+ mice. Furthermore, the inhibition of VASH2 normalized abnormal tumor vessels in adenocarcinoma.Citation29 It was also revealed in a recent study that Vasohibin 2 promotes human luminal breast cancer angiogenesis via transcriptional activation of fibroblast growth factor 2.43 The up-regulation of VASH2 by miR-200 was determined to promote EMT in hepatocellular carcinomaCitation44 and human breast cancer, via the activation of transforming growth factor β 1, and hypoxia dependent repression of GATA-binding factor 346 (). The results of all the above mentioned studies suggested that the inhibition of the VASH2 expressions could potentially become a strategy for future cancer therapy.
Figure 4. The VASH2 is released by both human aortic smooth muscle cells, and placenta trophoblasts, via PDGF. In a vice-versa manner, it decreases the release of VEGF and FGF2. The VASH2 is silenced by EZH2, and is trans-activated by Mic-200b, in order to promote EMT via vimentin up-regulation and E-cadherin down-regulation. This results in decreased apoptosis via the down-regulation of the wild-type of p53, Bax, and CCP-3; promotes invasions via the up-regulation of MMP2, CXRC4, and TGFβ1; and regresses the GATA-binding factor.
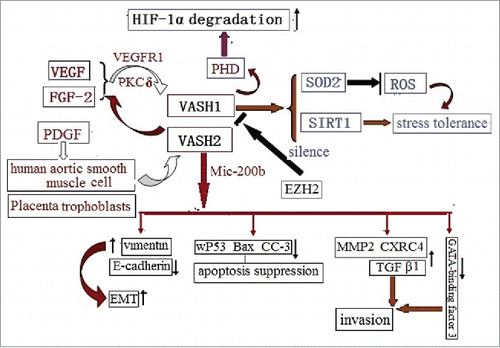
Although VASH1 is induced by a pro-angiogenesis factor, VASH2 has been found to enhance the expressions of FGF-2 and VEGF by nuclear factor-κB up-regulation in hepatocellular carcinoma (HCC) cells via an autocrine and paracrine modeCitation40 and breast cancer.Citation45 These findings imply a potential correlation between VASH1 and VASH2. Furthermore, VASH2 has displayed a potential role in apoptosis. For example, the up-regulation of VASH2 in hepatocellular carcinoma distinctly decreased the wild type p53 protein levels, and suppressed the expressions of the pro-apoptotic protein Bax and cleaved caspase-3 (CC-3).Citation40 VASH2 also appears to have a role in epithelial, in regard to the mesenchymal transition in ovarian serous adenocarcinoma cells. Furthermore, VASH2 expressions have been inversely correlated with the expression of miR-200bCitation46, which is known to repress the expression of ZEB1 and ZEB2, which are the key products for epithelial to mesenchymal transitions. It is also believed that the overexpression of VASH2 may promote EMT via increasing the levels of vimentin, MMP2, and CXRC444 (), activating ZEB1/2 (core transcriptional factor), and decreasing E-cadherin levels. In brief, decreasing VASH2 levels may promote apoptosis, as well as suppress EMT.
Although normal epithelial cells do not express vasohibin, an increasing number of cancer cells have been found to express vasohibin. The possible mechanism may be mediated by the methylation of its promoter region, or decreasing mir-200b, such as VASH2.Citation17,47 Recently, a neutralizing monoclonal antibody (mAb clone 1760) was established against human VASH2, which was found to almost completely inhibit the stimulatory effects of hVASH2 on the migration and tube formation by ECs. Also, the peritoneal injection of the clone 1760 antibody was determined to inhibit both the tumor growth and angiogenesis of a mouse xenograft model of human cancer cells, which suggested that it has a potential for future applications in anti-cancer treatments.Citation48 Moreover, VASH1 mutations have been observed in colon, rectal, and lung cancer tissues.Citation49
Conclusions
It was concluded in this study that, although the results of a large number of studies have shown that VASH proteins may be potential candidates for anti-angiogenic therapies, some questions have been revealed in regard to the clarity of these potentials, such as the unclear role of VASH receptors and downstream signaling. Therefore, the entire role of the VASH proteins requires further investigation in future analyses. The future observations, especially in regard to forms of cancer, could potentially indicate that vasohibins may have functions which go beyond their role in angiogenesis.
Acknowledgments
The authors would like to thank all those who contributed to this review, as well as the support shown by the Inner Mongolia Natural Science Foundation of China (2013MS1142), and the Ministry of Education Chunhui Plan (Z2012008).
Disclosure statement
The authors confirm no conflicts of interest.
References
- Kerbel RS. Vasohibin: the feedback on a new inhibitor of angiogenesis. J Clin Invest. 2004;114: 884–886. doi:10.1172/JCI23153.
- Sonoda H, Ohta H, Watanabe K, Yamashita H, Kimura H, Sato Y. Multiple processing forms and their biological activities of novel angiogenesis inhibitor vasohibin. Biochem Biophys Res Commun. 2006; 7;342(2):640–6. doi:10.1016/j.bbrc.2006.01.185.
- Takeda E, Suzuki Y, Sato Y. Age-associated down regulation of vasohibin-1 in vascular endothelial cells. Aging Cell. 2016;15(5):885–92. doi:10.1111/acel.12497.
- Shimizu K, Watanabe K, Yamashita H, Abe M, Yoshimatsu H, Ohta H, Sonoda H, Sato Y. Gene regulation of a novel angiogenesis inhibitor, vasohibin, in endothelial cells. Biochem Biophys Res Commun. 2005;327(3):700–6. doi:10.1016/j.bbrc.2004.12.073.
- Kern J, Bauer M, Rychli K, Wojta J, Ritsch A, Gastl G, Gunsilius E, Untergasser G. Alternative splicing of vasohibin-1 generates an inhibitor of endothelial cell proliferation, migration, and capillary tube formation. Arterioscler Thromb Vasc Biol. 2008;28(3):478–84. doi:10.1161/ATVBAHA.107.160432.
- Hegde RS and Kang SW. The concept of translocational regulation. J Cell Biol. 2008;182:225–232. doi:10.1083/jcb.200804157.
- Suzuki Y, Kobayashi M, Miyashita H, Ohta H, Sonoda H, Sato Y. Isolation of a small vasohibin-binding protein (SVBP) and its role in vasohibin secretion. J Cell Sci. 2010;123:3094–4101. doi:10.1242/jcs.067538.
- Kadonosono T, Yimchuen W, Tsubaki T, Shiozawa T, Suzuki Y, Kuchimaru T, Sato Y, Kizaka -Kondoh S. Domain architecture of vasohibins required for their chaperone-dependent unconventional extracellular release. Protein Sci. 2017;26(3):452–463. doi:10.1002/pro.3089.
- Shibuya T, Watanabe K, Yamashita H, Shimizu K, Miyashita H, Abe M, Moriya T, Ohta H, Sonoda H, Shimosegawa T, et al. Isolation of vasohibin-2 as a sole homologue of VEGF inducible endothelium-derived angiogenesis inhibitor vasohibin: a comparative study on their expressions. Arterioscler Thrombo Vasc Biol. 2006;26:1051–1057. doi:10.1161/01.ATV.0000216747.66660.26.
- Sato Y and Sonoda H. The vasohibin family: a negative regulatory system of angiogenesis genetically programmed in endothelial cells. Arterioscler Thrombo Vasc Biol. 2007;27: 37–41. doi:10.1161/01.ATV.0000252062.48280.61.
- Sato Y. The vasohibin family: A novel family for angiogenesis regulation. J Biochem. 2013;153:5–11. doi:10.1093/jb/mvs128.
- Kimura H, Miyashita H, Suzuki Y, Kobayashi M, Watanabe K, Sonoda H, Ohta H, Fujiwara T, Shimosegawa T, Sato Y. Distinctive localization and opposed roles of vasohibin-1 and vasohibin-2 in the regulation of angiogenesis. Blood. 2009;113: 4810–4818.doi:10.1182/blood-2008-07-170316.
- Sanchez-Pulido L, Ponting CP. Vasohibins: new transglutaminase-like cysteine proteases possessing a non-canonical Cys-His-Ser catalytic triad. Bioinformatics. 2016;32(10):1441–5. doi:10.1093/bioinformatics/btv761.
- Kozako T, Matsumoto N, Kuramoto Y, Sakata A, Motonagare R, Aikawa A, Imoto M, Toda A, Honda S, Shimeno H, et al. Vasohibin induces prolyl hydroxylase-mediated degradation of hypoxia-inducible factor-1α in human umbilical vein endothelial cells. FEBS Lett. 2012;586(7):1067–72.doi:10.1016/j.febslet.2012.03.007.
- Nimmagadda S, Geetha-Loganathan P, Pröls F, Scaal M, Christ B, Huang R.. Expression pattern of vasohibin during chick development. Dev Dyn. 2007;236:1358–1362. doi:10.1002/dvdy.21134.
- Kishlyansky M, Vojnovic J, Roudier E, Gineste C, Decary S, Forn P, Bergeron R, Desplanches D, Birot O. Striated muscle angio-adaptation requires changes in Vasohibin-1 expression pattern. Biochem Biophys Res Commun. 2010;399:359–364. doi:10.1016/j.bbrc.2010.07.076.
- Takahashi Y, Koyanagi T, Suzuki Y, Saga Y, Kanomata N, Moriya T, Suzuki M, Sato Y. Vasohibin-2 expressed in human serous ovarian adenocarcinoma accelerates tumor growth by promoting angiogenesis. Mol Cancer Res. 2012;10:1135–1146. doi:10.1158/1541-7786.MCR-12-0098-T.
- Hosaka T, Kimura H, Heishi T, Suzuki Y, Miyashita H, Ohta H, Sonoda H, Moriya T, Suzuki S, Kondo T, et al. Vasohibin-1 expressed in endothelium of tumor vessels regulates angiogenesis. Am J Pathol. 2009;175: 430–439. doi:10.2353/ajpath.2009.080788.
- Heishi T, Hosaka T, Suzuki Y, Miyashita H, Oike Y, Takahashi T, Nakamura T, Arioka S, Mitsuda Y, Takakura T, et al. Endogenous angiogenesis inhibitor vasohibin1 exhibits broad-spectrum antilymphangiogenic activity and suppresses lymph node metastasis. Am J Pathol. 2010;176:1950–1958. doi:10.2353/ajpath.2010.090829.
- Suenaga K, Kitahara S, Suzuki Y, Kobayashi M, Horie S, Sugawara J, Yaegashi N, Sato Y. Role of the vasohibin family in the regulation of fetoplacental vascularization and syncytiotrophoblast formation. Plos One. 2014;9: 1–10. doi:10.1371/journal.pone.0104728.
- Miyashita H, Watanabe T, Hayashi H, Suzuki Y, Nakamura T, Ito S, Ono M, Hoshikawa Y, Okada Y, Kondo T, et al. Angiogenesis inhibitor vasohibin-1 enhances stress resistance of endothelial cells via induction of SOD2 and SIRT1. Plos One. 2012;7(10):e46459. doi:10.1371/journal.pone.0046459.
- Yamashita H, Abe M, Watanabe K, Shimizu K, Moriya T, Sato A, Satomi S, Ohta H, Sonoda H, Sato Y. Vasohibin prevents arterial neointimal formation through angiogenesis inhibition. Biochem Biophys Res Commun. 2006;345:919–925. doi:10.1016/j.bbrc.2006.04.176.
- Wakusawa R, Abe T, Sato H, Yoshida M, Kunikata H, Sato Y, Nishida K. Expression of vasohibin, an antiangiogenic factor, in human choroidal neovascular membranes. Am J Ophthalmol. 2008;146: 235–243. doi:10.1016/j.ajo.2008.03.019.
- Sato H, Abe T, Wakusawa R, Asai N, Kunikata H, Ohta H, Sonoda H, Sato Y, Nishida K. Vitreous levels of vasohibin-1 and vascular endothelial growth factor in patients with proliferative diabetic retinopathy. Diabetologia. 2009;52:359–361. doi:10.1007/s00125-008-1229-z.
- Yoshinaga K, Ito K, Moriya T, Nagase S, Takano T, Niikura H, Yaegashi N, Sato Y.. Expression of vasohibin as a novel endothelium-derived angiogenesis inhibitor in endometrial cancer. Cancer Sci. 2008;99:914–919. doi:10.1111/j.1349-7006.2008.00777.x.
- Tamaki K, Moriya T, Sato Y, Ishida T, Maruo Y, Yoshinaga K, Ohuchi N, Sasano H. Vasohibin-1 in human breast carcinoma: a potential negative feedback regulator of angiogenesis. Cancer Sci. 2008;100: 88–94. doi:10.1111/j.1349-7006.2008.01015.x.
- Tamaki K, Sasano H, Maruo Y, Takahashi Y, Miyashita M, Moriya T, Sato Y, Hirakawa H, Tamaki N, Watanabe M, et al. Vasohibin-1 as a potential predictor of aggressive behavior of ductal carcinoma in situ of the breast. Cancer Sci. 2010;101:1051–1058. doi:10.1111/j.1349-7006.2009.01483.x.
- Tanaka T, Kohno H, Suzuki R, Hata K, Sugie S, Niho N, Sakano K, Takahashi M, Wakabayashi K. Dextran sodium sulfate strongly promotes colorectal carcinogenesis in Apc (Min/+) mice: inflammatory stimuli by dextran sodium sulfate results in development of multiple colonic neoplasms. Int J Cancer. 2006;118:25–34. doi:10.1002/ijc.21282.
- Kitahara S, Suzuki Y, Morishima M, Yoshii A, Kikuta S, Shimizu K, Morikawa S, Sato Y, Ezaki T. Vasohibin-2 modulates tumor onset in the gastrointestinal tract by normalizing tumor angiogenesis. Mol Cancer. 2014;13(99):1–15. doi:10.1186/1476-4598-13-99.
- Li D, Zhou K, Wang S, Shi Z, Yang Z. Recombinant adenovirus encoding vasohibin prevents tumor angiogenesis and inhibits tumor growth. Cancer Sci. 2010;101(2):448–452. doi:10.1111/j.1349-7006.2009.01388.x.
- Liu S, Han B, Zhang Q, Dou J, Wang F, Lin W, Sun Y, Peng G. Vasohibin-1 suppresses colon cancer. See comment in PubMed Commons belowOncotarget. 2015;6(10):7880–98. doi: 10.18632/oncotarget.3493.
- Lu C, Han HD, Mangala LS, Ali-Fehmi R, Newton CS, Ozbun L, Armaiz-Pena GN, Hu W, Stone RL, Munkarah A, et al. Regulation of tumor angiogenesis by EZH2. Cancer Cell. 2010;18:185–197. doi:10.1016/j.ccr.2010.06.016.
- Chan MS, Wang L, Chanplakorn N, Tamaki K, Ueno T, Toi M, Loo WT, Chow LW, Suzuki T, Sasano H.. Effects of estrogen depletion on angiogenesis in estrogen-receptor-positive breast carcinoma–an immunohistochemical study of vasohibin-1 and CD31 with correlation to pathobiological response of the patients in neoadjuvant aromatase inhibitor therapy. Expert Opinion Therapeutic Targets. 2012;16:69–78. doi:10.1517/14728222.2011.628938.
- Wang Q, Tian X, Zhang C, Wang Q. Upregulation of vasohibin-1 expression with angiogenesis and poor prognosis of hepatocellular carcinoma after curative surgery. Med oncol. 2012;29(4):2727–2736. doi:10.1007/s12032-011-0106-7.
- Kanomata N, Sato Y, Miyaji Y, Nagai A, Moriya T. Vasohibin-1 is a new predictor of disease-free survival in operated patients with renal cell carcinoma. J clin pathol. 2013;66(7): 613–619. doi:10.1136/jclinpath-2013-201444.
- Yazdani S, Miki Y, Tamaki K, Ono K, Iwabuchi E, Abe K, Suzuki T, Sato Y, Kondo T, Sasano H. Proliferation and maturation of intratumoral blood vessels in non-small cell lung cancer. Hum pathol. 2013;44(8):1586–1596. doi:10.1016/j.humpath.2013.01.004.
- Miyazaki Y, Kosaka T, Mikami S, Kikuchi E, Tanaka N, Maeda T, Ishida M, Miyajima A, Nakagawa K, Okada Y, et al. The prognostic significance of vasohibin-1 expression in patients with upper urinary tract urothelial carcinoma. Clin Cancer Res. 2012;18(15): 4145–4153. doi:10.1158/1078-0432.CCR-12-0073.
- Murakami K, Kasajima A, Kawagishi N, Sekiguchi S, Fujishima F, Watanabe M, Sato Y, Ohuchi N, Sasano H. The prognostic significance of vasohibin 1-associated angiogenesis in patients with hepatocellular carcinoma. Hum Pathol. 2014;45(3): 589–597. doi:10.1016/j.humpath.2013.10.028.
- Xue X, Gao W, Sun B, Xu Y, Han B, Wang F, Zhang Y, Sun J, Wei J, Lu Z, et al. Vasohibin 2 is transcriptionally activated and promotes angiogenesis in hepatocellular carcinoma. Oncogene. 2013;32:1724–1734. doi:10.1038/onc.2012.177.
- Kim JC, Kim KT, Park JT, Kim HJ, Sato Y, Kim HS. Expression of vasohibin-2 in pancreatic ductal adenocarcinoma promotes tumor progression and is associated with a poor clinical outcome. Hepatogastroenterology. 2015;62:251–256. doi:10.1038/onc.2012.177. PMID:25916042.
- Koyanagi T, Saga Y, Takahashi Y, Suzuki Y, Suzuki M, Sato Y. Downregulation of vasohibin-2, a novel angiogenesis regulator, suppresses tumor growth by inhibiting angiogenesis in endometrial cancer cells. Oncol Lett. 2013;5:1058–1062. doi:10.3892/ol.2013.1119.
- Tu M, Lu C, Lv N, Wei J, Lu Z, Xi C, Chen J, Guo F, Jiang K, Li Q, et al.Vasohibin 2 promotes human luminal breast cancer angiogenesis in a non-paracrine manner via transcriptional activation of fibroblast growth factor 2.Cancer Lett. 2016;383(2):272–281. doi:10.1016/j.canlet.2016.09.031.
- Xue X, Zhang Y, Zhi Q, Tu M, Xu Y, Sun J, Wei J, Lu Z, Miao Y, Gao W. MiR200-upregulated Vasohibin 2 promotes the malignant transformation of tumors by inducing epithelial-mesenchymal transition in hepatocellular carcinoma. Cell Commun Signal. 2014;12(62) :1–10. doi:10.1186/s12964-014-0062-x.
- Tu M, Li Z, Liu X, Lv N, Xi C, Lu Z, Wei J, Song G, Chen J, Guo F, et al. Vasohibin 2 promotes epithelial-mesenchymal transition in human breast cancer via activation of transforming growth factor β 1 and hypoxia dependent repression of GATA-binding factor 3. Cancer Lett. 2017; 1;388:187–197. doi:10.1016/j.canlet.2016.11.016.
- Tu M, Liu X, Han B, Ge Q, Li Z, Lu Z, Wei J, Song G, Cai B, Lv N, et al. Vasohibin-2 promotes proliferation in human breast cancer cells via upregulation of fibroblast growth factor-2 and growth/differentiation factor-15 expression. Mol Med Rep. 2014;10:663–669. doi:10.3892/mmr.2014.2317.
- Korpal M and Kang Y. The emerging role of miR-200 family of microRNAs in epithelial-mesenchymal transition and cancer metastasis. RNA Biol. 2008;5:115–119. doi:10.4161/rna.5.3.6558. PMID:19182522.
- Koyanagi T, Suzuki Y, Komori K, Saga Y, Matsubara S, Fujiwara H, Sato Y. Targeting human vasohibin-2 by a neutralizing monoclonal antibody for anti-cancer treatment. Cancer Sci. 2017;108(3):512–519. doi:10.1111/cas.13149.
- Liu J, Lee W, Jiang Z, Chen Z, Jhunjhunwala S, Haverty PM, Gnad F, Guan Y, Gilbert HN, Stinson J, et al. Genome and transcriptome sequencing of lung cancers reveal diverse mutational and splicing events. Genome Res. 2012;22(12): 2315–2327. doi:10.1101/gr.140988.112.
- Seshagiri S, Stawiski EW, Durinck S, Modrusan Z, Storm EE, Conboy CB, Chaudhuri S, Guan Y, Janakiraman V, Jaiswal BS, et al. Recurrent R-spondin fusions in colon cancer. Nature. 2012;488(7413):660–664. doi:10.1038/nature11282.
