ABSTRACT
Germline mutations in CDH1, the gene coding for the E-cadherin adhesion protein, are known to cause hereditary diffuse gastric cancer. We identified a new truncating germline mutation (p.Asp538Thrfs*19) in exon 11 of the CDH1 gene in a 41-year-old male with a diffuse gastric cancer. Although he had no parental history of gastric cancer, the co-segregation study in the family detected the same mutation in his healthy 31-year-old brother. The mutation affects one of the extracellular repeat (CAD repeats) domains which is essential for the homophilic binding specificity that directs “E-cadherin” to bind with itself each others. In this case, immunohistochemical analysis showed no expression of E-cadherin in the tumor sample and was a useful prescreening tool to genetic testing. This finding was associated with a poor response to trastuzumab-based treatment.
Introduction
Gastric cancer (GC) is the fourth leading cause of cancer death worldwide. GC incidence and mortality have clearly decreased during the last decades reaching an average incidence of about 14 cases per 100,000 individuals in Italy.Citation1 However, despite the favorable incidence trend and the gradually improved survival over the last 30 years, GC remains one of the most common causes of cancer death. The majority of GC patients present with either locally advanced or metastatic disease and chemotherapy remains the standard of care for these patients, but the sensitivity to treatment differs in everyone.
According to Lauren's criteria,Citation2 GC is classified into two main histological types: diffuse and intestinal, plus an uncommon variant: the undetermined mixed type. The intestinal and diffuse types exhibit numerous differences in pathology, epidemiology, etiology and prognosis with the diffuse type generally being thought to have a worse prognosis. Since the publication of the WHO classification of GC in 1990, signet-ring cell carcinoma (SRCC), previously classified as diffuse type according to Lauren's classification, constitutes a specific histotype.Citation3 However, some aspects of the worse prognosis and lower chemosensitivity of signet-ring tumor carcinoma than non-SRCC are still controversial. Regarding early SRCC, most studies have reported that this tumor type has less risk of lymph node metastasis and favorable prognosis compared with other types,Citation4 on the contrary other studies reported that SRCC had an unfavorable risk of lymph node metastasis, and thus a worse prognosis after surgical resection, compared to other GC types.Citation5,Citation6 Moreover in advanced GC, SRCC was reported as an independent predictor of poor prognosis in multivariate analysis in some studies,Citation6,Citation7 while it was not significant in other studies also performed by multivariate analysis after adjustment for the stage since the presence of SRCC are more frequently found at an advanced stage in GC than in other GC types.Citation8,Citation9,Citation10,Citation11,Citation12 While the incidence of GC has decreased worldwide in recent decades, the incidence of SRCC is constantly increasing in Asia, United States and Europe, accounting for 8% to 30% of GC cases in recent studies.Citation13 SRCC is associated with germline CDH1-mutations and may be a hereditary form. Hereditary diffuse gastric cancer (HDGC), defined by multidisciplinary workshop criteria updated in 2015, is an autosomal dominant disorder.Citation14 Approximately 40% of HDGC families have germline mutations in the CDH1 gene and the cumulative risk of GC for CDH1 mutation carriers by the age of 80 years is reported to be 70% for men and 56% for women.Citation15 Female carriers also have a risk of breast cancer of about 50% with lobular cancer being the most characteristic.Citation15
SRCC has two forms: early gastric cancer, which can be resected endoscopically in some cases and has a better outcome than non-SRCC, and advanced GC, which is generally thought to have a worse prognosis and lower chemosensitivity than non-SRCC. The CDH1 gene is present in a gene cluster with other members of the cadherin family on chromosome 16.
With regard to pathogenic CDH1 germline mutations, there are a large number of truncating mutations, which do not lead to the production of a functional protein. Rare large exonic deletions exist, with a frequency of about 5%. To date, more than 180 pathogenic germline variants have been reported in HDGC families in a diverse range of ethnic groups. Somatic CDH1 alterations have also been found in approximately 30% of all patients with GC, both diffuse and intestinal types.Citation16
As CDH1 is a tumor suppressor gene, a second somatic hit is needed for tumor initiation, which most frequently includes promoter methylation, and less frequently somatic mutation or loss of heterozygosity.Citation17
Due to their high number and the difficulty to demonstrate their functional role in vivo, most mutations remain clinically uncertain and must still be clarified. Nonetheless, although uncommon, GC associated with CDH1 mutation constitutes an important health issue due to its severity, high penetrance, early age at presentation and the unavailability of effective screening tools.
Herein, we present an affected 41-year-old man diagnosed with metastatic GC with signet ring cells, who harbored a novel germline mutation in the CDH1 gene but who did not meet the clinical criteria for a genetic screening.
Clinical case report
In January 2016, a 41-year-old male of Italian origin who developed diffuse GC was admitted to our hospital. The biopsy from gastroscopy, performed at an outside institution was assessed as poorly differentiated SRCC.
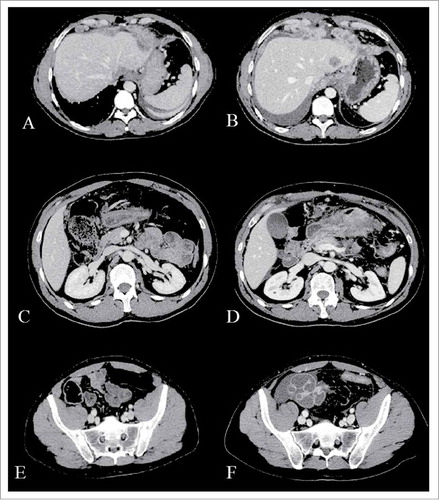
In order to determine the extent of disease a computerized axial tomography scan (CT) and a laparoscopy were performed. Diagnostic laparoscopy showed a below diaphragmatic peritoneal involvement while the CT scan detected a pancreatic and hepatic infiltration (, , ). The multiple biopsies performed in our Institute confirmed the previously found histotype.
Figure 1. Computerized axial tomography (CAT) scans. Baseline CT-scan (A, C, E) compared with that performed after two cycles of chemotherapy (B, D, F) shows a significant gastric, peritoneal and hepatic progression.
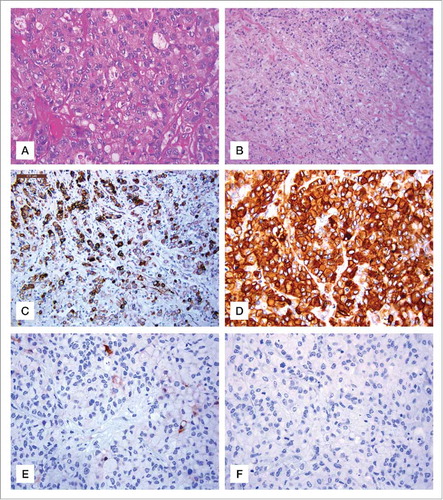
Immunohistochemical staining showed the metastatic tumor tissue to be positive for pancytokeratin (, ), cytokeratin 7 () cytokeratin 20 and CEA () with a human epidermal growth factor receptor 2 (HER2) overexpression (score 3+) in rare neoplastic cells ().
Figure 2. Hematoxylin and eosin stain gastric sections and immunohistochemical staining for Ck7, CEA, HER2 and E-cadherin. (A) Neoplastic cells showing diffuse solid growth and focal vague glandular appearances. H&E, original magnification 200x. (B) Signet-ring morphology of most neoplastic cells with infiltrative growth H&E, original magnification, 100x. (C) Ck7 immunopositive neoplastic cells, (IHC, Haematoxylin counterstain, original magnification 100x). (D) Strong cytoplasmic expression of CEA in neoplastic cells (IHC Haematoxylin counterstain, original magnification 200x). (E) Rare neoplastic Her 2 neu positive cells (IHC, Haematoxylin counterstain, original magnification 200x). (F) Absent expression of E cadherin in signet ring neoplastic cells (IHC, Haematoxylin counterstain, original magnification 200x).
Thus, according to our phase II protocol (code CRO: CRO-2011-2012 Code EUDRACT: 2011-001720-37) the patient received a chemotherapy consisting of 60 mg/m2 oxaliplatin on day 1 and 8, 30 mg/m2 of docetaxel on day 1 and 8 every three weeks and fluoropyrimidine for 14 days every three weeks (capecitabine 1000 mg/m2/day) associated with trastuzumab 8mg/kg loading dose and then 4 mg/kg every three weeks.
After three cycles of treatment, a CT scan showed a clear increase of peritoneal metastases and pancreatic and hepatic involvement (, , ).
The patient rapidly worsened without undergoing further treatments. Although no positive GC history was reported in the patient's family, due to the clinicopathological characteristics of this tumor (patient's young age at diagnosis, the clinical aggressiveness of the case, and the histopathological phenotype of the tumor i.e. diffuse adenocarcinoma with SRCCs) we evaluated the E-cadherin expression level. Result revealed the complete loss of protein expression in the tumor sample (). After genetic counseling and obtaining informed consent from the patient, we screened for germline CDH1 mutations.
Results
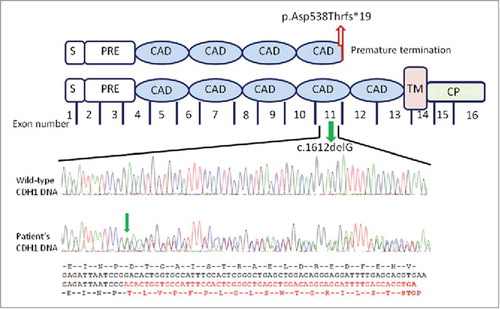
We found a novel mutation in the CDH1 gene (GenBank KX271351, Clin Var accession number SCV000588228). The mutation consists in a heterozygous guanine deletion in exon 11 (c.1612delG) () leading to a frameshift with a premature E-cadherin protein truncation at codon 556 (p.Asp538Thrfs*19) in the extracellular fourth repeat (EC4) region which is essential for the homophilic binding specificity of E-cadherin.
Figure 3. Schematic representation of the CDH1 gene mutation and parallel sequencing of exon 11 of the CDH1 gene. The mutation located in the central region of the CDH1gene encodes for the fourth protein extracellular domain containing a calcium binding site. The c.1612G deletion (green arrow) in the exon 11 causes a frameshift of amino acids change resulting in a premature stop codon (p.Asp538Thrfs*19; red arrow). Structurally, the E-cadherin comprises a number of domains: a signal sequence (S); a propeptide of around 130 residues (PRE); 5 tandemly repeated extracellular cadherin domains (ECAD); a single transmembrane domain (TM) and a N-terminal cytoplasmic domain (CP). Analysed four-color sequencing electropherograms. Top: partial CDH1 exon 11 wild-type sequence; bottom: the parallel CDH1 exon 11 sequence from the patient's DNA. Red sequence shows the deletion resulting in the relative loss of wild-type CDH1 allele with a stop codon.
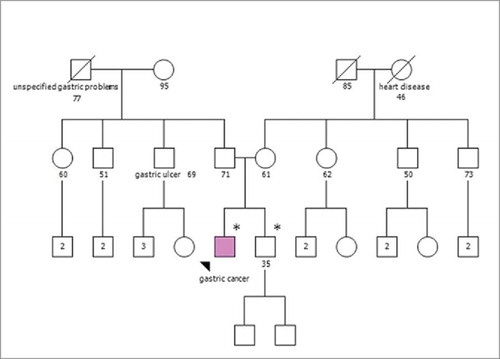
The pedigree analysis () shows healthy parents with no relevant positive family history. Genetic testing was offered to his healthy 31 year-old brother whereas tumor unaffected parents aged 63 and 72 y, respectively, were not available for CDH1 mutation carrier testing. The brother showed the same heterozygous germline mutation. Overall, these findings may suggest that one of the parents is carrier of mutation showing incomplete penetrance or, in alternative, that a germinal (gonadal) mosaicism is present in one of the parents. Currently, since neither parens released consent for CDH1 testing, we cannot exclude either of these hypotheses.
Figure 4. Pedigree of the individual's family. Squares indicate males; circles indicate females. Solid symbol indicates the gastric cancer patient. Symbols with a slash indicate deceased individuals. The numbers below squares and circles indicate age at the time family members were analyzed. Number inside a symbol indicate number of children. An asterisk (*) marks the examined individual found to carry the germline CDH1 mutation.
Moreover, both of the brothers gave negative results for multiplex ligation-dependent probe amplification (MLPA) analysis and showed a difference in a point mutation in the CDH1 promoter region (position c.-285 C>A, rs16260 ), with the proband being homozygous for the wild type C/C genotype and the brother being heterozygous for the minor A allele.
Discussion
In this report, we describe a 41-year-old male with metastatic GC characterized by the presence of signet ring cells, the expression of HER2 and the loss of E-cadherin. Molecular analysis of the germline CDH1 gene identified a mutation that had not been previously described (Clin Variant accession number SCV000588228 and LOVD database submitted) which conforms to an accepted pathogenic mechanism since it produces a stop codon in the EC4 region of the gene. His brother (age 31), with no diagnosis of GC, was found to carry the same germline CDH1mutation, thus ruling out the possibility of a de novo origin of the mutation.
In the light of both molecular and clinical data, two observations are outstanding.
First, this family did not fit any classification proposed by the updated guidelines for the diagnosis of syndrome HDGC,Citation18 but the presence of the same mutation in the brother led to the suspicion of a hereditary disease due to a new mutation of the CDH1gene. In this case, the absence of the CDH1expression on the immunohistochemistry (ICH) test was particularly useful. The present case underlined that IHC screening for CDH1 expression is an advantageous tool in suspicious cases like this that do not meet CDH1 testing criteria but due to the young age of the patient and the non-response to therapy lead to the hypothesis of a potential CDH1 mutation. This is of importance since almost 100% of CDH1 mutation carriers who had performed prophylactic gastrectomies revealed the presence of microscopic cancer loci in the tissue samplesCitation14 and the prognosis of GC, still the fourth most common cause of death from cancer, remained strongly related to the early stage at diagnosis.
Second, HER2-positivity is predominantly seen in Lauren's intestinal type with a low prevalence in DGC (32% vs. ∼5%)Citation19 and a much lower prevalence in the SRCC type (1.9%). Thus, our patient's presentation is rare given that his tumor is of SRCC type and was strongly positive for HER2, although in few cells. This aspect is particularly important to discriminate unnecessary trastuzumab treatment in SRCC patients. To date, testing for HER2 is recommended in patients with inoperable, locally advanced, recurrent or metastatic disease. HER2 positivity seems to help to select the patients most likely to obtain benefit from HER2 target therapy since trastuzumab showed a significant overall survival benefit for patients with HER2 positivity advanced stage GC compared with those treated with only cisplatin/fluoropyrimidine (5FU) chemotherapy in the ToGA study. Median overall survival was 13·8 months in patients treated with trastuzumab plus chemotherapy compared with 11·1 months in those assigned to chemotherapy alone.Citation20 However, in ToGA trial only 9% of patients had a diffuse GC type and these patients have poor response to trastuzumab.Citation20 Thus, if HER2 overexpression is to be considered an optimal patient selection biomarker for anti-HER2 therapy, the efficacy of this treatment in the SRCC tumor setting remains challenging.
Interestingly, our patient experienced a rapid progression after 3 months of HER2 target therapy showing that HER2-positivity alone is not always a relevant predictive biomarker of response to HER2-targeted agents.
In this scenario, the role of the CDH1 mutation and HER2 over expression combination in SRCC that we found to be associated with a particularly severe clinical presentation of the tumor and with a lack of response to treatment remain to be elucidated.
Materials and methods
Immunohistochemistry
A formalin-fixed, paraffin-embedded tumor block was cut into 4-μm-thick sections for H&E and immunostaining. Immunohistochemistry was performed by using the mouse monoclonal antibody against human E-cadherin (clone 36, Ventana Medical System, Tucson, AZ), the mouse monoclonal antibody against pankeratin (clone AE1/AE3 & PCK26, Ventana Medical System, Tucson, AZ) and against CEA (clone CEA31, Ventana Medical System, Tucson, AZ ) and rabbit monoclonal antibodies against CK7 (clone SP52, Ventana Medical System, Tucson, AZ), against CK20 (clone SP33, Ventana Medical System, Tucson, AZ) and against HER 2 (clone 4B5, Ventana Medical System, Tucson, AZ).
Germline CDH1 mutation screening
Genomic DNA was extracted from peripheral blood leukocytes, using the EZ1 DNA Blood kit and the BioRobot EZ1 Workstation (QIAGEN Inc., Valencia, CA, USA). Mutation analysis was performed by primers specific PCRCitation21 and bidirectional Sanger sequencing (ABI BigDye Terminator Sequencing Kit, Applied Biosystems) of all 16 CDH1 coding exons, including the intron-exon boundaries on the ABI Prism 3130xl Genetic Analyzer (Applied Biosystems). An additional independent PCR and sequencing reaction confirmed the presence of the mutation.
Multiplex ligation-dependent probe amplification (MLPA) to identified large genomic alterations at the CDH1 locus was performed using the SALSA MLPA P083-C2 CDH1 (MRC-Holland, Amsterdam, Holland) according to the manufacturer's instructions. The fragments were separated by capillary electrophoresis using the ABI 3130 xl Genetic Analyzer (Applied Biosystems GS500 LIZ size standard) and comparative analyses were performed using the Coffalyser software from MRC-Holland.
Ethics statement
Ethical approval was obtained from the Medical Research Ethics Committee of the Centro di Riferimento Oncologico, IRCCS, Aviano, Italy. All the participants in the study were asked to accept and sign the written informed consent.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors would like to thank Dr. ssa Anna Vallerugo for her professional English language editing of the manuscript.
Funding
Funding for this research was provided by 5 x mille CRO, intramural Grant.
References
- Inghelmann R, Grande E, Francisci S, Verdecchia A, Micheli A, Baili P, Capocaccia R, De Angelis R. Regional estimates of stomach cancer burden in Italy. Tumori. 2007;97:367–73.
- Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. an attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi:10.1111/apm.1965.64.1.31.
- Pernot S, Voron T, Perkins G, Lagorce-Pages C, Berger A, Taieb J. Signet-ring cell carcinoma of the stomach: Impact on prognosis and specific therapeutic challenge. World J Gastroenterol. 2015;21:11428–38. doi:10.3748/wjg.v21.i40.11428.
- Kim BS, Oh ST, Yook JH, Kim BS. Signet ring cell type and other histologic types: differing clinical course and prognosis in T1 gastric cancer. Surgery. 2014;155:1030–5. doi:10.1016/j.surg.2013.08.016.
- Bozkaya Y, Erdem GU, Ozdemir NY, Demirci NS, Hocazade C, Yazıcı O, Zengin N. Comparison of clinicopathological and prognostic characteristics in patients with mucinous carcinoma and signet ring cell carcinoma of the stomach. Curr Med Res Opinion. 2017;33:109–16. doi:10.1080/03007995.2016.1239192.
- Lu M, Yang Z, Feng Q, Yu M, Zhang Y, Mao C, Shen L, Tang J. The characteristics and prognostic value of signet ring cell histology in gastric cancer: A retrospective cohort study of 2199 consecutive patients. Medicine (Baltimore). 2016;95:e4052. doi:10.1097/MD.0000000000004052.
- Piessen G, Messager M, Leteurtre E, Jean-Pierre T, Mariette C. Signet ring cell histology is an independent predictor of poor prognosis in gastric adenocarcinoma regardless of tumoral clinical presentation. Ann Surg. 2009;250:878–87. doi:10.1097/SLA.0b013e3181b21c7b.
- Theuer CP, Nastanski F, Brewster WR, Butler JA, Anton-Culver H. Signet ring cell histology is associated with unique clinical features but does not affect gastric cancer survival. Am Surg. 1999;65:915–21.
- Heger U, Blank S, Wiecha C, Langer R, Weichert W, Lordick F, Bruckner T, Dobritz M, Burian M, Springfeld C, et al. Is preoperative chemotherapy followed by surgery the appropriate treatment for signet ring cell containing adenocarcinomas of the esophagogastric junction and stomach? Ann Surg Oncol. 2014;21:1739–48. doi:10.1245/s10434-013-3462-z.
- Zu H, Wang H, Li C, Xue Y. Clinicopathologic characteristics and prognostic value of various histological types in advanced gastric cancer. Int J Clin Exp Pathol. 2014;7:5692–700.
- Kim DY, Park YK, Joo JK, Ryu SY, Kim YJ, Kim SK, Lee JH. Clinicopathological characteristics of signet ring cell carcinoma of the stomach. ANZ J Surg. 2004;74:1060–4. doi:10.1111/j.1445-1433.2004.03268.x.
- Li C, Kim S, Lai JF, Hyung WJ, Choi WH, Choi SH, Noh SH. Advanced gastric carcinoma with signet ring cell histology. Oncology. 2007;72:64–8. doi:10.1159/000111096.
- Taghavi S, Jayarajan SN, Davey A, Willis AI. Prognostic significance of signet ring gastric cancer. J Clin Oncol. 2012;30:3493–8. doi:10.1200/JCO.2012.42.6635.
- van der Post RS, Vogelaar IP, Carneiro F, Guilford P, Huntsman D, Hoogerbrugge N, Caldas C, Schreiber KEC, Hardwick RH, Ausems MGEM, et al. Hereditary diffuse gastric cancer: updated clinical guidelines with an emphasis on germline CDH1 mutation carriers. J Med Genet. 2015;52:361–74. doi:10.1136/jmedgenet-2015-103094.
- Hansford S, Kaurah P, Li-Chang H, Woo M, Senz J, Pinheiro H, Schrader KA, Schaeffer DF, Shumansky K, Zogopoulos G, et al. Hereditary diffuse gastric cancer syndrome: CDH1 mutations and beyond. JAMA Oncol. 2015;1:23–32. doi:10.1001/jamaoncol.2014.168.
- Corso G, Carvalho J, Marrelli D, Vindigni C, Carvalho B, Seruca R, Roviello F, Oliveira C. Somatic mutations and deletions of the E-cadherin gene predict poor survival of patients with gastric cancer. J Clin Oncol. 2013;31:868–75. doi:10.1200/JCO.2012.44.4612.
- Fitzgerald RC, Caldas C. Clinical implications of E-cadherin associated hereditary diffuse gastric cancer. Gut. 2004;53:775–8. doi:10.1136/gut.2003.022061.
- Fitzgerald RC, Hardwick R, Huntsman D, Carneiro F, Guilford P, Blair V, Chung DC, Norton J, Ragunath K, Van Krieken JH, et al. Hereditary diffuse gastric cancer: updated consensus guidelines for clinical management and directions for future research. J Med Genet. 2010;47:436–44. doi:10.1136/jmg.2009.074237.
- Kunz PL, Mojtahed A, Fisher GA, Ford JM, Chang DT, Balise RR, Bangs CD, Cherry AM, Pai RK. HER2 expression in gastric and gastroesophageal junction Adenocarcinoma in a US population: clinicopathologic analysis with proposed approach to HER2 Assessment. Applied Immunohistochem Mol Morphol. 2012;20:13–24. doi:10.1097/PAI.0b013e31821c821c.
- Bang Y-J, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2012;376:687–97. doi:10.1016/S0140-6736(10)61121-X.
- Garziera M, Canzonieri V, Cannizzaro R, Geremia S, Caggiari L, De Zorzi M, Maiero S, Orzes E, Perin T, Zanussi S, et al. Identification and characterization of CDH1 germline variants in sporadic gastric cancer patients and in individuals at risk of gastric cancer. Plos One. 2013;8:e77035. doi:10.1371/journal.pone.0077035.
