ABSTRACT
Cytotoxic chemotherapy can induce a systemic inflammatory response which has been proposed to be an underlying mechanism of cancer treatment related fatigue. Dexamethasone, a synthetic glucocorticoid that has potent anti-inflammatory effects, is incorporated into chemotherapy regimens to prevent chemotherapy-induced nausea and vomiting (CINV). The purpose of this study was to determine whether by suppressing cytotoxic chemotherapy-induced inflammation, dexamethasone could ameliorate chemotherapy induced fatigue/lethargy in tumor free mice. The effect of dexamethasone (DEX) on Cytoxan-Adriamycin (CA)-induced inflammation was assessed by measuring circulating levels of IL-1β, TNF-α, IL-6, GCSF, KC, and MCP-1 twenty-four-hours post CA injection. Decline in voluntary wheel running activity (VWRA) from baseline (used as a proxy for fatigue/lethargy), body weight and composition, and food intake were monitored in mice administered four cycles of CA every two weeks with or without DEX. CA increased circulating levels of IL-6, GCSF, KC, and MCP-1 and caused a rapid decline in VWRA and body weight immediately following CA-injection. Although the acute CA-induced decline in VWRA and body weight was not evident in mice administered CA + DEX, DEX alone had a suppressive effect on VWRA, and body weight continued to decline in mice administered both CA and DEX while it returned to baseline in CA-treated mice. CA or DEX alone had no long term impact on VWRA but DEX exacerbated lethargy and weight loss in CA-treated mice. Despite dampening the systemic inflammatory response to chemotherapy, dexamethasone failed to ameliorate acute or long term chemotherapy related fatigue/lethargy. Our pre-clinical findings suggest that supportive therapies like dexamethasone used to acutely control nausea and vomiting in cancer patients may actually contribute to overall symptom burden in cancer patients.
| Abbreviations | ||
| BMC | = | bone mineral content |
| BDNF | = | brain derived neurotropic factor |
| CA | = | Cytoxan-Adriamycin |
| CAF | = | Cytoxan-Adriamycin-5-fluorouracil |
| CINV | = | chemotherapy-induced nausea and vomiting |
| CTRS | = | cancer treatment-related symptoms |
| DEX | = | dexamethasone |
| FM | = | fat mass |
| HPA | = | hypothalamic pituitary adrenal |
| LM | = | lean mass |
| LSD | = | least significant difference |
| NS | = | normal saline |
| SSRI | = | selective serotonin reuptake inhibitor |
| VWRA | = | voluntary wheel running activity |
Background
Fatigue is a common symptom experienced by cancer patients undergoing cancer chemotherapy regardless of tumor type or chemotherapy type.Citation1–6 Fatigue can persist after tumor control or treatment cessation.Citation7–9 Fatigue affects physical functioning, quality of life, and intervention adherence.Citation2,3,10 The cause of cancer treatment-related fatigue has not been fully delineated, but it is likely multifactorial and influenced by other treatment related side effects including anemia, skeletal muscle atrophy, cardiopulmonary, hepatic or renal impairment, malnutrition, impaired sleep quality, depression, anxiety, and cognitive dysfunction.Citation11,12 The induction of inflammatory cytokines by cytotoxic chemotherapy is a proposed etiological mechanism of cancer treatment related fatigue and related symptoms.Citation13 Fatigue, or lethargy as it is often referred to in rodents, is a predominant symptom of sickness behavior, a cluster of symptoms that occur following immune challenge that are triggered by the production of the pro-inflammatory cytokines IL-1β and TNF-α. These two cytokines are called initiator cytokines because they induce the production of several other inflammatory cytokines and chemokines including GCSF, IL-6, KC, and MCP-1. This acute inflammatory response activates the hypothalamic pituitary adrenal (HPA) axis resulting in the production of endogenous glucocorticoids: cortisol in humans and corticosterone in mice which have anti-inflammatory properties.Citation14 In rodent models of sickness behavior, an immune challenge such as injection with bacterial endotoxin causes a decrease in locomotor function, used frequently as a proxy for cytokine-induced lethargy, hypophagia, transient weight loss, and behavioral depression.Citation15–19 These behavioral changes coincide with increased production of inflammatory cytokines and chemokines in the peripheral circulation and brain including the hypothalamus, hippocampus, and cerebellum. A similar inflammatory and behavioral response occurs in rodents injected with cytotoxic chemotherapy which led us and others to conclude that the chemotherapy-induced inflammatory response may contribute to the burden of acute cancer treatment-related symptoms (CTRS) in cancer patients.Citation13,20,21
Dexamethasone is a synthetic glucocorticoid and, like all glucocorticoids, has potent anti-inflammatory effects: blocking the synthesis of histamines and prostaglandins in vivo.Citation22,23 and inflammatory cytokines in vitro.Citation24–26 It is prescribed to treat a variety of inflammatory conditions and is effective at reducing inflammation in variety of rodent inflammatory disease models.Citation27–30 Low dose dexamethasone is routinely used to prevent chemotherapy-induced nausea and vomiting (CINV) in cancer patients exposed to highly emetic chemotherapeutic agents. Adriamycin and Cytoxan are commonly used agents used in the adjuvant treatment of early stage breast cancer. When combined, these drugs are highly emetogenic, and as such are administered with dexamethasone and other antiemetic agents.Citation31 Oral dexamethasone (4 mg) is usually administered prior to the start of chemotherapy infusion and then daily for up to 4 d post each chemotherapy infusion.Citation31 Given the potential link between cytotoxic chemotherapy-induced inflammation and treatment related fatigue, it is possible that by dampening chemotherapy-induced inflammation dexamethasone could ameliorate acute treatment related fatigue and other CTRS. Indeed Yennurajalingam and colleagues found that a two-week course of dexamethasone significantly reduced physical fatigue and improved appetite relative to placebo in advanced cancer patients although participants were not in active chemotherapy treatment.Citation32
While beneficial at reducing CINV and potentially fatigue, negative side effects of dexamethasone include insomnia and anxiety both of which are associated with fatigue in disease free breast cancer survivors during and following primary treatment.Citation33–38 Glucocorticoids also have catabolic effects on skeletal muscleCitation39 and it is possible that dexamethasone when used as an antiemetic during multiple cycles of chemotherapy could contribute to weight loss observed in cancer patients undergoing cytotoxic chemotherapy.Citation40–43
The impact that dexamethasone, alone or in combination with cytotoxic chemotherapy, has on fatigue and other CTRS has not been investigated in the clinical setting because dexamethasone is incorporated into chemotherapy regimens as standard clinical care. Thus, the purpose of this observational study was to use a previously established mouse model of breast cancer chemotherapy symptoms to examine the impact of dexamethasone alone or in combination with cytotoxic chemotherapy on inflammatory signaling, fatigue/lethargy, food intake, body weight and composition in tumor free female mice.
Methods
Mice
All animal procedures were performed according to protocols approved by the Institutional Animal Care & Use Committee at Massachusetts General Hospital. Female C57BL/6 mice, 10–12 weeks old (18–21 grams) were purchased from Jackson Laboratories (Cat #000664, Bar Harbor, ME) and unless stated otherwise were group housed in rooms with 12-h light-dark cycle with ad lib access to mouse chow and water. Mice for CTRS assessment were housed singly in a cage fitted with a running wheel (diameter 11.5 cm). All mice had ad lib access to food and drinking water supplemented with antibiotics (1.3 mg/ml sulfamethoxazole and 0.3 mg/ml trimethoprim) to prevent infection related to neutropenia, secondary to chemotherapy-induced bone marrow suppression. Mice were monitored daily for signs of morbidity due to the experimental procedure and were removed from the study if they; 1) lost ≥ 20% of their body weight or developed a body condition score of 1.Citation44 Mice displaying these signs were removed from the study and sacrificed according to protocols established at the MGH Department of Comparative Medicine.
Drug administration in mice
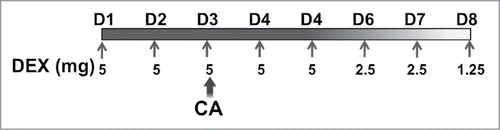
Mice were administered CA, a combination of cyclophosphamide (Cytoxan) and doxorubicin (Adriamycin), at concentrations of 167 mg/kg and 4 mg/kg, respectively. CA was chosen because it is currently a common adjuvant regimen used in the treatment of breast cancer. CA was administered to mice by intraperitoneal (i.p.) injection in a volume of 1 mL of normal saline (NS). Control mice were injected with the same volume of NS without drug. DEX was administered by subcutaneous (s.q.) injection in the flank in a volume of 200 µL NS. Control mice were injected with 200 µL of NS alone. The dosage of DEX and CA injected into mice were based on the human equivalent dose according to the body surface area normalization method.Citation45 In Experiment 2, DEX was administered daily for 8 d (See for treatment outline). Briefly, mice were injected with 5 mg/kg DEX on days one to five, 2.5 mg/kg DEX on D 6 and 7, and 1.25 mg/kg on D 8. This tapering DEX regimen was chosen to reduce the likelihood of adrenal suppression and to approximate the multi-day dosing schema used in the clinical setting. Control mice were injected with the same volume of NS without DEX.
Figure 1. Mice were separated into four treatment groups; Sham, DEX, CA, and DEX + CA as described in the methods section. Mice in the Sham and CA groups were injected subcutaneously with NS on D1 to D8 of each treatment cycle (filled arrows). Mice in the DEX and DEX + CA groups were injected sq with 5 mg/kg DEX on days one to five, 2.5 mg/kg DEX on d 6 and 7, and 1.25 mg/kg on D 8. On Treatment D 3 (D3) mice in the CA and DEX + CA groups were injected i.p. with Cytoxan + Adriamycin (CA) while mice in the Sham and DEX groups were injected with the same volume of normal saline (NS).
Experiment 1: Assessment of the effect of DEX on CA-induced inflammatory cytokine and chemokine expression.
For the measurement of circulating cytokines 48 mice were separated equally into four treatment groups; NS + NS (Sham), DEX + NS (DEX), DEX + CA, and 4) NS + CA (CA). Mice in the DEX and DEX + CA groups were injected with 5 mg/kg DEX or NS for 3 d. On the 3rd D mice were injected with either CA or NS. Approximately 24-hours after the CA injection, mice in all groups were terminally sedated and peripheral blood collected by cardiac puncture. Peripheral blood collected by cardiac puncture was allowed to clot for 1 hour and then centrifuged at 8000 RPM for 10 minutes at room temperature (RT). Serum was removed, aliquoted and immediately stored at -80°C until analysis. Levels of IL-1β, TNF-α, IL-6, G-CSF, KC, and MCP-1 were measured in duplicate using a magnetic bead-based immunoassay (Cat# MCYTOMAG-70 K, EMD Millipore, Billerica, MA). Data were collected and analyzed using the Luminex-200 system Version IS (Luminex, Austin, TX). A four or five-parameter regression formula was used to calculate the sample concentrations from the standard curves.
Experiment 2: Assessing the effect of DEX and CA on CTRS
Mice were housed singly in cages fitted with running wheels to allow daily monitoring of voluntary wheel running activity (VWRA), which was used as a proxy for fatigue. Wheel turns were collected automatically in 60 min bins with a magnetic reed switch (MiniMitter, Bend, OR) and the Vital View Data Acquisition System (Vital View, Bend, OR). After acclimation for 10 d baseline wheel running activity, food intake, and body weight were measured daily for 14 d after which time mice were separated into four treatment groups (n = 10 per group): Sham, DEX, CA, and DEX + CA. Mice underwent four treatment cycles at 18–20 d intervals. Approximately 4 weeks after the last treatment cycle mice underwent body composition assessment by dual-energy X-ray absorptiometry (DEXA) densitometry using a Lunar PIXImus II (software version 1.42.006.010; Lunar Corp, Madison, Wisconsin).
Analysis
Kaplan-Meier analysis was used to estimate survival rate. Log-rank testing was used to evaluate the equality of survival curves. In Experiment 1, IL-1β and IL-6 serum level data were not normally distributed and were log10 transformed prior to analysis. Two-way ANOVA with DEX and CA as the independent variables was used to examine DEX x CA interaction effects on serum cytokine and chemokine levels. For Experiment 2, VWRA during the 12 hour dark phase was used to calculate dark phase time on wheel, total VWRA, average speed, and peak speed. Total VWRA was the number of wheel turns in 12 hours; time on wheel was the number of 1 hour intervals where wheel rotations were ≥ 600 (i.e. ≥ 10 turns per minute), average speed was total VWRA divided by time on wheel, and peak speed was the maximum speed reached during a 60 minute interval. One-way ANOVA with Fisher's least significant difference (LSD) post-hoc tests were used to examine group differences in outcome variables with Bonferroni correction for multiple comparisons as indicated (.05/number of comparisons).
Results
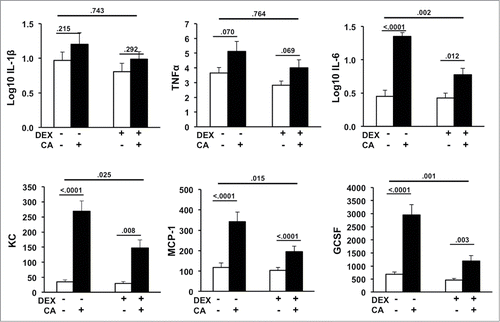
In the first experiment we sought to determine whether DEX could dampen the expected CA-induced increase in circulating inflammatory cytokine and chemokines. shows serum levels of IL-1β, TNF-α, IL-6, MCP-1, GCSF, and KC in the Sham, CA, DEX, and DEX + CA treatment groups. In contrast to IL-1β and TNF-α, serum levels of KC, GCSF, IL-6, and MCP-1 were significantly increased in CA-treated mice compared to sham-treated mice injected with NS alone () Two-way ANOVA with DEX and CA as the independent variables revealed a significant interaction DEX x CA effect for serum MCP-1 (F(3,44) = 6.507, p = .015), KC (F(3,46) = 5.394, p = .025), GCSF (F(3,47) = 12.072, p = .001), IL-6 (F(3,46) = 11.225, p = .002) but not IL-1β (F(3,46) = .109, p = .743) or TNF-α (F(3,46) = .091, p = .764). Although levels of MCP-1, KC, GCSF, and IL-6 were significantly higher in DEX + CA treated mice relative to their DEX-treated counterparts, the magnitude of the CA-induced inflammatory response was dampened by approximately 2-fold in DEX treated animals (Fig.2).
Figure 2. Serum levels of IL-1β, TNF-α, IL-6, GCSF, KC, and MCP-1 in mice 24-hours after CA or NS injection in the presence or absence of DEX (12 mice per group). Each bar represents the mean ± SEM of each value. P-values derived from one-way or two-way ANOVA are indicated in each figure. The threshold for detection was 3.2 pg/mL. P-values derived from one-way or two-way ANOVA are indicated in each figure.
In Experiment 2 we examined the effect of DEX either alone or in combination with cytotoxic chemotherapy on fatigue, food intake, body weight and composition in mice administered four treatment cycles. Mice were separated into four treatment groups; Sham, DEX, CA, and DEX + CA as described in the methods section. Daily VWRA, body weight, and food intake were measured daily throughout 4 cycles of treatment and up to three weeks thereafter. Body composition was assessed before and then three weeks after the final treatment cycle by DEXA. All of the mice in the Sham and DEX alone groups completed the four treatment cycles, whereas one CA-treated mouse and three CA + DEX treated mice met criteria for removal from the study after the 3rd and 4th treatment cycles. There was no statistically significant difference in survival between the CA and DEX + CA treatment groups (p = .182).
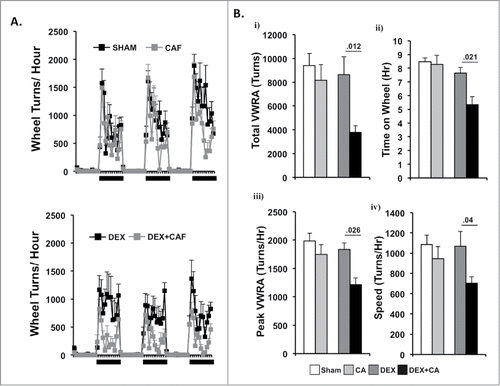
shows plots of hourly VWRA during a 9-d period; 1-d prior to (B) and throughout the first 8-d treatment cycle (D1 to D8). DEX and/or CA did not cause a transient shift in the circadian pattern of VWRA throughout the treatment cycle since mice in each treatment group initiated VWRA at the onset of the dark phase. In we show further analysis of dark phase VWRA during B and then D1 to D8 of the treatment cycle; i) total VWRA, ii) time on wheel, iii) peak VWRA, and iv) average speed (see methods section for further description of these variables). One-way ANOVA with Bonferroni correction for multiple comparisons (p<.006) was used to detect significant group differences in VWRA outcome variable for each treatment day. Significant group differences were only observed for total VWRA (F(3,36) = 12.832; p<0.001), time on wheel (F(3,36) = 18.141; p<0.001), peak VWRA (F(3,36) = 4.764; p = 0.007), and speed (F(3,36) = 7.448; p = 0.001) on treatment Day 3 (D3) which corresponded to the day of CA injection. To determine which treatment groups differed on D3, we conducted follow up post-hoc tests. We found that mice in the DEX alone treatment group spent significantly less time on their wheels (p = .016), and ran at a lower speed (p = .003) than mice in the Sham-treatment group which resulted in a lower total VWRA (p = .001). Peak speed however was not significantly different between these two groups (p = .082). Comparing CA versus sham treatment groups we found that CA-treated mice ran significantly less during the dark phase (p<.001) because they spent significantly less time on their wheels (p<.001), ran at slower speeds (p<.001), and achieved a lower peak speed (p<.001). In contrast, we did not observe a significant difference between the DEX and DEX + CA treatment groups in total VWRA (p = .0576), time on wheel (p = .633), peak (p = .498), or speed (p = .467). Comparing the CA and DEX + CA treatment groups we found no significant difference in total VWRA (p = .221), peak (p = .285), or speed (p = .682), but mice in the CA + DEX group spent less time on their wheels than mice in the CA only treatment group (p < .001).
Figure 3. Number of wheel turns per hour in the dark and light phase one day prior to (B) and throughout the first 8-d treatment cycle (D1 to D8) in sham and CA treated mice (Top Panel) and Dex and DEX + CA treated mice (Bottom panel). Solid arrows indicate the timing of s.q. normal saline or dexamethasone injection. Open arrows indicate the timing of CA or NS i.p. injection. Each data point represents the mean ± SEM of each value.
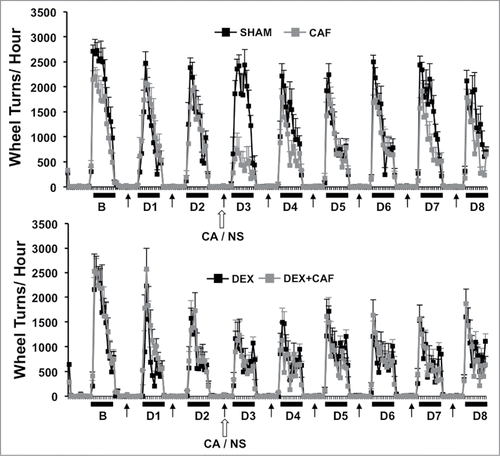
Figure 4. i) total VWRA, ii) time on wheel, iii) peak VWRA, and iv) average speed (see methods section for further description of these variables) in Sham and CA-treated mice (Top Panel) and DEX and DEX+CA treated mice (Bottom Panel). Each data point represents the mean ± SEM of each value. Open arrows indicate the timing of CA or NS i.p. injection. Group differences on each day of treatment were examined by one-way ANOVA with Bonferroni correction for multiple comparisons. Follow-up post hoc tests (LSD) were performed to determine which groups differed (See results for details). Significant differences between Sham and CA-treated mice (Top panel) or DEX and DEX+CA treated mice are indicated by a *.
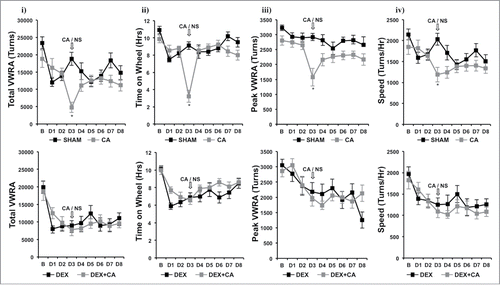
shows plots of hourly VWRA during a 3-d period 2 weeks after the 4th treatment cycle. All of the mice in the Sham and DEX groups completed the four treatment cycles, whereas two CA-treated mice and three CA + DEX treated mice group met criteria for removal from the study after the 3rd and 4th treatment cycle and their data were therefore not included in the analysis. Again we did not observe any shift in the circadian onset of VWRA in that mice in all groups initiated VWRA at the beginning of the dark phase. shows i) total VWRA, ii) time on wheel, iii) peak VWRA, and iv) and speed averaged across the 3-d. One-way ANOVA using treatment group as the independent variable revealed a significant group difference in time on wheel (F(3,34) = 5.599, p = .003), total VWRA (F(3,34) = 3.606, p = .024), peak (F(3,34) = 2.985, p = .046) but no group difference in speed (F(3,34) = .2.047, p = .128). Follow up post-hoc tests revealed no significant difference between mice in the Sham and CA treatment groups for any of the outcome variables. Similarly there were no significant differences between mice in the Sham and DEX treatment groups. In contrast, mice in the DEX + CA treatment group spent less time on their running wheels (p = .021), and when they did run they reached a lower peak speed (p = .026), a lower average speed (p = .04), and as a result had a lower total VWRA (p = .012) than mice treated with DEX alone ().
Figure 5. (A) Number of wheel turns per hour in the dark and light phase during a 3-d period two- weeks after the 4th treatment cycle in sham and CA treated mice (Top Panel) and DEX and DEX + CA treated mice (Bottom panel). Each data point represents the mean ± SEM. (B) i) total VWRA, ii) time on wheel, iii) peak VWRA, and iv) speed averaged across the 3-d. Each bar represents the mean ± SEM of each value. Group differences were examined by one-way ANOVA. Significant group differences are indicated by p-values.
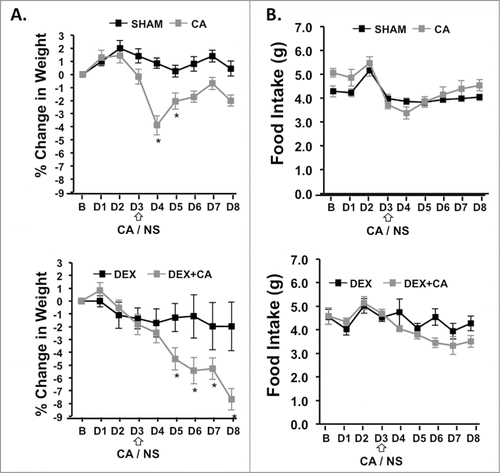
shows percentage change in body weight from baseline (B) for each day of the 1st treatment cycle (D1 to D8). One-way ANOVA with Bonferroni correction for multiple comparisons (p<.006) with treatment group as the independent variable revealed a significant group difference in body weight change on treatment D4 to D8 (p < .006). Compared to sham-treated mice CA-treated mice showed a significant difference in body weight only on D4 which corresponded to the day following CA-injection (p<.001). On this day CA-treated mice lost approximately 5% of their body weight. Mice in the DEX only group did not show any significant changes in body weight throughout the treatment cycle when compared to sham-treated mice. In contrast, weight loss was significantly greater for mice in the DEX + CA treatment group than for mice in the DEX alone or CA alone treatment groups (p<.05). On D8 mean weight loss in DEX + CA mice was approximately 8% compared to 2% in the DEX alone and CA alone groups respectively. shows food intake from baseline throughout the 8-d treatment cycle. One-way ANOVA with Bonferroni correction revealed a group difference in food intake only for D3 (F(3,38) = 6.285, p = .002). Follow up post hoc tests showed that compared to shams, DEX-treated mice ate significantly more on this day (p = .034), while there was no significant difference in food intake between sham and CA alone treated mice (p = .321), DEX and DEX + CA mice (p = .551), or CA and DEX + CA treated mice (p = .321).
Figure 6. (A) Percent change in body weight and daily food intake from the day before treatment (B) and each treatment day (D1 to D8) of the 1st cycle in sham and CA-treated mice (Top panel) and DEX and DEX+CA treated mice (Bottom Panel). Open arrows indicate the timing of CA or NS i.p. injection. Each data point represents the mean ± SEM of each value. Group differences on each day of treatment were examined by one-way ANOVA with Bonferroni correction for multiple comparisons. Follow-up post hoc tests (LSD) were performed to determine which groups differed. Significant differences between Sham and CA-treated mice (Top panel) or DEX and DEX+CA treated mice are indicated by a *.
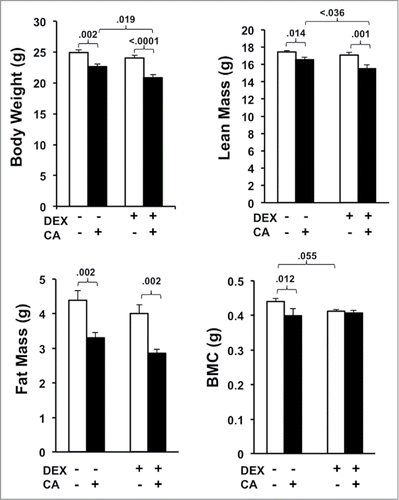
shows group differences in body weight and composition two weeks after the 4th treatment cycle (). One-way ANOVA revealed a significant group difference in body weight (F(3,34) = 13.196, p<.0001), fat mass (FM) (F(3,34) = 8.676, p < .0001), and lean mass (LM) (F(3,34) = 6.797, p = .001) and a trend towards a significant group difference in bone mineral content (BMC) (F(3,34) = 2.853, p = .053). Follow up post-hoc tests revealed that mice injected with CA alone weighed significantly less than sham-treated mice at the end of the treatment regimen, which was related to reduced FM (p = .002), LM (p = .014), and BMC (p = .012). Compared to the DEX alone group mice in the DEX + CA group had reduced body weight (p < .0001), LM (p = .001), and FM (p = .002) compared to mice in the DEX only group but no statistically significant difference in BMC. Comparing CA and CA + DEX treated mice we observed a significant difference in body weight (p = .019) and LM (p = .036). These significant group differences remained after controlling for group differences in average daily VWRA and food intake throughout the experiment (statistics not shown). DEX treatment alone did not significantly affect body weight, LM, or FM. There was, however, a trend towards a significant decline in BMC in DEX treated mice compared to Sham-treated mice (p = .055).
Figure 7. Average body weight, fat mass (FM), lean mass (LM), and bone mineral content (BMC) derived from DEXA scans performed three-weeks after the 4th treatment cycle. Each bar represents the mean ± SEM of each value. Group differences were examined using one way ANOVA with post-hoc tests. Significant group differences are indicated by the p-values.
Discussion
Here we demonstrate the impact that dexamethasone alone or in combination with cytotoxic chemotherapy has on chemotherapy-induced fatigue/lethargy and other CTRS in tumor free mice. Consistent with our previous work we found that cytotoxic chemotherapy (CA) increased circulating levels of inflammatory markers. The dexamethasone regimen used in the present study, chosen to reflect a similar regimen used clinically for the management of CINV, did not completely block the acute CA-induced inflammatory response but reduced it by approximately 2-fold. In our recent study using mice lacking both IL-1 and TNF-receptors, we found that complete blockade of the chemotherapy-induced inflammatory response impairs survival.Citation17 In the present study we did not observe a significant difference in survival between the CA and CA + DEX treatment groups, which suggest that the dexamethasone regimen used in the present study did not appear to impair recovery from cytotoxic injury.
Breast cancer chemotherapy regimens in the past included 5-fluorouracil (5-FU), a pyrimidine analogue that was frequently combined with CA (CAF). We used CAF in prior studies to examine patterns of lethargy in tumor free mice throughout multiple drug doses thereby mirroring the clinical scenario. We found that CAF caused a rapid increase in lethargy following each of the first three CAF doses which gradually resolved over several days post-treatment. After the fourth CAF dose however, fatigue became persistent and several weeks post-CAF remained significantly higher than in sham-treated control mice. In the present study we excluded 5-FU from the drug regimen because CAF is no longer a commonly used treatment regimen for early stage breast cancer. Compared to CAF-treatment, where mice displayed reduced VWRA for several days post-injection, CA only caused a transient decline in VWRA during the active period immediately following injection. Moreover, while repeated rounds of CAF caused a persistent decline in VWRA, this did not occur in mice treated with CA without 5-FU. Based on earlier studies in which short term dexamethasone decreased endotoxin-induced inflammatory signaling in rodents and attenuated endotoxin-induced sickness behavior,Citation46,47 we reasoned that DEX would similarly attenuate CA-mediated fatigue. Indeed, the acute drop in VWRA immediately following CA-injection was absent in CA + DEX treated mice. However, DEX treatment alone had a suppressive effect on VWRA. Several prior studies have shown that dexamethasone has a suppressive effect on locomotor activity in rodents.Citation48,49,50 In the majority of studies movement around the home cage or in an open field apparatus was used as a measure of locomotor activity rather than VWRA. In addition to general locomotor supression, chronic DEX exposure increases immobility time in the Forced Swim and Tail Suspension tests and decreases consumption of sucrose solution all of which model depressive behavior in mice.Citation49 These DEX driven behavioral effects have been shown to result from the impaired synthesis of brain derived neurotropic factor (BDNF), a neurotrophic signaling molecule that plays a central role in neurogenesis.Citation51 The hippocampus, the brain region responsible for coordinating learning, memory, and emotion is one of the few brain regions to undergo neurogenesis into adulthood.Citation52 Rodent studies suggest that by reducing BDNF synthesis, DEX impairs hippocampal neurogenesis leading to behavioral suppression.Citation53,54 There is growing appreciation that the antidepressant effects of selective serotonin reuptake inhibitor (SSRI) antidepressants are due in part to their ability to increase BDNF levels.Citation55 Using the SSRI fluvoxamine, Terada and colleagues were able to prevent the DEX-mediated decrease in open field locomotor activity and immobility in the forced swim test and this effect was associated with an increase in BDNF function.Citation48 Given the impact that DEX exposure has on locomotor activity it is unclear why persistent fatigue observed in DEX + CA mice was not observed in mice injected with DEX alone. Decreased VWRA in the DEX + CA treatment group was not due to increased morbidity in the remaining mice in this group in that all of the surviving mice in the DEX + CA treatment group survived for several months after the last treatment cycle (data not shown).
The impact of CA and DEX on body weight and composition may explain the persistent decline in VWRA in mice injected with both CA and DEX since DEX combined with CA appeared to exacerbate weight loss. We found that CA also caused an acute drop in body weight which did not occur in CA-treated mice that were also treated with DEX. Body weight continued to decline in DEX + CA treated mice during the days post-CA injection whereas in CA-treated mice it returned towards baseline. Taken together, we cannot conclude that DEX, by dampening CA-induced inflammation, blunted the acute CA-induced decline in VWRA and weight loss. In prior experiments using CA combined with 5-fluorouracil (CAF) we found that mice injected with multiple cycles of CAF developed persistent weight loss in that their body weight failed to return to baseline three weeks after a fourth CAF injection.Citation17 Persistent weight loss in these mice was associated with the loss of fat, lean, and bone-mass in CAF-treated mice. In the present study we found that CA injected without 5-fluorouracil also caused a reduction in lean-, fat-, and bone-mass. Chemotherapy-induced inflammatory signaling has been implicated in skeletal muscle atrophy.Citation14 By activating the HPA axis, inflammatory cytokines like IL-6 increase production of endogenous glucocorticoids: cortisol in humans and corticosterone in mice. Braun and colleagues showed that CAF administration increased circulating levels of corticosterone and caused a significant loss of skeletal muscle mass in mice.Citation14 The effect of CAF on skeletal muscle mass was blocked in mice lacking a functional glucocorticoid receptor in skeletal muscle, which suggests that CAF-induced skeletal muscle atrophy is mediated by corticosterone. Similarly mice lacking IL-6, a potent activator of the HPA axis, are protected from CAF-induced loss of lean body mass.Citation56 Given the impact of glucocorticoids on skeletal muscle atrophy, it is not surprising that mice in the CA + DEX group had the lowest body weight and LM of all the other treatment groups. Nonetheless, body weight and composition was not significantly different between DEX-treated mice versus shams.
Because dexamethasone is incorporated into chemotherapy regimens as standard clinical care to prevent CINV, no one had previously investigated the effect of fatigue/lethargy on patients undergoing active cytotoxic chemotherapy treatment. Our current pre-clinical data demonstrated that, despite its ability to suppress circulating levels of inflammatory cytokines, dexamethasone when applied in combination with CA reduced body weight and LM compared to CA alone and exacerbated fatigue/lethargy during the course of treatment.
Disclosure of potential conflicts of interest
The authors declare no conflict of interest
Competing interests
The authors declare that they have no competing interests.
Author contributions
LJW conceived of and designed the study. JW and LTT performed the experiments. LJW and JW performed the statistical tests. LJW, JW, and KAL drafted the manuscript and figures. All authors read and approved the final manuscript.
Acknowledgments
Funding for this study was provided by NINR R01NR013171 to LJW.
References
- Aktas A, Walsh D, Rybicki L. Symptom clusters and prognosis in advanced cancer. Support Care Cancer. 2012;20(11):2837–43. doi:10.1007/s00520-012-1408-9.
- Curt GA. The impact of fatigue on patients with cancer: Overview of FATIGUE 1 and 2. oncologist. 2000;5 Suppl 2:9–12. doi:10.1634/theoncologist.5-suppl_2-9.
- Hofman M, Ryan JL, Figueroa-Moseley CD, Jean-Pierre P, Morrow GR. Cancer-related fatigue: the scale of the problem. oncologist. 2007;12 Suppl 1:4–10. doi:10.1634/theoncologist.12-S1-4.
- Holmes D. Trying to unravel the mysteries of chemobrain. LancetNeurology. 2013;12(6):533–4.
- Myers JS, Pierce J, Pazdernik T. Neurotoxicology of chemotherapy in relation to cytokine release, the blood-brain barrier, and cognitive impairment. Oncology nursing forum. 2008;35(6):916–20. doi:10.1188/08.ONF.916-920.
- Thiagarajan M, Chan CM, Fuang HG, Beng TS, Atiliyana MA, Yahaya NA. Symptom Prevalence and Related Distress in Cancer Patients Undergoing Chemotherapy. Asian Pac J Cancer Prev. 2016;17(1):171–6. doi:10.7314/APJCP.2016.17.1.171.
- Berger AM, Gerber LH, Mayer DK. Cancer-related fatigue: implications for breast cancer survivors. Cancer. 2012;118(8 Suppl):2261–69. doi:10.1002/cncr.27475.
- Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J Clin Oncol. 2000;18(4):743–53. doi:10.1200/JCO.2000.18.4.743.
- Cella D, Davis K, Breitbart W, Curt G, Fatigue Coalition. Cancer-related fatigue: prevalence of proposed diagnostic criteria in a United States sample of cancer survivors. J Clin Oncol. 2001;19(14):3385–91. doi:10.1200/JCO.2001.19.14.3385.
- Luctkar-Flude MF, Groll DL, Tranmer JE, Woodend K. Fatigue and physical activity in older adults with cancer: A systematic review of the literature. Cancer Nurs. 2007;30(5):E35–45. doi:10.1097/01.NCC.0000290815.99323.75.
- Cheville AL. Cancer-related fatigue. Phys Med Rehabil Clin N Am. 2009;20(2):405–16. doi:10.1016/j.pmr.2008.12.005.
- Radbruch L, Strasser F, Elsner F, Gonçalves JF, Løge J, Kaasa S, Nauck F, Stone P. Fatigue in palliative care patients – an EAPC approach. Palliat Med. 2008;22(1):13–32. doi:10.1177/0269216307085183.
- Wood LJ, Weymann K. Inflammation and neural signaling: Etiologic mechanisms of the cancer treatment-related symptom cluster. Curr Opin Support Palliat Care. 2013;7(1):54–59. doi:10.1097/SPC.0b013e32835dabe3.
- Braun TP, Szumowski M, Levasseur PR, Grossberg AJ, Zhu X, Agarwal A, Marks DL. Muscle atrophy in response to cytotoxic chemotherapy is dependent on intact glucocorticoid signaling in skeletal muscle. PLoS One. 2014;9(9):e106489. doi:10.1371/journal.pone.0106489.
- Zhu W, Cao FS, Feng J, Chen HW, Wan JR, Lu Q, Wang J. NLRP3 inflammasome activation contributes to long-term behavioral alterations in mice injected with lipopolysaccharide. Neuroscience. 2017;343:77–84. doi:10.1016/j.neuroscience.2016.11.037.
- Grossberg AJ, Zhu X, Leinninger GM, Levasseur PR, Braun TP, Myers MG Jr, Marks DL. Inflammation-induced lethargy is mediated by suppression of orexin neuron activity. J Neurosci. 2011;31(31):11376–86. doi:10.1523/JNEUROSCI.2311-11.2011.
- Smith LB, Leo MC, Anderson C, Wright TJ, Weymann KB, Wood LJ. The role of IL-1beta and TNF-alpha signaling in the genesis of cancer treatment related symptoms (CTRS): A study using cytokine receptor-deficient mice. Brain Behav Immun. 2014;38:66–76. doi:10.1016/j.bbi.2013.12.022.
- Weymann KB, Wood LJ, Zhu X, Marks DL. A role for orexin in cytotoxic chemotherapy-induced fatigue. Brain Behav Immun. 2014;37:84–94. doi:10.1016/j.bbi.2013.11.003.
- McDonald TL, Hung AY, Thomas CR, Wood LJ. Localized External Beam Radiation Therapy (EBRT) to the Pelvis Induces Systemic IL-1Beta and TNF-Alpha Production: Role of the TNF-Alpha Signaling in EBRT-Induced Fatigue. Radiat Res. 2016;185(1):4–12. doi:10.1667/RR14072.1.
- Bower JE. Cancer-related fatigue–mechanisms, risk factors, and treatments. Nat Rev Clin Oncol. 2014;11(10):597–609. doi:10.1038/nrclinonc.2014.127.
- Kruse JL, Strouse TB. Sick and tired: Mood, fatigue, and inflammation in cancer. Curr Psychiatry Rep. 2015;17(3):555. doi:10.1007/s11920-015-0555-3.
- Floman Y, Zor U. Mechanism of steroid action in inflammation: Inhibition of prostaglandin synthesis and release. Prostaglandins. 1976;12(3):403–413. doi:10.1016/0090-6980(76)90021-6.
- Yamasaki H, Yamamoto T. Inhibitory Effect of Adrenal Glucocorticoids on Histamine Release. Jpn J Pharmacol. 1963;13:223–4. doi:10.1254/jjp.13.223.
- Almawi WY, Beyhum HN, Rahme AA, Rieder MJ. Regulation of cytokine and cytokine receptor expression by glucocorticoids. J Leukoc Biol. 1996;60(5):563–572.
- Piccolella E, Lombardi G, Vismara D, Del Gallo F, Colizzi V, Dolei A, Dianzani F. Effects of dexamethasone on human natural killer cell cytotoxicity, interferon production, and interleukin-2 receptor expression induced by microbial antigens. Infect Immun. 1986;51(2):712–14.
- Wahl SM, Altman LC, Rosenstreich DL. Inhibition of in vitro lymphokine synthesis by glucocorticosteroids. Journal of immunology (Baltimore, Md: 1950). 1975;115(2):476–481.
- Xu T, Qiao J, Zhao L, He G, Li K, Wang J, Tian Y, Wang H. Effect of dexamethasone on acute respiratory distress syndrome induced by the H5N1 virus in mice. Eur Respir J. 2009;33(4):852–60. doi:10.1183/09031936.00130507.
- Wen SH, Wu HJ, Lin L, Chong L, Zhu LL, Zhang WX, Zhang HL, Li CC. Adjunctive dexamethasone therapy improves lung injury by inhibiting inflammation and reducing RIP3 expression during Staphylococcus aureus pneumonia in mice. Int Immunopharmacol. 2014;23(2):709–18. doi:10.1016/j.intimp.2014.10.027.
- Rocksen D, Lilliehook B, Larsson R, Johansson T, Bucht A. Differential anti-inflammatory and anti-oxidative effects of dexamethasone and N-acetylcysteine in endotoxin-induced lung inflammation. Clin Exp Immunol. 2000;122(2):249–56. doi:10.1046/j.1365-2249.2000.01373.x.
- Bersani-Amado LE, Dantas JA, Damiao MJ, Rocha BA, Besson JC, Bastos RL, Silva LN, Bersani-Amado CA, Cuman RK. Involvement of cytokines in the modulation and progression of renal fibrosis induced by unilateral ureteral obstruction in C57BL/6 mice: Effects of thalidomide and dexamethasone. Fundam Clin Pharmacol. 2016;30(1):35–46. doi:10.1111/fcp.12162.
- Ettinger DS, Bierman PJ, Bradbury B, Comish CC, Ellis G, Ignoffo RJ, Kirkegaard S, Kloth DD, Kris MG, Lim D, et al. Antiemesis. J Natl Compr Canc Netw. 2007;5(1):12–33. doi:10.6004/jnccn.2007.0004.
- Yennurajalingam S, Frisbee-Hume S, Palmer JL, Delgado-Guay MO, Bull J, Phan AT, Tannir NM, Litton JK, Reddy A, Hui D, et al. Reduction of cancer-related fatigue with dexamethasone: A double-blind, randomized, placebo-controlled trial in patients with advanced cancer. J Clin Oncol. 2013;31(25):3076–82. doi:10.1200/JCO.2012.44.4661.
- Vardy J, Chiew KS, Galica J, Pond GR, Tannock IF. Side effects associated with the use of dexamethasone for prophylaxis of delayed emesis after moderately emetogenic chemotherapy. Br J Cancer. 2006;94(7):1011–15. doi:10.1038/sj.bjc.6603048.
- Savard J, Villa J, Ivers H, Simard S, Morin CM. Prevalence, natural course, and risk factors of insomnia comorbid with cancer over a 2-month period. J Clin Oncol. 2009;27(31):5233–39. doi:10.1200/JCO.2008.21.6333.
- Savard J, Liu L, Natarajan L, Rissling MB, Neikrug AB, He F, Dimsdale JE, Mills PJ, Parker BA, Sadler GR, et al. Breast cancer patients have progressively impaired sleep-wake activity rhythms during chemotherapy. Sleep. 2009;32(9):1155–60. doi:10.1093/sleep/32.9.1155.
- Palesh OG, Roscoe JA, Mustian KM, Roth T, Savard J, Ancoli-Israel S, Heckler C, Purnell JQ, Janelsins MC, Morrow GR. Prevalence, demographics, and psychological associations of sleep disruption in patients with cancer: University of Rochester Cancer Center-Community Clinical Oncology Program. J Clin Oncol. 2010;28(2):292–98. doi:10.1200/JCO.2009.22.5011.
- Minton O, Stone PC. A comparison of cognitive function, sleep and activity levels in disease-free breast cancer patients with or without cancer-related fatigue syndrome. BMJ Support Palliat Care. 2012;2(3):231–38. doi:10.1136/bmjspcare-2011-000172.
- Minton O, Alexander S, Stone PC. Identification of factors associated with cancer related fatigue syndrome in disease-free breast cancer patients after completing primary treatment. Breast Cancer Res Treat. 2012;136(2):513–20. doi:10.1007/s10549-012-2284-1.
- Bodine SC, Furlow JD. Glucocorticoids and Skeletal Muscle. Adv Exp Med Biol. 2015;872:145–76. doi:10.1007/978-1-4939-2895-8_7.
- Eriksson S, Nilsson JH, Strandberg Holka P, Eberhard J, Keussen I, Sturesson C. The impact of neoadjuvant chemotherapy on skeletal muscle depletion and preoperative sarcopenia in patients with resectable colorectal liver metastases. HPB (Oxford). 2017;19(4):331–37. doi:10.1016/j.hpb.2016.11.009.
- Rutten IJ, van Dijk DP, Kruitwagen RF, Beets-Tan RG, Olde Damink SW, van Gorp T. Loss of skeletal muscle during neoadjuvant chemotherapy is related to decreased survival in ovarian cancer patients. J Cachexia Sarcopenia Muscle. 2016;7(4):458–66. doi:10.1002/jcsm.12107.
- Reisinger KW, Bosmans JW, Uittenbogaart M, Alsoumali A, Poeze M, Sosef MN, Derikx JP. Loss of Skeletal Muscle Mass During Neoadjuvant Chemoradiotherapy Predicts Postoperative Mortality in Esophageal Cancer Surgery. Ann Surg Oncol. 2015;22(13):4445–52. doi:10.1245/s10434-015-4558-4.
- Takayoshi K, Uchino K, Nakano M, Ikejiri K, Baba E. Weight Loss During Initial Chemotherapy Predicts Survival in Patients With Advanced Gastric Cancer. Nutr Cancer. 2017;69(3):408–15. doi:10.1080/01635581.2017.1267774.
- Ullman-Cullere MH, Foltz CJ. Body condition scoring: a rapid and accurate method for assessing health status in mice. Lab Anim Sci. 1999;49(3):319–23.
- Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22(3):659–61. doi:10.1096/fj.07-9574LSF.
- de Paiva VN, Lima SN, Fernandes MM, Soncini R, Andrade CA, Giusti-Paiva A. Prostaglandins mediate depressive-like behaviour induced by endotoxin in mice. Behav Brain Res. 2010;215(1):146–51. doi:10.1016/j.bbr.2010.07.015.
- Hanaa-Mansour A , Hassan WA, Georgy GS. Dexamethazone protects against Escherichia coli induced sickness behavior in rats. Brain Res. 2016;1630:198–207. doi:10.1016/j.brainres.2015.10.049.
- Terada K, Izumo N, Suzuki B, Karube Y, Morikawa T, Ishibashi Y, Kameyama T, Chiba K, Sasaki N, Iwata K. Fluvoxamine moderates reduced voluntary activity following chronic dexamethasone infusion in mice via recovery of BDNF signal cascades. Neurochem Int. 2014;69:9–13. doi:10.1016/j.neuint.2014.02.002.
- Sigwalt AR, Budde H, Helmich I, Glaser V, Ghisoni K, Lanza S, Cadore EL, Lhullier FL, de Bem AF, Hohl A, et al. Molecular aspects involved in swimming exercise training reducing anhedonia in a rat model of depression. Neuroscience. 2011;192:661–74. doi:10.1016/j.neuroscience.2011.05.075.
- Casarotto PC, Andreatini R. Repeated paroxetine treatment reverses anhedonia induced in rats by chronic mild stress or dexamethasone. Eur Neuropsychopharmacol. 2007;17(11):735–42. doi:10.1016/j.euroneuro.2007.03.001.
- Foltran RB, Diaz SL. BDNF isoforms: A round trip ticket between neurogenesis and serotonin? J Neurochem. 2016;138(2):204–21. doi:10.1111/jnc.13658.
- Kempermann G, Song H, Gage FH. Neurogenesis in the Adult Hippocampus. Cold Spring Harb Perspect Biol. 2015;7(9):a018812. doi:10.1101/cshperspect.a018812.
- Ruksee N, Tongjaroenbuangam W, Mahanam T, Govitrapong P. Melatonin pretreatment prevented the effect of dexamethasone negative alterations on behavior and hippocampal neurogenesis in the mouse brain. J Steroid Biochem Mol Biol. 2014;143:72–80. doi:10.1016/j.jsbmb.2014.02.011.
- Kim JB, Ju JY, Kim JH, Kim TY, Yang BH, Lee YS, Son H. Dexamethasone inhibits proliferation of adult hippocampal neurogenesis in vivo and in vitro. Brain Res. 2004;1027(1-2):1–10. doi:10.1016/j.brainres.2004.07.093.
- Bjorkholm C, Monteggia LM. BDNF – a key transducer of antidepressant effects. Neuropharmacology. 2016;102:72–79. doi:10.1016/j.neuropharm.2015.10.034.
- Elsea CR, Kneiss JA, Wood LJ. Induction of IL-6 by Cytotoxic Chemotherapy Is Associated With Loss of Lean Body and Fat Mass in Tumor-free Female Mice. Biological research for nursing. 2015;17(5):549–57. doi:10.1177/1099800414558087.
