ABSTRACT
Ovarian cancer is the third most common cancer in the female reproductive organs and epithelial ovarian cancer has the highest lethality of all gynecological cancers. Pomegranate fruit juice (PFJ) has been shown to inhibit the growth of several types of cancer other than ovarian cancer. In this study, we exposed the ovarian cancer cell line A2780 to PFJ and two of its components (ellagic acid and luteolin). MTT and wound healing assays demonstrated that all three treatments suppressed the proliferation and migration of the ovarian cancer cells. In addition, western blotting and ELISA assays showed that the expression levels of MMP2 and MMP9 gradually decreased after treatment with increasing concentrations of ellagic acid and luteolin. To confirm our findings in the in vitro experiments, we used another ovarian cancer cell line, ES-2, in nude mice experiments. All three treatments inhibited tumor growth without obvious side-effects. Furthermore, compared with the control group, the expression levels of MMP2 and MMP9 were depressed. Ellagic acid induced a greater effect than luteolin, suggesting that ellagic acid might be a promising candidate for further preclinical testing for treatment of human ovarian cancer.
Abbreviations
| DMSO | = | dimethyl sulfoxide |
| ELISA | = | enzyme-linked immunosorbent assay |
| EA | = | ellagic acid |
| HRP | = | horseradish peroxidase |
| HE staining | = | hematoxylin-eosin staining |
| L | = | luteolin |
| MMPs | = | matrix metalloproteinases |
| MTT3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide | = | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| PFJ | = | pomegranate fruit juice |
| PBS | = | phosphate buffered saline |
Introduction
Ovarian cancer, one of the three major malignant tumors in women, has high incidence and mortality. It is hard to diagnose until very late stages and the five-year survival rate is highly unfavorable,Citation1 due especially to its distant metastasis. The Cancer Statistics 2016 estimated 22,280 new cases of ovarian cancer with 14,240 deaths for year 2016 in the United States.Citation2 If ovarian cancer is diagnosed early and appropriate surgeries and interventional therapies are provided, 90% of patients will survive for more than five years. However, with advanced and metastasized ovarian cancer, less than 30% of the patients may survive for five years.Citation3 Although death rate of ovarian cancer was higher in North America and Europe than in Asia and Africa, the difference is narrowing.Citation4 While the specific etiology of ovarian cancer is still unclear, it might be related to age, fertility, blood type, and mental and environment factors.Citation5–7 Chemotherapy is currently the most common treatment to inhibit ovarian cancer, but long-term use could result in tumor recurrence and further progression, frequently leading to chemotherapeutic resistance.Citation8,9 In order to consolidate curative effect, immune therapy and traditional Chinese medicine therapy have been adopted by some authors.Citation10,11 Paclitaxel, a natural antitumor agent, is in the standard front-line treatment and has significant effects on advanced malignancies including ovarian cancer.Citation12 Substances like paclitaxel are good examples of how natural compounds may be used to treat cancers, inspiring the discovery of safe and effective approaches in ovarian cancer prevention and therapy.
Matrix metalloproteinases (MMPs) are a family of Ca2+-dependent Zn2+-containing endopetidases, which are capable of degrading extracellular matrix proteins to promote cancer cell migration, invasion and metastasis.Citation13 Among the more than 20 members of MMPs, MMP2 and MMP9 are correlated with the aggressiveness of cancer.Citation14 MMPs are regulated by hormones, growth factors, and cytokines, which are all involved in ovarian cancer.Citation15–17 Thus, MMPs have been considered as significant targets for ovarian cancer therapy.
Pomegranate has been used as medicine in many cultures throughout history but is usually consumed as fresh fruit or commercial fruit juice. It possesses many pharmacological effects, including anti-inflammatory, anti-oxidant, anti-bacterial and estrogenic activities.Citation18,19 All biological activities are generally attributed to the high phenol, flavonoid, anthocyanin, and tannin contents of the juice, seed and peel.Citation20 Recent studies have demonstrated that pomegranate is a potent anti-carcinogenic agent that inhibits multiple signaling pathways, inducing apoptosis and cell cycle arrest.Citation21–24 Additionally pomegranate can significantly inhibit angiogenesis and metastasis in cancer development progress.Citation25
Luteolin (2-(3,4-dihydroxyphenyl)chromenylium-5,7-diol, L) is a nontoxic flavonoid compound that has been used in Chinese traditional medicine to treat various pathologies.Citation26 In different cancers, luteolin can act as an MMPs inhibitor, attenuating MMPs expression by suppressing the ERK/NF-κB pathway or directly inhibiting its activity.Citation27,28 Ellagitannins, one subclass of hydrolysable tannins, are broken down into free ellagic acid (2, 3, 7, 8-Tetrahydroxy-chromeno [5, 4, 3-cde] chromene-5, 10-dione, EA), which can be absorbed by stomach.Citation29 When the pomegranate juice is processed, each fruit produces a minimum of 2g/L of ellagitannins.Citation30 EA has shown anti-proliferation activity in breast cancer and antioxidant activity through inhibiting inflammatory factors such as TNF-α.Citation31 However, controversial results have been reported on liver cancer, in which EA was demonstrated to promote hepatocarcinogenesis or perform no effect on hepatocarcinoma.Citation32,33
While it is confirmed that pomegranate has significant effects in breast, prostate and colon cancers,Citation34–36 there are few detailed reports on ovarian cancer. This study focuses on the pharmacological effects and anti-cancer mechanism of pomegranate fruit juice (PFJ) and two of its main components, EA and L on ovarian cancer to provide theoretical basis for new anti-cancer drug development. Furthermore, for the first time, we compared the efficacy of EA to L and found that EA performed stronger effects than L on ovarian cancer.
Results
PFJ, EA and L could inhibit the proliferation of human ovarian carcinoma cell line A2780 cells
We examined different concentrations of PFJ, EA and L to establish whether they might have the ability to inhibit the proliferation of cancer cells. As shows, they all significantly suppressed the growth of A2780 cells in 12, 24 and 48 h, compared to the control group. Among the treatments (n = 3), dose- and time-dependent responses were observed in L (), while EA showed a dose-dependent response only at 48h (). In order to confirm the results of MTT assays, we performed crystal violet assays to verify the proliferation inhibitive effect of PFJ, EA and L. Cells were stained by crystal violet after 48h treatments with PFJ, EA or L at different concentrations and OD values were compared. We found that PFJ, EA and L all could decrease the cell number of A2780 in a dose-dependent manner (). Furthermore, by triple repeats of each treatment, we found that the inhibitive effect of EA was more obvious than L after 48h treatment (, ), which was consistent with the results from MTT assays at the 48h time point.
Figure 1. Different concentrations of PFJ, EA andL showed an inhibition effect on cell proliferation according to the MTT values. The inhibiting cancer cell proliferation activity of different concentration of EA (), L () and PFJ (). Treated after 12, 24 and 48 h, all of the three show obvious suppression features, both EA and L presented a desired dose- and time-dependent manner at 48 h. Results were obtained from three separate experiments. Student t test was used for statistical tests, # represents P < 0.01 and * represents P < 0.05 when compared with ctrl.
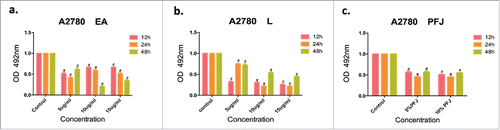
Figure 2. Cell numbers inhibition by different concentrations of PFJ, EA and Lin A2780 cell line by Crystal Violet assay. After treating with different concentrations of EA, L and PFJ, A2780 cells were stained with crystal violet (, , ). Cell number was determined by OD570 value after treating with different concentration of EA, L and PFJ. After data analysis, each of the three compounds could reduce cell number remarkably, moreover, EA performed a most effectively and does-dependent inhibity function compared to L. PFJ could also reduce cell numbers as the concentraions increased. Results were obtained from three independent experiments, # represents P < 0.01 and * represents P < 0.05 when compared with control.
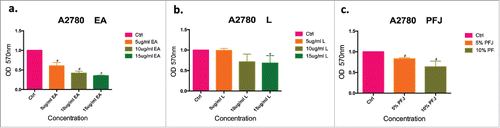
PFJ, EA and L could inhibit the migration of human ovarian carcinoma cell line A2780 cells
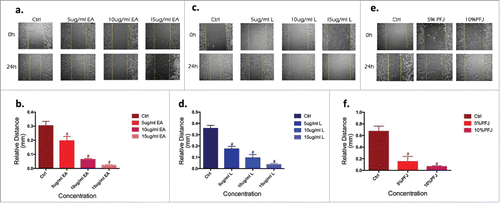
Migration is an initial step for a malignant tumor to make the disease rapidly deteriorating. As shown in , EA (), L () and PFJ () significantly inhibited tumor migration in a dose-dependent manner. Consistent with MTT and crystal violet assay results, treatments with PFJ, EA and L all inhibited cell motility into a wounded area of confluent cultures in a dose-dependent manner ().
Figure 3. The migration of ovarian cancer cells, quantified by Wound Healing, was significantly suppressed by EA and L. Wound healing in the cell vitro experiments is typically characterized by the remaining distance of the scar after treated with three compounds in 24 h cells comparing to 0 h. EA (, ), L (, ) and PFJ (, ) show dose- and time-dependent and preliminary demonstrate our hypothesis. Results were obtained from three separate experiments. Student t test was used for statistical tests, # represents P < 0.01 and * represents P < 0.05 when compared with Ctrl.
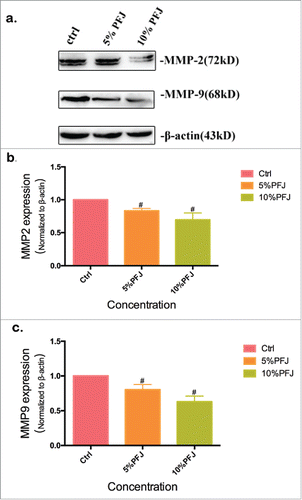
The expression levels of MMP2 and MMP9 were markedly down-regulated by PFJ, EA and L
To establish the mechanism of the three compounds to inhibit cancer cell migration, we used MMP2 and MMP9 as markers of metastasis. MMP2 (72kDa type IV collagenase) is intimately linked with the invasion and metastasis of ovarian cancer, while MMP9 (68kDa type IV collagenase) is a useful serum marker of ovarian cancer.Citation37 To determine whether EA or L might regulate the expression of MMPs, we conducted western blot analysis and evaluated the expression intensity of MMPs in A2780 cells after treatment with the products for 24 hours. Compared with the control (, and ), EA at concentrations of 10–15 μg/mL markedly down-regulated MMP2 and MMP9 expression in a dose-dependent manner. L could slightly reduce MMP2 and MMP9 expression at the concentration of 5 μg/mL, but at increased concentrations (10–15 μg/mL) the inhibitory effects became much higher. As shown in , decreased expression levels of MMP2 () and MMP9 () were observed when the cells were treated with 5% and 10% PFJ. Decreased MMP2 and MMP9 expression was also observed as PFJ at different concentrations in western blot analysis ().
Figure 4. The amount of MMP2 and MMP9 was significantly reduced by EA, L. To illustrate the metastasis inhibition mechanism of EA (, ) and L (, ), western blot show the down-regulation of MMP2 and MMP9 in a dose-dependent tendency. But at the lower concentration (5 μg/mL) of EA(), the inhibiting activity not markedly compared with control group. Results were obtained from three separate experiments. Student t test was used for statistical tests, # represents P < 0.01 and * represents P < 0.05 when compared with Ctrl.
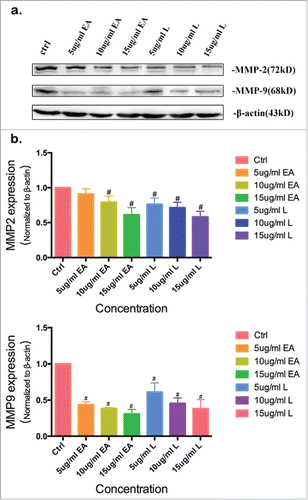
Figure 5. The expression level of MMP2 and MMP9 was inhibited by PFJ. At the same time, different concentrations of PFJ also exerts an influence on inhibiting the expression of MMPs. Compared with corresponding ctrl group, both MMP2 and MMP9 expression levels were restrained by 5% and 10% PFJ at does-denpendent manners. Results were obtained from three separate experiments. Student t test was used for statistical tests, # represents P < 0.01 and * represents P < 0.05 when compared with Ctrl.
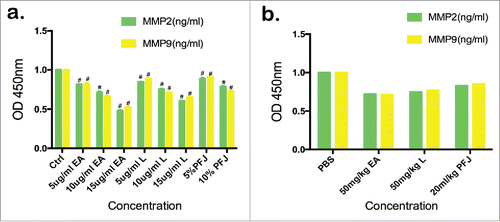
The contents of MMP2 and MMP9 in the cell supernatant decreased in a dose-dependent manner upon treatment with EA, L and PFJ as examined using ELISA assays ().
Figure 6. The contents of MMP2 and MMP9 in the supernatant fluid of cultured cancer cells and mice serum were detected by ELISA. In cell supernatant ELISA assay (), we measured the OD value at 450 nm, and it shows the same trend with western blot. In nude mice serum ELISA assay (), each sample contains corresponding the number of mice group. And it also presents a trend that EA, L and PFJ can decrease the expression level of MMP2 and MMP9. Cell supernatant ELISA assay were performed by three separate experiments. Student t test was used for statistical tests, # represents P < 0.01 and * represents P < 0.05 when compared with Ctrl.
PFJ, EA and L inhibit tumor growth in vivo
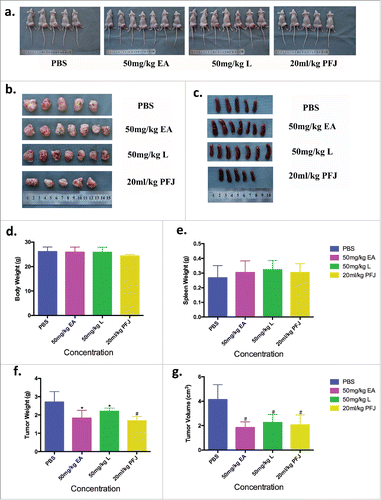
To better understand the impacts of EA and L on ovarian cancer, we injected ES-2 cells into the right hind leg of female nude mice. Two weeks later, all mice could be found bearing a tumor and were randomly assigned into four experimental groups (EA, L, PFJ, and PBS). As body weight curve shown, all animals gained body weight gradually (). Interestingly, the body weight of PBS group suddenly dropped during 15–20 days and recovered as we improved the living environment. Compared with that in the PBS group, the tumor volumes increased more slowly in the other three groups (). At the end of experiment, all mice were sacrificed for histological examinations of the tumor tissues. We found that all three treatments reduced both tumor weight and volume (, , and ) with no effects on body weight () or spleen weight (, ). EA showed a greater reduction of tumor weight and volume than L, suggesting that it could be a better candidate for a future anti-cancer drug.
Figure 7. Tumor growth and body weight changes as time during EA, L and PFJ treatments after the tumor mass could be touched. Treated three compounds with bearing ovarian cancer nude mice to clarify the in-vivo effect of them. In the everlasting 40 days, nude mice were treated with PBS, 50 mg/kg EA, 50 mg/kg L and 20 mL/kg PFJ as experimental design. Body weight and tumor volume were started to measure after the tumor mass could be touched. At 15th-19th (), due to the surrounding was worse, the body weight of PBS group was drop drastically before the living environment was improved, subsequently, the body weight of PBS group was recovered and performed as our result shown. All groups were stably increased during the 40 days. Tumor volume were measured once every two days and the tumor sizes were calculated according to the formula V = 0.5 ab.Citation2 As shown by the tumor volume curve (), the tumor volumes were increasing slowly in these three compound groups compared with PBS.
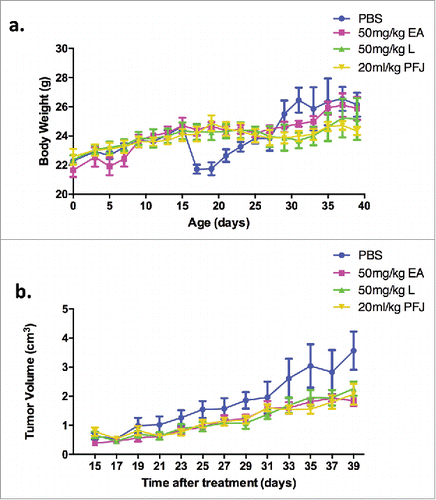
Figure 8. The final body weight, spleen weight, tumor weight and tumor volume were measured and recorded at necroscopy (the 40th day). Nude mice were sacrificed then gathered the solid tumor and spleen at the end of experiment (, , ). And contrast the value of body weight, tumor weight and tumor volume in different groups (, , , ), EA shows more advantaged and less side effect in vivo. It seems that EA could be used as a medicine in the future treatment.
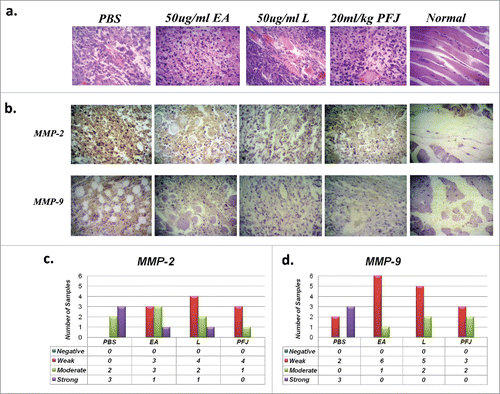
PFJ, EA and L inhibited MMP2 and MMP9 expression: Histological and biochemical evidence
Hematoxylin-Eosin staining showed dark and basophilic materials in the cytoplasm of most tumor cells compared to cells of control tissues (). The HE staining results indicate that the three products all had anti-cancer effects through transforming cell structures. We then quantified the expression of MMPs in solid tumor paraffin sections by immunohistochemistry and found that the expression levels of MMP2 and MMP9 were significantly reduced compared to the PBS group (, ). These results indicate that EA, L and PFJ all had anti-cancer activities through down regulating MMPs expression. Staining of MMPs was strongest in the PBS groups but either ‘weak’ or ‘moderate’ after treatment, suggesting that all three treatments had therapeutic effects (, ). Moreover, serum ELISA analysis demonstrated that EA, L and PFJ had suppressive activities on MMP9 and MMP2 (), further confirming the anti-tumor characteristics of the three products.
Figure 9. Immunohistochemistry staining for MMP2 and MMP9 and Hematoxylin-Eosin staining in different nude mice ovarian carcinoma tissues. The HE and immunohistochemistry staining in solid tumor paraffin sections. The result of MMP2: PBS (n = 5; moderate:2, strong:3), EA (n = 7; weak:3, moderate:3, strong:1), L (n = 7; weak:4, moderate:2, strong:1) and PFJ (n = 5; weak:4, moderate:1). And MMP9: PBS (n = 5; weak:2, strong:3), EA (n = 7; weak:6, moderate:1), L (n = 7; weak:5, moderate:2) and PFJ (n = 5; weak:3, moderate:2). It seems that all of three compounds may inhibit MMP9 expression strongly than MMP2.
Discussion
Ovarian cancer remains a serious threat to the health and lives of women due to its high mortality. Basic and clinical researchers are currently seeking effective antineoplastic agents without side effects for more accurate and efficient use in diagnosis, treatment or prognosis of ovarian cancer. In this study, we demonstrated the ability of pomegranate fruit juice (PFJ) and two of its main components, ellagic acid (EA) and luteolin (L), to suppress the proliferation, migration and progression of ovarian cancer through down-regulating the expression of MMP2 and MMP9.
A previous study in prostate cancer showed that pomegranate could exert anticancer activity, which was attributed to its high content of polyphenols.Citation38 Another research group confirmed that EA, L, and ursolic acid extracted from pomegranate caused a concentration dependent decrease in PANC-1 cell proliferation.Citation39 Consistently, a study in prostate cancer indicated that PFJ components EA, L and punicic acid together inhibited the growth of both hormone-dependent and -independent prostate cancer cells and inhibited their migration, progression and metastasis. Similarly, EA has also been demonstrated to exert in vivo anti-angiogenic effect and inhibit MMP2 activity, both obviously contributing to antitumor activities.Citation40 L acts as an anti-metastatic agent by suppressing MMP2 and MMP9 production and downregulating expression in azoxymethane-induced colorectal cancer.Citation28 Yuan-Chiang and colleagues first investigated the effects of EA on ovarian cancer and pointed out that EA may be a potential novel chemoprevention and treatment assistant agent for human ovarian carcinoma.Citation43 We sought to clarify the anti-tumor mechanism of EA, L and PFJ in ovarian cancer, moreover, the efficay of each treatment was compared in our study.
We found EA and L to significantly reduce the proliferation, migration and invasion of ovarian cancer both in vivo and in vitro. The growth of tumour cells was suppressed by PFJ, EA and L and the inhibitory effect became even stronger with increasing concentrations of the fruit products. Both EA and L showed a time- and dose-dependent manner, EA performed a more obviously cell inhibitive effect comparing to L. Additionally, Wound Healing assays showed PFJ, EA and L to have a dose-dependent inhibition of cell migration. MMP2 and MMP9, both important markers in tumor migration and invasion, showed the effects of treatments on protein levels. Intensity of MMP2 and MMP9 expression decreased with increasing concentrations of the compounds tested.
During our in vitro experiments, we noticed that EA seemed to have superior anti-cancer effects over L. To date, there is no publication comparing the antitumor activities of EA and L. Our findings may guide further study of the role played by EA in resisting ovarian cancer. In our experiment, PFJ was squeezed directly from fresh fruit, presumably containing anti-cancer substances such as anthocyanins. Therefore, the anticancer effect of PFJ should not be simply attributed to the effects of EA and L together. Because neither splenomegaly nor intense changes in body weight was observed, EA, L and PFJ did not induce severe side effects in nude mice.
In addition to pomegranate, EA and L can be extracted from many other plants, including various berries, pineapple, broccoli, bird chili, and onion leaves.Citation44,45 For further usages of these two compounds, our study encourages their dietary and medicinal applications.
Finally, our study demonstrated that EA, L and PFJ suppressed the proliferation and migration of ovarian cancer through down regulating the expression of MMP2 and MMP9, both in vivo and in vitro. We reported for the first time that EA had greater effects than L, suggesting that EA may be a promising candidate for further preclinical testing for treatment of human ovarian cancer.
Conclusion
Natural plants or fruit-derived metabolites are of great resources for adjunct therapies to complement conventional treatment. In our study, PFJ, EA and L markedly inhibited the metastasis of ovarian cancer cells through down-regulating the expression of MMPs. All three products slowed down the growth of solid tumors in our in vivo experiments. Our results indicate great potentials of using a broad variety of natural products such as those reported in this study to improve the prognosis of ovarian cancer.
Materials and methods
Reagents and antibodies
DMEM and McCoy's 5A medium were purchased from GE Healthcare Life Sciences, HyClone Laboratories. Histostain-Plus Kits (SP-9001, SP-9002) and Mouse Anti-β actin mAb(TA-09) were purchased from ZSGB-Bio, Beijing, China. Luteolin (≥98%, L9283) and ellagic acid (≥95%, E2250) were purchased from Sigma-Aldrich (USA). DAB Horseradish Peroxidase Color Development Kit (P0203), BeyoECL Plus (P0018) and Hematoxylin Staining Solution (C0107) were purchased from Beyotime Institute of Biotechnology. MMP2 (BMO569) and MMP9 (PB0709) were purchased as primary antibodies from Boster Biological Technology Co., LTD.Citation46 HRP-linked rabbit- and mouse-anti IgG (7074s, 7076s) were chosen from CST (USA). Mouse MMPs ELISA Kit (DM-X6142, DM-X6008) was purchased from Baomanbio, Shanghai, China.
Cell culture
A2780 and ES-2, two human epithelial ovarian cancer cell lines, were purchased from Procell, Wuhan, China. A2780 cells were cultured in DMEM supplemented with 10% FBS and 0.1% 1000*penicillin-streptomycin solution and maintained in monolayer culture at 37˚C and 5% CO2 in a humidified incubator. Cells were passaged twice weekly using 0.05% trypsin. Similarity, ES-2 cells were cultured in McCoy's 5A with 10% FBS and 0.1% 1000*penicillin-streptomycin solution.
MTT assay
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), used to estimate the cytotoxicity of drugs, is a standard colorimetric assay for measuring cellular proliferation. For the cytotoxicity assay, cells were passaged into 96-well plates at 5,000 (A2780) cells per well and grown to >80% confluence, before being treated with EA, L (5 μg/mL, 10 μg/mL, 15 μg/mL) or PFJ (5%, 10%). The viable cells were determined 12, 24 or 48 h later by the MTT assay. A total of 20 μl of MTT was added to each well at the indicated time points and 150 μl of DMSO was added to dissolve the formed formazan crystals. MTT has been validated to be an accurate measure of the viable cell population. DMSO at the concentrations used had no effect on cell viability.
Crystal violet assay
Crystal violet staining is a colorimetric indirect determination to detect maintained adherence of cells. A2780 cells at 45000 cells per 500ul were seeded in 24-well plates and treated with different concentrations of PFJ (5, 10%), EA (5, 10, 15 ug/ml) and L (5, 10, 15 ug/ml) overnight. After incubation for 48 hours, the medium was aspirated from the wells and 300ul 4% PFA was added per well to fix the cells. To removed remaining liquid, inverted the plate on filter paper, then dyed cells with 1% crystal violet 300ul/well for 5 minutes and washed with gentle stream of tap water. After added 1% SDS 300ul per well, the plate was incubated in room temperature for 1–3 hours on a bench rocker with a frequency of 20 oscillations/minute, then measured the optical density of each well at 570 nm a plate reader.
Wound healing assay
In order to evaluate the migration ability, cells were passaged into 24-well plates at 300,000 (A2780) cells per well and grown to >80% confluence. 20 μl pipette tips were used to make a straight, 1-mm-wide scratch and the scattered cells were washed away by PBS for twice. Next, cells were treated with serum-free medium (control), EA (5 μg/mL, 10 μg/mL, 15 μg/mL), L (5 μg/mL, 10 μg/mL, 15 μg/mL), or PFJ (5%, 10%) and cultured for another 24 hours. The scratch gaps were photographed at time points of 0, 24 hours under a light microscope and analyzed using the Digimizer Version 4.6.1 software.
Western blot analysis
The expression level of MMP2 and MMP9 in ovarian cancer cells were determined by western blot analysis, proteins extracted from A2780 cells were heat-inactivated and transferred to the PVDF membrane by electrophoresis (150 mA for 80 and 70 minutes for MMP2 and MMP9 respectively). 5% skim milk was used to block the other interfering proteins. The PVDF membrane was then washed with 1 × TBST three times, dyed in a darkroom, and imaged. A 1:400 dilution of primary antibody in 5% skim milk and a 1:500 dilution of secondary antibody in 1xTBST were used. Finally, proteins grey-level was measured by Quantity One (Bio-Rad Quantity One version 4.6.2).
Nude mice In vivo experiments
With the animal experiment approved by the Institutional Ethics Committee of Harbin Medical University, 24 female nude mice, weighing 16 ± 18 g and 4–8 weeks old, were purchased from VRL, Beijing, China. All mice were raised on purified, laminar air flow shelves in the sterile laboratory, at a constant temperature of 25 ± 2°C. Humidity was maintained between 45% to 50%. We injected human ovarian cancer ES-2 cells (80 μl, 4.09× 106/μl) into the right hind leg and monitored body weight every alternate day. All tumor mass could be touched 15 days after inoculation. The mice were randomly divided into four groups, to be treated with 50 mg/kg EA (n = 7), 50 mg/kg L (n = 7), 20 mL/kg PFJ (n = 5) or 1× PBS (n = 5). All mice were executed to examine tissue invasion around the tumor cells and the metastasis of superficial lymph node and viscera. The tumor tissues were used for histological examinations by HE and IHC staining. We also cut mice tails to draw blood for ELISA to detect the concentration of MMP2 and MMP9.
ELISA analysis
Seven different concentrations of standard MMP2 and MMP9 were pipetted into pre-coated plates. Supernatant from A2780 cell culture and serum from nude mice were used as antigens. Next, biotin-labeled anti-human MMP2 and MMP9 were added to the plates and the reaction was allowed to continue for 60 minutes at 37°C. After washing three times, Avidin-Biotin-enzyme Complex (ABC) was added to stain. After a 15-minute incubation at room temperature, stop buffer was added and the optical density was measured at 450 nm.
HE staining
Paraffin sections were dipped in xylene, followed by submersion in 100% and then in 80% ethanol solutions. Slides were washed with distilled water for 5 minutes, before being dyed with hematoxylin and eosin. The slides then underwent ethanol dehydration, being submerged in 85% and 90% ethanol, and then by carbol xylol. Finally, the slides were mounted using neutral balsam.
Immunohistochemical staining
Previously prepared paraffin-fixed nude mice tissue sections (3 μm) (normal and tumor) were processed for peroxidase (DAB) immunohistochemistry. After deparaffinization and rehydration using xylene and a series of weakening concentration of ethanol (95%, 80%, 70%), 50 μl of 1:200 dilution MMP2 and MMP9 primary antibody was added to each sample. The samples were stored overnight at 4°C. After being washed with water for 5 minutes, addition of peroxidase labeled polymer and substrate allowed the brown staining of the target proteins to be observed. The samples were counterstained by hematoxylin for 30 seconds.
Statistical analysis
Data are presented as the mean of triplicate or quadruplicate determinants with standard error (s.e.). Assays were repeated at least three times. Statistical analysis was performed to assess the difference between the means of the untreated and treated samples using Student's t-test, Chi square test and Spearman's Rank correlation analysis with SPSS statistical software version 17.0 and GraphPad Prism software. P-value <0.05 was considered statistically significant. Our study closely followed the line of randomness and preciseness to ensure reproducibility.
Author contributions statement
H.D.L. designed the study and drafted the manuscript, Z.Z. and Y.F.L. analyzed and interpreted data, E.M., Q.H.L, and X.Y.W. participated in sample collection, Z.Z., S.W.W., T.L., Q.H.L., Y.F.L., W.L., S.J.H., S.G., M.Y., Y.Y.Q., Z.H.S., H.Y.W., L.Q.D. and T.T.G. conducted experiments, H.X.B. and Y.J.Z. contributed reagents/ materials/analysis tools. R.N.J contributed reagents/materials/analysis tools and supervised H.D.L. as a co-supervisor at University of Calgary. R.N.J. and S.L.L. revised this article. S.L.L. finalized the manuscript. All authors read and approved the final manuscript.
Competing interests
There are no competing interest concerns with any of the authors.
Acknowledgments
The authors thank all the colleagues of Genomics Research Center for comments on earlier versions of this manuscript. This work was supported by grants of the National Natural Science Foundation of China (NSFC30970119, 81030029, 81271786, NSFC-NIH 81161120416, 81671980) and College Students' Innovation & Entrepreneurship project in Heilongjiang Province (201410226047 J.J.K, D.Y.; 201510226020 D.S.L, L.Y., T.L., L.Q., L.L.G.; 201610226095 H.Y.W., Z.H.S. T.T.G., S.J.H., S.G.; 201610226094 Y.Y.Q., M.Y.). H.D.L. is supported by a scholarship from China Scholarship Council, CSC No. 201508230143, for an academic visit to University of Calgary (Univ. of Calgary ID number: 30016355). Health and Family Planning Commission of Heilongjiang Province (2016–188). The Fundamental Research Funds for the Provincial Universities (2017JCZX57). Sincerely gratitude to Tegen Etosha Dunnill Jones for revising this article. The authors thank all the colleagues of Genomics Research Center for comments on earlier versions of this manuscript. Sincerely gratitude to the Open Access Authors Fund by University of Calgary to cover the article-processing charges and Arnie Charbonneau Cancer Institute provided laboratory and experimental equipments.
Funding
China Scholarship Council (CSC). [grant number 201508230143], The Fundamental Research Funds for the Provincial Universities (grant number 2017JCZX57). National Natural Science Foundation of China (NSFC). [grant numbers NSFC30970119, 81030029, 81271786].
References
- Kanis MJ, Kolev V, Getrajdman J, Zakashansky K, Cohen C, Rahaman J. Carcinosarcoma of the ovary: a single institution experience and review of the literature. Eur J Gynaecol Oncol. 2016;37(1):75–9.
- Siegel RL, Miller KD, and Jemal A, Cancer statistics, 2016. Ca A Cancer Journal for Clinicians. 2016;66(1):10–29. doi:10.3322/caac.21332.
- Schummer M, Drescher C, Forrest R, Gough S, Thorpe J, Hellström I, Hellström KE, Urban N. Evaluation of ovarian cancer remission markers HE4, MMP7 and Mesothelin by comparison to the established marker CA125. Gynecologic Oncology. 2012;125(1):65–9. doi:10.1016/j.ygyno.2011.11.050.
- Sung PL, Chang YH, Chao KC, Chuang CM; Task Force on Systematic Review and Meta-analysis of Ovarian Cancer. Global distribution pattern of histological subtypes of epithelial ovarian cancer: a database analysis and systematic review. Gynecol Oncol. 2014;133(2):147–54. doi:10.1016/j.ygyno.2014.02.016.
- Cetin I, Cozzi V, and Antonazzo P, Infertility as a cancer risk factor − a review. Placenta. 2008;29 Suppl B:169–77. doi:10.1016/j.placenta.2008.08.007.
- Hung LJ, Chan TF, Wu CH, Chiu HF, Yang CY. Traffic air pollution and risk of death from ovarian cancer in Taiwan: fine particulate matter (PM2.5) as a proxy marker. J Toxicol Environ Health A. 2012;75(3):174–82. doi:10.1080/15287394.2012.641200.
- Hannibal CG, Vang R, Junge J, Frederiksen K, Kurman RJ, Kjaer SK. A nationwide study of ovarian serous borderline tumors in Denmark 1978−2002. Risk of recurrence, and development of ovarian serous carcinoma. Gynecol Oncol. 2017;114(1):147–80. doi:10.1016/j.ygyno.2017.11.007.
- Weidle UH, Birzele F, Kollmorgen G, Rueger R. Mechanisms and Targets Involved in Dissemination of Ovarian Cancer. Cancer Genomics Proteomics. 2016;13(6):407–423. doi:10.21873/cgp.20004.
- Monk BJ, Herzog TJ, and Tewari KS, Evolution of Chemosensitivity and Resistance Assays as Predictors of Clinical Outcomes in Epithelial Ovarian Cancer Patients. Curr Pharm Des. 2016;22(30):4717–4728. doi:10.2174/1381612822666160505114326.
- Zhang DM, Xu HG, Wang L, Li YJ, Sun PH, Wu XM, Wang GJ, Chen WM, Ye WC. Betulinic Acid and its Derivatives as Potential Antitumor Agents. Medicinal research reviews. 2015;35(6):1127–55. doi:10.1002/med.21353. Epub 2015 Jun 2.
- Hao J, Zhu C, Cao R, Yang X, Ding X, Man Y, Wu X. [Purple-bluish tongue is associated with platelet counts, and the recurrence of epithelial ovarian cancer]. J Tradit Chin Med. 2016;36(3):321–5. doi:10.1016/S0254-6272(16)30044-9.
- Ai B, Bie Z, Zhang S, Li A. Paclitaxel targets VEGF-mediated angiogenesis in ovarian cancer treatment. Am J Cancer Res. 2016;6(8):1624–35.
- Verma RP and Hansch C, Matrix metalloproteinases (MMPs): Chemical–biological functions and (Q)SARs. Bioorganic & Medicinal Chemistry. 2007;15(6):2223–68. doi:10.1016/j.bmc.2007.01.011.
- Coussens LM and Werb Z, Matrix metal loproteinases and the development of cancer. Chemistry & Biology, 1996;3(11):895–904. doi:10.1016/S1074-5521(96)90178-7.
- Cheung LWT, Leung PCK, and Wong AST, Gonadotropin-Releasing Hormone Promotes Ovarian Cancer Cell Invasiveness through c-Jun NH2-Terminal Kinase–Mediated Activation of Matrix Metalloproteinase (MMP)-2 and MMP-9. Cancer Res. 2006;66(22):10902–10. doi:10.1158/0008-5472.CAN-06-2217.
- Lu YM, Rong ML, Shang C, Wang N, Li X, Zhao YY, Zhang SL. Suppression of HER-2 via siRNA interference promotes apoptosis and decreases metastatic potential of SKOV-3 human ovarian carcinoma cells. Oncology Reports. 2012;29(3):1133–9. doi:10.3892/or.2012.2214.
- Yu Y, Li H, Xue B, Jiang X, Huang K, Ge J, Zhang H, Chen B. SDF-1/CXCR7 axis enhances ovarian cancer cell invasion by MMP-9 expression through p38 MAPK pathway. Dna & Cell Biology. 2014;33(8):543–9. doi:10.1089/dna.2013.2289.
- Spilmont M, Léotoing L, Davicco MJ, Lebecque P, Mercier S, Miot-Noirault E, Pilet P, Rios L, Wittrant Y, Coxam V. Pomegranate and its derivatives can improve bone health through decreased inflammation and oxidative stress in an animal model of postmenopausal osteoporosis. Eur J Nutr. 2014;53(5):1155–64. doi:10.1007/s00394-013-0615-6.
- Costantini S, Rusolo F, De Vito V MS, Picariello G, Capone F, Guerriero E, Castello G, Volpe MG. Potential anti-inflammatory effects of the hydrophilic fraction of pomegranate (Punica granatum L.) seed oil on breast cancer cell lines. Molecules. 2014;19(6):8644–60. doi:10.3390/molecules19068644.
- Faria A, Calhau C, The bioactivity of pomegranate: impact on health and disease. Critical Reviews in Food Science & Nutrition. 2011;51(51):626–34. doi:10.1080/10408391003748100.
- Amin AR, Kucuk O, Khuri FR, Shin DM. Perspectives for cancer prevention with natural compounds. Journal of Clinical Oncology. 2009;27(16):2712–25. doi:10.1200/JCO.2008.20.6235.
- Syed DN, Afaq F, Mukhtar H, Pomegranate derived products for cancer chemoprevention. Seminars in Cancer Biology. 2007;17(5):377–85. doi:10.1016/j.semcancer.2007.05.004.
- Jurenka JS, Therapeutic applications of pomegranate (Punica granatum L.): a review. Alternative Medicine Review A Journal of Clinical Therapeutic. 2008;13(2):128–44.
- Seeram NP, Adams LS, Henning SM, Niu Y, Zhang Y, Nair MG, Heber D. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. Journal of Nutritional Biochemistry. 2005;16(6):360–7. doi:10.1016/j.jnutbio.2005.01.006.
- Turrini E, Ferruzzi L, and Fimognari C, Potential Effects of Pomegranate Polyphenols in Cancer Prevention and Therapy. Oxidative Medicine & Cellular Longevity. 2015;2015:1–19. doi:10.1155/2015/938475.
- Lópezlázaro M, Distribution and biological activities of the flavonoid luteolin. Mini Reviews in Medicinal Chemistry. 2009;9(1):31–59. doi:10.2174/138955709787001712.
- Amrutha K, Nanjan P, Shaji SK, Sunilkumar D, Subhalakshmi K, Rajakrishna L, Banerji A. Discovery of lesser known flavones as inhibitors of NF-κB signaling in MDA-MB-231 breast cancer cells—A SAR study. Bioorganic & Medicinal Chemistry Letters. 2014;24(19):4735–4742. doi:10.1016/j.bmcl.2014.07.093.
- Pandurangan AK, Dharmalingam P, Sadagopan SK, Ganapasam S. Luteolin inhibits matrix metalloproteinase 9 and 2 in azoxymethane-induced colon carcinogenesis. Human & Experimental Toxicology. 2014;33(11):1176–85. doi:10.1177/0960327114522502.
- Seeram NP, Henning SM, Zhang Y, Suchard M, Li Z, Heber D. Pomegranate juice ellagitannin metabolites are present in human plasma and some persist in urine for up to 48 hours. Journal of Nutrition. 2006;136(10):2481–5.
- Gil MI, Tomás-Barberán FA, Hess-Pierce B, Holcroft DM, Kader AA. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. Journal of Agricultural & Food Chemistry. 2000;48(10):4581–9. doi:10.1021/jf000404a.
- Mehta R and Lansky EP, Breast cancer chemopreventive properties of pomegranate (Punica granatum) fruit extracts in a mouse mammary organ culture. European Journal of Cancer Prevention. 2004;13(4):345–348. doi:10.1097/01.cej.0000136571.70998.5a.
- Tsuda H, Uehara N, Iwahori Y, Asamoto M, Iigo M, Nagao M, Matsumoto K, Ito M, Hirono I. Chemopreventive effects of beta-carotene, alpha-tocopherol and five naturally occurring antioxidants on initiation of hepatocarcinogenesis by 2-amino-3-methylimidazo[4,5-f]quinoline in the rat. Jpn J Cancer Res. 1994;85(12):1214–9. doi:10.1111/j.1349-7006.1994.tb02932.x.
- Tharappel JC, Lehmler HJ, Srinivasan C, Robertson LW, Spear BT, Glauert HP. Effect of antioxidant phytochemicals on the hepatic tumor promoting activity of 3,3′,4,4′-tetrachlorobiphenyl (PCB-77). Food Chem Toxicol. 2008;46(11):3467–74. doi:10.1016/j.fct.2008.08.023.
- Shirode AB, Kovvuru P, Chittur SV, Henning SM, Heber D, Reliene R. Antiproliferative effects of pomegranate extract in MCF-7 breast cancer cells are associated with reduced DNA repair gene expression and induction of double strand breaks. Mol Carcinog. 2014;53(6):458–70. doi:10.1002/mc.21995.
- Wang L and Martins-Green. M, Pomegranate and its components as alternative treatment for prostate cancer. Int J Mol Sci. 2014;15(9):14949–66. doi:10.3390/ijms150914949.
- Jaganathan SK, Vellayappan MV, Narasimhan G, Supriyanto E. Role of pomegranate and citrus fruit juices in colon cancer prevention. World J Gastroenterol. 2014;20(16):4618–25. doi:10.3748/wjg.v20.i16.4618.
- Mieszalo K, Lawicki S, and Szmitkowski M, [The utility of metalloproteinases (MMPs) and their inhibitors (TIMPs) in diagnostics of gynecological malignancies]. Pol Merkur Lekarski. 2016;40(237):193–7.
- Turrini E, Ferruzzi L, and Fimognari C, Potential Effects of Pomegranate Polyphenols in Cancer Prevention and Therapy. Oxidative Medicine & Cellular Longevity. 2014;2015:1–19. doi:10.1155/2015/938475.
- Nair V, Dai Z, Khan M, Ciolino HP. Pomegranate extract induces cell cycle arrest and alters cellular phenotype of human pancreatic cancer cells. Anticancer Research. 2011;31(9):2699–704.
- Huang ST, Wang CY, Yang RC, Wu HT, Yang SH, Cheng YC, Pang JH. Ellagic Acid, the Active Compound of Phyllanthus urinaria, Exerts In Vivo Anti-Angiogenic Effect and Inhibits MMP-2 Activity. Evidence-based complementary and alternative medicine: eCAM. 2011;2011(5):296–297.
- Dammer EB, Göttle M, Duong DM, Hanfelt J, Seyfried NT, Jinnah HA. Consequences of impaired purine recycling on the proteome in a cellular model of Lesch-Nyhan disease. Mol Genet Metab. 2015;114(4):570–9. doi:10.1016/j.ymgme.2015.02.007.
- Zhu TX, Lan B, Meng LY, Yang YL, Li RX, Li EM, Zheng SY, Xu LY. ECM-related gene expression profile in vascular smooth muscle cells from human saphenous vein and internal thoracic artery. J Cardiothorac Surg. 2013;8:155. doi:10.1186/1749-8090-8-155.
- Chung YC, Lu LC, Tsai MH, Chen YJ, Chen YY, Yao SP, Hsu CP. The inhibitory effect of ellagic Acid on cell growth of ovarian carcinoma cells. Evidence-based Complementary and Alternative Medicine. 2013;2013(2):386–386.
- Amakura Y, Okada M, Tsuji S, Tonogai Y. High-performance liquid chromatographic determination with photodiode array detection of ellagic acid in fresh and processed fruits. Journal of Chromatography A. 2000;896(1–2):87–93. doi:10.1016/S0021-9673(00)00414-3.
- Miean KH and Mohamed S, Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. Journal of Agricultural & Food Chemistry. 2001;49(6):3106–12. doi:10.1021/jf000892m.
- Langers AM, Verspaget HW, Hawinkels LJ, Kubben FJ, van Duijn W, van der Reijden JJ, Hardwick JC, Hommes DW, Sier CF. MMP-2 and MMP-9 in normal mucosa are independently associated with outcome of colorectal cancer patients. Br J Cancer. 2012;106(9):1495–8. doi:10.1038/bjc.2012.80.
