Abstract
The abscopal effect is defined as the clearance of distant tumors after applying localized irradiation to a particular tumor site. It has been proposed that a mechanism for the abscopal effect might be the activation of the immune system, which leads to immunogenic tumor cell death. Here, we describe a woman with advanced breast cancer that received modified ablative radiation therapy that targeted her primary breast tumor. She experienced an apparent regression of metastatic mass in the thoracic spine. This case supported the hypothesis that the abscopal effect might be attributable to an activation of the systemic immune response.
Introduction
Radiation therapy (RT) can induce a phenomenon known as an abscopal effect. This effect is observed in the treatment of metastatic cancer, where a distant non-irradiated tumor regresses after localized radiation therapy is delivered to a particular tumor site. The mechanisms underlying the regulation of abscopal events remain unclear. Recent evidence has suggested that the immune system is a major process in mediating the abscopal effect.Citation1 RT is thought to induce DNA damage and immunogenic cell death, which can lead to dendritic-cell activation in the tumor microenvironment. Then, these dendritic cells cross-present the tumor antigens to prime a tumor-specific T cell response.Citation2,Citation3
Accumulating evidence has shown that high radiation dose with few fractions, such as stereotactic ablative body radiotherapy (SABR), could induce anti-tumor immunity,Citation3,Citation4 modify tumor phenotypes, and change the tumor microenvironment.Citation4–7 Therefore, SABR may produce a beneficial outcome superior to conventional RT fractionation. With the continued development of new radiation technology, SABR is increasingly used in a variety of malignancies and at a variety of sites, including renal cell carcinoma, melanoma, lung cancer, and hepatocellular carcinoma.Citation8–15 Only two studies conducted in Japan reported an abscopal effect. The effect was observed in metastatic lymph nodes and infiltrative lymphocytes after applying a few fractions of large- dose irradiation to treat breast carcinoma.Citation16–17 Here, we describe a patient with stage IV, invasive ductal breast carcinoma. This patient may have benefitted from an abscopal effect of multiple fractions of high radiation dose delivered locally and precisely targeted the breast tumor and caused limited damage to the surrounding normal tissue.
Case presentation
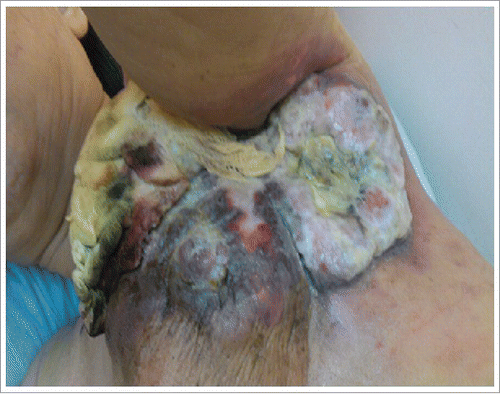
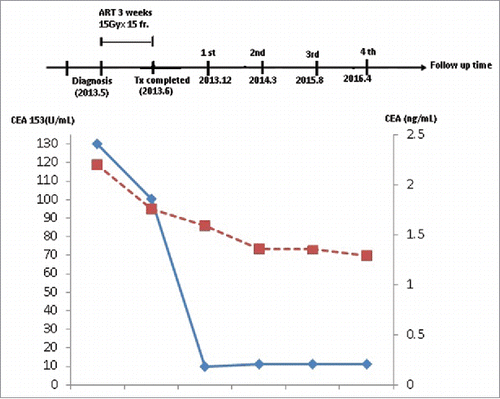
A 65-year-old woman presented with a necrotic mass on her right breast, which bled and produced an exudate with an offensive smell. The mass on the breast was first noticed 3 years prior to the examination. She refused any conventional treatment, including surgery, chemotherapy, or hormone therapy for breast cancer with an unknown cause. She visited our outpatient clinic of Radiation Oncology in May 2012. She complained of exaggerated bleeding from her right breast, an exudate with an offensive smell, and severe pain. On physical examination, an ulcerated, bleeding, necrotic mass was noted in the upper outer quadrant of the right breast (). A laboratory examination on patient's blood sample revealed a low hemoglobin concentration (8.3 mg/dL). Tumor markers, CEA and CEA-125, were within normal ranges (CEA <3 ng/mL); but the CA 153 level was high, at 130.2 U/mL (normal range <38 U/mL). Computed tomography (CT) of the breast showed a large, fungating mass, about 16 cm in diameter, over the left anterior chest wall and axilla. A whole body bone scan showed a metastasis in the 8th thoracic vertebra (A). The tumor was diagnosed as a estrogen receptor(ER+), HER-2/neu-positive, progesterone-negative, cT4N3bM1, stage IV, invasive ductal carcinoma, with metastases to the 8th thoracic vertebra and axillary lymph nodes.
Figure 2. (A) Bone scan before palliative radiation. (B) Bone scan after 2 years, a resolution of bone lesion.
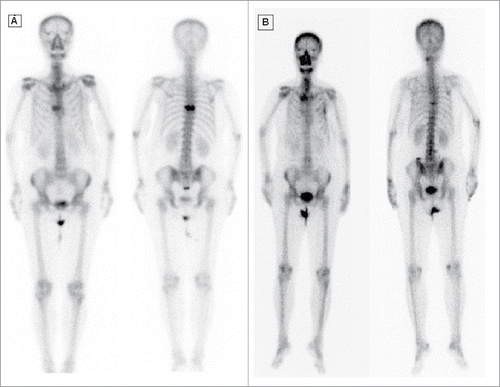
Based on data from previous animal and clinical studies,Citation18–20 we developed an ablative RT with an electron beam. The shaped electron beams were delivered precisely and conformally; they targeted the local breast tumor and the involved axillary lymph nodes. A total dose of 225 Gy was delivered to the clinically involved areas in 15 fractions (15 Gy/fraction) with minimal damage to normal tissues or organs at risk. The fractions were delivered over 3 weeks, and RT was completed in June 2013. Next, based on published literature and our clinical experience, we prescribed a 50 Gy dose, delivered in 25 fractions (2 Gy/ fraction) to the metastatic thoracic bone lesion, as palliative RT.Citation21–23 After 2 years, a follow-up whole body bone scan showed resolution of the bone metastatic lesion ().
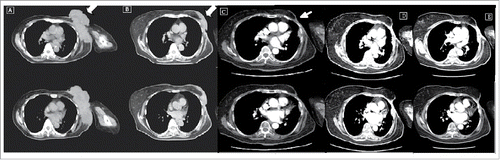
After initiation of a personalized ablative RT, the follow-up breast CT images at 3 months, 12 months, and 4 years showed a remarkable shrinkage and nearly complete regression of the breast tumor (-). Additionally, a remarkable reduction in the axillary lymph nodes was also noted. From December 2013 to present, the patient has reported feeling very well, and no further tumor recurrence was reported. Laboratory findings showed normal levels of tumor markers. Continuous checking showed that the CA15-3(EIA) and CEA levels decreased to the normal range (). No abnormal finding was observed in routine follow-up with abdominal ultrasound and CT. We also acquired a biopsy at the local radiation site in 2015 that confirmed no recurrence. The biopsy report indicated no sign of tumor cells, except fibrosis. The patient continues to survive at present, she has returned to work, and reports a good quality of life.
Figure 3. The axial computed tomography of the upper chest and axilla.(A) before radiotherapy; (B) Three months after radiosurgery, shrinkage of the local breast tumor (C) CT image after 1 year, nearly complete regression of locally breast tumor (D) CT image after 4 years, complete remission of the local breast tumor, we supposed that an abscopal effect had occurred. (E) Follow-up CT after 5 years confirmed a complete remission and the biopsy result showed fibrosis at the local tumor site.
Discussion
The patient of this study was the first to receive multiple fractions of high radiation dose that directly targeted a local breast tumor with minimal damage to normal tissues or organs at risk. This technique of delivering multiple fractions of high radiation dose was designed for a particular patient that refused all standard treatments. We have termed this technique “modified ablative RT”; it resembles radiosurgery, except that we delivered more than 5 dose fractions.
The mechanisms underlying the abscopal effect of RT have not been clearly understood. Some proposed mechanisms include immune-mediated tumor regression, cytokine effects, tumor growth inhibition, or tumoricidal effects.Citation24–27 The immunological activation and the response against non-irradiated malignant lesions is in part due to the release of tumor antigens in the tumor microenvironment and the release of inflammatory factors induced by irradiation at the target tumor site.Citation28–30 Another mechanism proposed was that radiation-elicited immunogenic cell death acts synergistically with other biological effects. Many proposed mechanisms have been supported by animal studies.Citation30–35 Other proposed mechanisms include the promotion of T-cell recruitment and function associated with irradiation, and the involvement of other mediators, such as TGF-β antagonists or p53.Citation36–40 More recently, several clinical trials have tested immune check point inhibitors, such as anti CTLA-4, anti PD-1, and anti PD-L1/L2 agents in a variety of tumor types with applicable results.Citation41–42 Some studies have suggested that a dual immune checkpoint blockade combined with RT might elicit an optimal abscopal response.Citation43–44
The tumor immunity induced by RT may be influenced by the radiation dose and fractionation. High-dose radiation has greater capacity than low-dose radiation for inducing host anti-cancer immune defenses.Citation45 Recently, with the advanced technology of online image guidance systems and complex planning techniques, the intensify radiotherapy can now be delivered precisely in a small number of high (5–30 Gy) individual doses to a targeted tumor volume to minimize the local recurrence.Citation44–47 Clinical studies have indicated that conventional fractions (1.8 or 2.0 Gy/fraction) or hypofractionated (3 × 9.5 Gy, 5 × 6 Gy) RT could induce host anti-cancer immune defenses to strengthen the systemic efficacy of immunotherapeutic strategies for treating different cancers.Citation48–52 Despite increasing evidence in support of the hypothesis that an abscopal effect arises from the initiation of an immune-mediated process triggered by high-dose radiation, several clinical trials investigating the combination of immunotherapy and RT in breast cancer patients are actively ongoing.Citation53 This approach represents a promising option for cancer treatment in clinical practice.
Conclusions
We described a rare abscopal effect, where metastasis was ameliorated at a site distant from the primary site of tumor irradiation. We postulate that a strong immunogenic response and subsequent abscopal regression in the remaining site of disease may due to the substained inflammatory response in this case.
Author contributions
AC conceived the study and drafted the manuscript; HL participated in the study design and interpretation. All authors read and approved the final manuscript.
Conflict of interest
The authors declare no conflict of interest related to this study
Sponsorship
No funding or grant supported this study
Authors' submitted files for original images
Below are the links to the authors’ submitted files for the original images
Ethics approval and consent to participate
This work did not require any written patient consent.
Consent for publication
Not applicable.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article.
Acknowledgments
We would like to thank the patient for her willingness to share her case for this study.
Funding
This work did not receive any specific funding.
References
- Grass GD, Krishna N, Kim S. The immune mechanisms of abscopal effect in radiation therapy. Curr Probl Cancer. 2016;40(1):10–24. doi: 10.1016/j.currproblcancer.2015.10.003.
- Spiotto M, Fu YX, Weichselbaum RR. The intersection of radiotherapy and immunotherapy: Mechanisms and clinical implications. Sci Immunol. 2016;1(3). pii: EAAG1266. doi: 10.1126/sciimmunol.aag1266.
- Sologuren I, Rodríguez-Gallego C, Lara PC. Immune effects of high dose radiation treatment: Implications of ionizing radiation on the development of bystander and abscopal effects. Translational Cancer Research. 2014;3(1):18–31.
- Kaur P, Asea A. Radiation-induced effects and the immune system in cancer. Front Oncol. 2012;2:191. doi: 10.3389/fonc.2012.00191
- Deloch L, Derer A, Hartmann J, Frey B, Fietkau R, Gaipl US. Modern radiotherapy concepts and the impact of radiation on immune activation. Front Oncol. 2016 20;6:141. doi: 10.3389/fonc.2016.00141
- Golden EB, Apetoh L. Radiotherapy and immunogenic cell death. Semin Radiat Oncol. 2015;25(1):11–7. doi: 10.1016/j.semradonc.2014.07.005.
- Gaipl US, Multhoff G, Scheithauer H, Lauber K, Hehlgans S, Frey B, Rödel F. Kill and spread the word: Stimulation of antitumor immune responses in the context of radiotherapy. Immunotherapy. 2014;6(5):597–610. doi: 10.2217/imt.14.38.
- Abuodeh Y, Venkat P, Kim S. Systematic review of case reports on the abscopal effect. Curr Probl Cancer. 2016;40(1):25–37. doi: 10.1016/j.currproblcancer.2015.10.001.
- Siva S, MacManus MP, Martin RF, Martin OA. Abscopal effects of radiation therapy: A clinical review for the radiobiologist. Cancer Lett. 2015;356(1):82–90. doi: 10.1016/j.canlet.2013.09.018
- K. Okuma, H. Yamashita, Y. Niibe, K. Hayakawa, K. Nakagawa. Abscopal effect of radiation on lung metastases of hepatocellular carcinoma: A case report. J Med. Case Reports. 2011;5:111. doi: 10.1186/1752-1947-5-111.
- Cotter SE, Dunn GP, Collins KM, Sahni D, Zukotynski KA, Hansen JL, O'Farrell DA, Ng AK, Devlin PM, Wang LC. Abscopal effect in a patient with metastatic merkel cell carcinoma following radiation therapy: Potential role of induced antitumor immunity. Arch. Dermatol. 2011;147:870–2. doi: 10.1001/archdermatol.2011.176.
- J. Brock, S. Ashley, J. Bedford, E. Nioutsikou, M. Partridge, M. Brada. Review of hypofractionated small volume radiotherapy for early-stage non-small cell lung cancer. Clin Oncol (R Coll Radiol). 2008 Nov;20(9):666–76. doi: 10.1016/j.clon.2008.06.005.
- Fuss M1, Thomas CR Jr. Stereotactic body radiation therapy: An ablative treatment option for primary and secondary liver tumors. Ann Surg Oncol. 2004;11(2):130–8.
- Siva S, MacManus M, Ball D. Stereotactic radiotherapy for pulmonary oligometastases: A systematic review. J Thorac Oncol. 2010;5(7):1091–9. doi: 10.1097/JTO.0b013e3181de7143.
- Wersäll PJ, Blomgren H, Pisa P, Lax I, Kälkner KM, Svedman C. Regression of non-irradiated metastases after extracranial stereotactic radiotherapy in metastatic renal cell carcinoma. Acta Oncol. 2006;45(4):493–7.
- Konoeda K. Therapeutic efficacy of pre-operative radiotherapy on breast carcinoma: In special reference to its abscopal effect on metastatic lymph-nodes]. Nihon Gan Chiryo Gakkai Shi. 1990;25(6):1204–14.
- Mikuriya S, Saito T, Konoeda K, Matsuba T, Torii A, Sato O, Adachi H, Yokohari R, Yamada K, Asano T, Matsumura K, Seto T, Takatani O. Evaluation of abscopal effect observed in advanced cancer of breast treated with preoperative radiotherapy (author's transl). Nihon Gan Chiryo Gakkai Shi. 1979;14(6):997–1008.
- Lee Y, Auh SL, Wang Y, Burnette B, Wang Y, Meng Y, Beckett M, Sharma R, Chin R, Tu T, Weichselbaum RR, Fu YX. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: Changing strategies for cancer treatment. Blood. 2009;114(3):589–95. doi: 10.1182/blood-2009-02-206870.
- Arthur DW, Schmidt-Ullrich RK, Friedman RB, Wazer DE, Kachnic LA, Amir C, Bear HD, Hackney MH, Smith TJ, Lawrence W Jr. Accelerated superfractionated radiotherapy for inflammatory breast carcinoma: Complete response predicts outcome and allows for breast conservation. Int J Radiat Oncol Biol Phys. 1999;44(2):289–96.
- Brown L, Harmsen W, Blanchard M, Goetz M, Jakub J, Mutter R, Petersen I, Rooney J, Stauder M, Yan E, Laack N. Once-daily radiation therapy for inflammatory breast cancer. Int J Radiat Oncol Biol Phys. 2014;89(5):997–1003. doi: 10.1016/j.ijrobp.2014.01.054.
- Majumder D, Patra NB, Chatterjee D, Mallick SK, Kabasi AK, Majumder A. Prescribed dose versus calculated dose of spinal cord in standard head and neck irradiation assessed by 3-D plan. South Asian J Cancer. 2014;3(1):22–7. doi: 10.4103/2278-330X.126510.
- Yamada S, Hoshi A, Takai Y, Hoshino F, Mochizuki Y, Kanehira C, Kaneta K. Radiation tolerance dose of the spinal cord following conventionally fractionated irradiation. Gan No Rinsho. 1987;33(10):1189–92.
- Lo SS, Chiang EL. Clinical spinal cord tolerance to radiosurgery. Radiology key. [accessed 2017 May 30]. https://radiologykey.com/clinical-spinal-cord-tolerance-to-radiosurgery.
- Ishiyama H, Teh BS, Ren H, Chiang S, Tann A, Blanco AI, Paulino AC, Amato R. Spontaneous regression of thoracic metastases while progression of brain metastases after stereotactic radiosurgery and stereotactic body radiotherapy for metastatic renal cell carcinoma: Abscopal effect prevented by the blood—brain barrier? Clin Genitourin Cancer. 2012;10(3):196–8. doi: 10.1016/j.clgc.2012.01.004.
- Demaria S, Formenti SC. Radiation as an immunological adjuvant: Current evidence on dose and fractionation. Front Oncol. 2012;2:153. doi: 10.3389/fonc.2012.00153.
- Kaminski JM, Shinohara E, Summers JB, Niermann KJ, Morimoto A, Brousal J. The controversial abscopal effect. Cancer Treat Rev. 2005;31:159–72.
- Rastogi S, Coates PJ, Lorimore SA, Wright EG. Bystander-type effects mediated by long-lived inflammatory signaling in irradiated bone marrow. Radiat Res. 2012;177:244–50.
- Demaria S1, Ng B, Devitt ML, Babb JS, Kawashima N, Liebes L, Formenti SC. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys 2004;58:862–70.
- Shiraishi K, Ishiwata Y, Nakagawa K, Yokochi S, Taruki C, Akuta T, Ohtomo K, Matsushima K, Tamatani T, Kanegasaki S. Enhancement of antitumor radiation efficacy and consistent induction of the abscopal effect in mice by ECI301, an active variant of macrophage inflammatory protein-1alpha. Clin Cancer Res. 2008;14:1159–66.
- Kaur P, Asea A. Radiation-induced effects and the immune system in cancer. Front Oncol 2012;2:191. doi: 10.3389/fonc.2012.00191.
- Van der Meeren AMP, Vandamme M, Squiban C, Wysocki J, Griffiths N. Abdominal radiation exposure elicits inflammatory responses and abscopal effects in the lungs of mice. Radiat Res. 2005;163:144–52.
- Aravindan S, Natarajan M, Ramraj SK, Pandian V, Khan FH, Herman TS, Aravindan N. Abscopal effect of low-LET gamma-radiation mediated through Rel protein signal transduction in a mouse model of nontargeted radiation response. Cancer Gene Ther. 2014;21:54–9. doi: 10.1038/cgt.2013.72.
- Hodge JW, Sharp HJ, Gameiro SR. Abscopal regression of antigen disparate tumors by antigen cascade after systemic tumor vaccination in combination with local tumor radiation. Cancer Biother Radiopharm. 2012;27:12–22. doi: 10.1089/cbr.2012.1202.
- Yasuda K, Nirei T, Tsuno NH, Nagawa H, Kitayama J. Intratumoral injection of interleukin-2 augments the local and abscopal effects of radiotherapy in murine rectal cancer. Cancer Sci. 2011;102:1257–63. doi: 10.1111/j.1349-7006.2011.01940
- Reynders K, Illidge T, Siva S, Chang JY, De Ruysscher D. The abscopal effect of local radiotherapy: Using immunotherapy to make a rare event clinically relevant. Cancer Treat Review. 2015;41(6):503–10. doi: 10.1016/j.ctrv.2015.03.011.
- Demaria S, Formenti SC. Role of T lymphocytes in tumor response to radiotherapy. Front Oncol. 2012;2:95. doi: 10.3389/fonc.2012.00095.
- Vanpouille-Box C, Diamond JM, Pilones KA, Zavadil J, Babb JS, Formenti SC, Barcellos-Hoff MH, Demaria S. TGFβ Is a master regulator of radiation therapy-induced a ntitumor immunity. Cancer Res. 2015;75:2232–42. doi: 10.1158/0008-5472.
- Camphausen K, Moses MA, Ménard C, Sproull M, Beecken WD, Folkman J, O'Reilly MS. Radiation abscopal antitumor effect is mediated through p53. Cancer Res. 2003;63(8):1990–93.
- Lorimore SA, Rastogi S, Mukherjee D, Coates PJ, Wright EG. The influence of p53 functions on radiation-induced inflammatory bystander-type signaling in murine bone marrow. Radiat Res. 2013;179:406–15. doi: 10.1667/RR3158.2.
- Strigari L, Mancuso M, Ubertini V, Soriani A, Giardullo P, Benassi M, D'Alessio D, Leonardi S, Soddu S, Bossi G. Abscopal effect of radiation therapy: Interplay between radiation dose and p53 status. Int J Radiat Biol. 2014;90(3):248–55. doi: 10.3109/09553002.2014.874608.
- Levy A, Massard C, Soria JC, Deutsch E. Concurrent irradiation with the anti-programmed cell death ligand-1 immune checkpoint blocker durvalumab: Single centre subset analysis from a phase 1/2 trial. Eur J Cancer. 2016;68:156–162. doi: 10.1016/j.ejca.2016.09.013.
- Ribeiro Gomes J, Schmerling RA, Haddad CK, Racy DJ, Ferrigno R, Gil E, Zanuncio P, Buzaid AC. Analysis of the abscopal effect with Anti-PD1 therapy in patients with metastatic solid tumors. J Immunother. 2016;39(9):367–372.
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012 Mar 22;12(4):252–64. doi: 10.1038/nrc3239.
- Ng J, Dai T. Radiation therapy and the abscopal effect: A concept comes of age. Ann Transl Med. 2016 Mar;4(6):118. doi: 10.21037/atm.2016.01.32.
- Haikerwal SJ, Hagekyriakou J, MacManus M, Martin OA, Haynes NM. Building immunity to cancer with radiation therapy. Cancer Lett. 2015;368(2):198–208. doi: 10.1016/j.canlet.2015.01.009.
- Glide-Hurst CK, Chetty IJ1.C.K. Improving radiotherapy planning, delivery accuracy, and normal tissue sparing using cutting edge technologies. J Thorac Dis. 2014;6(4):303–18. doi: 10.3978/j.issn.2072-1439.2013.11.10.
- Brown JM, Carlson DJ, Brenner DJ. The tumor radiobiology of SRS and SBRT: Are more than the 5 Rs involved? Int J Radiat Oncol Biol Phys. 2014 Feb 1;88(2):254–62. doi: 10.1016/j.ijrobp.2013.07.022.
- Deloch L, Derer A, Hartmann J, Frey B, Fietkau R, Gaipl US. Modern radiotherapy concepts and the impact of radiation on immune activation. Front Oncol. 2016;6:141. doi: 10.3389/fonc.2016.00141
- Brody JD, Ai WZ, Czerwinski DK, Torchia JA, Levy M, Advani RH, Kim YH, Hoppe RT, Knox SJ, Shin LK. In situ vaccination with a TLR9 agonist induces systemic lymphoma regression:a phase I/II study. J. Clin. Oncol. 28 (2010) 4324–4332. J Clin Oncol. 2010;28(28):4324–32. doi: 10.1200/JCO.2010.28.9793
- Gulley JL, Arlen PM, Bastian A, Morin S, Marte J, Beetham P, Tsang KY, Yokokawa J, Hodge JW, Ménard C, et al. Combining a recombinant cancer vaccine with standard definitive radiotherapy in patients with localized prostate cancer. Clin Cancer Res. 2005;11(9):3353–62.
- Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, Mu Z, Rasalan T, Adamow M, Ritter E, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012 Mar 8;366(10):925–31. doi: 10.1056/NEJMoa1112824.
- Golden EB, Demaria S, Schiff PB, Chachoua A, Formenti SC. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer, Cancer Immunol. Cancer Immunol Res. 2013;1(6):365–72. doi: 10.1158/2326-6066.CIR-13-0115.
- Hu ZI, McArthur HL, Ho AY. The Abscopal Effect of Radiation Therapy: What is it and how can we use it in breast cancer? Curr Breast Cancer Rep. 2017;9(1):45–51. doi: 10.1007/s12609-017-0234-y.