ABSTRACT
A 67-year-old male presented with anasarca and persistent non-pruritic rash of lower extremities. Physical examination was positive for subcutaneous edema with a non-blanching rash of abdomen and lower extremities. Labs showed leukocytosis, lymphocytosis, anemia and thrombocytopenia. He also had acute kidney injury and high anion gap (AG) metabolic acidosis with elevated lactic acid (11.3 mg/dL). Computerized tomography (CT) of abdomen and pelvis showed hepatosplenomegaly, ascites and abdominal lymphadenopathy. Peripheral blood (PB) smear showed blastiod appearing lymphocytes. He was started on bicarbonate infusion due to persistent lactic acidosis (LA), however showed no significant improvement. He was started on IV dexamethasone on 3rd day of hospitalization based on preliminary result of peripheral picture which led to some improvement in LA. Following the confirmation of mantle cell lymphoma (MCL) on bone marrow (BM) biopsy and immunophenotyping, the patient started receiving VR-CAP regimen (bortezomib, rituximab, cyclophosphamide, doxorubicin, and prednisone) which led to significant improvement in LA and leukocytosis. After discharge, he received further chemotherapy with resolution of the LA and normalization of blood counts. Restaging tests confirmed a complete remission with resolution of the skin rash, resolution of the pathological lymphadenopathy and hepatosplenomegaly on imaging, and absence of lymphoma on a repeat BM biopsy.
Introduction
Malignancy-related lactic acidosis (LA) is a rare oncological emergency that can be fatal if not promptly identified and treated. It has been associated with both hematological (85%) and solid (15%) cancers.Citation1 Among hematologic cancers, lymphoma, predominantly non-Hodgkin's type, accounts for 50% cases. We present a literature review of the topic and a case of type B LA in a patient with newly diagnosed mantle cell lymphoma that resolved after initiation of effective anti-lymphoma therapy.
Case summary
A 67-year-old male presented to a regional hospital with complaints of progressive anasarca and a persistent non-pruritic lower extremity rash of 2–3 months duration. He also reported worsening exertional dyspnea and weight gain of 50 pounds over the same period. He had a prior history of gout and non-insulin dependent diabetes for which he was taking metformin. Vitals were normal. Physical examination was remarkable for subcutaneous pitting edema of the abdominal wall, scrotum, and extremities with a non-blanching rash involving the lower limbs and abdomen. Complete blood count showed leukocytosis (WBC count of 83,900/mm3 with absolute lymphocyte count of 75,800 /mm3), anemia and thrombocytopenia (Hb of 8.7 g/dL, platelets of 76,000/mm3). He was also found to have non-oliguric kidney injury (creatinine of 1.57 mg/dL with unknown baseline), with hyperkalemia of 6.6 mmol/L and a high AG metabolic acidosis. Lactic acid was 11.3 mmol/L, LDH was 691 U/L, and uric acid was 11.7 mg/dL. The patient was transferred to a tertiary care center for further management.
Upon admission, chemistry panel was similar with an AG of 14 mmol/L, potassium of 5.4 mmol/L (3.5–5.2), bicarbonate of 16 mmol/L and glucose of 91 mg/dL (65–99). The liver panel revealed mild liver dysfunction with albumin of 3.0 g/dL (3.5–5.5), ALP of 157 U/L (39–117), ALT of 47 U/L (0–32), AST of 97 U/L (0–40) and LDH of 260 U/L (121–224). On venous blood gas, he had a pH of 7.23, pCO2 of 42 mmHg, pO2 of 42 mmHg and bicarbonate of 17 mmol/L. His lactic acid was persistently elevated (9.6 mmol/L, normal range 0.5–2.2). His infectious workup was negative, and vitals were within normal limits with no signs of sepsis, hypovolemia or hypoperfusion. The DIC panel was done for the possibility of acute promyelocytic leukemia and was negative. Transthoracic echocardiogram showed normal cardiac function. Upon admission, metformin was stopped due to its potential contribution to the development of LA.
However, LA was persistently elevated. The PB smear showed abnormal blastoid-appearing lymphocytes. Non-contrast CT of abdomen and pelvis revealed hepatomegaly measuring 21.9 cm and splenomegaly measuring 19.7 cm, as well as ascites, and a few prominent sub-centimeter lymph nodes in the gastrohepatic and retroperitoneal regions.
Due to persistent hyperuricemia with possible tumor lysis, the patient received a single dose of rasburicase and was started on IV fluids and oral allopurinol. Intravenous infusion of bicarbonate was also initiated because of unresolved LA.
Based on preliminary results of PB flow cytometry suggesting blastoid MCL, the patient was treated with IV dexamethasone 40 mg daily for 4 days starting on the third day of hospitalization while awaiting BM and final immunophenotyping results. This led to some improvement in leukocytosis and LA.
Final results of PB flow cytometry with immunophenotyping showed a CD5-positive kappa light chain-restricted mature monoclonal B-cell population which was dim to negative for CD23, consistent with MCL. BM biopsy showed a hypercellular (>90%) marrow with extensive infiltration (>60%) by small lymphocytes in a nodular and diffuse pattern with an atypical aggregate of lymphocytes that stained positive with cyclin D1, CD20 and CD5. Punch biopsy of the skin rash revealed mild perivascular lymphoid infiltrates of atypical cells (CD 20+ and BCL1+) with reactive T-cells consistent with lymphomatous skin involvement.
Cytogenetic analysis of the skin biopsy revealed loss of chromosomes or segments 1p31.3p13.2, 9 and 13 in a mosaic state representing about 70% of cells. Moreover, tumor suppressor gene CDKN2A was homozygously deleted in the tissue specimen.
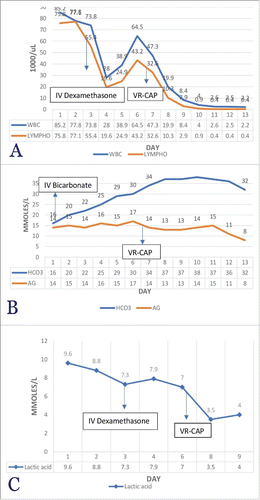
Following the confirmation of the diagnosis of blastoid variant MCL, the patient was started on the VR-CAP regimenCitation2 (bortezomib, rituximab, cyclophosphamide, doxorubicin, and prednisone) which he tolerated very well. There was a significant reduction in leukocytosis after chemotherapy. Similarly, lactic acid level, which dropped from 9.6 to 7 mmol/L over six days while on steroids and bicarbonate, decreased significantly from 7 to 3.5 mmol/L over 48–72 hours ().
Figure 1. Showing the temporal Trends of (A) Bicarbonate and AG, (B) WBC count, Lymphocyte count, and (C) Lactic acid Levels over the course of hospital Stay. Figure A: temporal trend of Bicarbonate and AG. On Day 1, Bicarbonate Infusion was Started, and on Day 7, Chemotherapy was Started. Figure B: A Downward Trend of Leukocytosis and Lymphocytosis with IV Dexamethasone (Day 3) and VR-CAP (Day 7). Figure C: Declining Trend of Lactic Acid after IV Dexamethasone, Sodium Bicarbonate and Chemotherapy (VR-CAP).
The patient was discharged home after two weeks in stable condition for outpatient follow-up. He has since received further chemotherapy (VR-CAP x2 alternating with R-MTX/Ara-C x2) with resolution of the LA and normalization of his blood counts and renal function. Restaging tests confirmed a complete remission with resolution of the skin rash, resolution of the pathological lymphadenopathy and hepatosplenomegaly on imaging, and absence of lymphoma on a repeat bone marrow biopsy.
Discussion
Luft and colleagues defined LA as a condition with pH ≤ 7.35 and blood lactic acid level more than 5 mmol/L.Citation3 Lactic acid, an organic molecule, is generated because of glucose fermentation and exists as two optical isomers of L-lactic acid and D-lactic acid in humans and bacteria respectively. An imbalance of overproduction and underutilization of lactic acid results in LA and is subdivided into types A, B, and D lactic acidosis. Type A lactic acidosis commonly occurs in hypoxemic, hypovolemic or septic states due to impaired tissue oxygenation shifting metabolism from aerobic to anaerobic. In contrast, the etiology of type B lactic acidosis is diverse and includes malignancy, medical conditions, medications and inherited enzyme deficiencies and occurs despite adequate oxygenation ().Citation4
Table 1. Types and causes of lactic acidosis.
Although the exact mechanism remains unclear, the pathophysiology of type B lactic acidosis is complex and likely multifactorial. One suggested mechanism is the “Warburg effect”, which postulates that the needs of malignant cells are distinctly different than a cell in a homeostatic state, and therefore malignant cells exhibit higher aerobic glycolytic activity compared to healthy cells leading to the conversion of pyruvate to lactic acid and then to lactic acidosis. In essence, this may provide an advantage to tumor cells by providing the necessary cellular components (lipids, amino acids and nucleotides) for cell proliferation.Citation5 However, the reverse Warburg effect described by Martinez-Outschoorn and colleagues suggests that that there is “metabolic coupling” between stromal and parenchymal tumor cells, such that the stromal cells generate and export lactic acid and the tumor cells import the lactic acid where it is utilized in mitochondrial oxidative phosphorylation. These cellular mechanisms are evidenced by high expression of various markers, 1) PKM1 and LDH-B in stromal cells showing increased aerobic glycolysis in the microenvironment of the tumor rather than in the cancer cell itself and 2) TOMM20 and BCL6 reflecting oxidative phosphorylation and resistance to apoptosis in the tumor cells.Citation6
Additionally, insulin-like growth factor (IGF) and insulin-like growth factor binding protein (IGFBP) derangements in the setting of hematologic cancers may contribute to LA. Silos et al. measured the levels of IGFs, IGFBPs and TNF-α in three cases of hematologic cancers including lymphoma and leukemia and found that levels of LA and TNF-alpha correlated with disease activity. Furthermore, IGF-1 and IGF-2 were inversely related whereas IGFBP-1 and IGFBP-2 were directly related to disease activity. The composite picture may be one of inducing overexpression of glycolytic enzyme hexokinase.Citation7 In addition, hepatic dysfunction in malignancies can impair the utilization and clearance of lactic acid due to reduced gluconeogenesis or Cori lactic acid shuttle.Citation1 Other potential mechanisms, which might play a role in LA, include tumor lysis syndrome, tumor micro-emboli, local tumor ischemia due to vascular compromise and vitamin deficiencies including thiamine and riboflavin.
More than 50% of reported lymphoma cases associated with LA in hematologic cancers are related to advanced stage high-grade non-Hodgkin's lymphoma. Type B lactic acidosis in MCL has rarely been reported and we identified only one other case report of a patient with blastoid variant MCL presenting with LA, who also responded to chemotherapy and showed complete remission with resolution of the LA.Citation8
Prompt treatment of LA includes taking care of metabolic derangements with appropriate fluids and exogenous bicarbonate to prevent acidosis-related complications. Even more important is the prompt identification and treatment of the inciting etiology and remains the only successful approach to reversing the LA. This implies the use of chemotherapy in lymphomatous tumors.Citation9
In general, the diagnosis of type B lactic acidosis in hematologic malignancy appears to be an ominous prognostic sign which has been reported to occur both at the time of first diagnosis and relapse.Citation10 Most patients with LA die within hours to months after the diagnosis of malignancy-related LA, and only a few patients respond to chemotherapy with remission and permanent resolution of LA.Citation6 Hence, lactic acidosis reflects aggressive disease activity and can be interpreted as a poor prognostic marker that is associated with a high risk of recurrence, progression or treatment refractoriness.Citation9
Conclusion
Our case illustrates a typical presentation of malignancy-associated type B lactic acidosis and emphasizes that prompt treatment of the underlying malignancy can result in resolution in some patients.
References
- Ruiz JP, Singh AK, Hart P. Type B lactic acidosis secondary to malignancy: case report, review of published cases, insights into pathogenesis, and prospects for therapy. Scientific World Journal. 2011;11:1316–1324. doi:10.1100/tsw.2011.125.
- Robak T, Huang H, Jin J, Zhu J, Liu T, Samoilova O, Pylypenko H, Verhoef G, Siritanaratkul N, Osmanov E, et al. Bortezomib-based therapy for newly diagnosed mantle-cell lymphoma. N Engl J Med. 2015;372(10):944–953. doi:10.1056/NEJMoa1412096.
- Luft D, Deichsel G, Schmulling RM, Stein W, Eggstein M. Definition of clinically relevant lactic acidosis in patients with internal diseases. Am J Clin Pathol. 1983;80(4):484–489. doi:10.1093/ajcp/80.4.484.
- Fall PJ, Szerlip HM. Lactic acidosis: from sour milk to septic shock. J Intensive Care Med. 2005;20(5):255–271. doi:10.1177/0885066605278644.
- Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. doi:10.1126/science.1160809.
- Martinez-Outschoorn UE, Whitaker-Menezes D, Valsecchi M, Martinez-Cantarin MP, Dulau-Florea A, Gong J, Howell A, Flomenberg N, Pestell RG, Wagner J, et al. Reverse Warburg effect in a patient with aggressive B-cell lymphoma: is lactic acidosis a paraneoplastic syndrome? Semin Oncol. 2013;40(4):403–418. doi:10.1053/j.seminoncol.2013.04.016.
- Sillos EM, Shenep JL, Burghen GA, Pui C, Behm FG, Sandlund JT. Lactic acidosis: a metabolic complication of hematologic malignancies. Cancer. 2001;92(9):2237–2246. doi:10.1002/1097-0142(20011101)92:9<2237::AID-CNCR1569>3.0.CO;2-9.
- Ohtsubo K, Imamura R, Seki R, Ohshima K, Hashiguchi M, Yakushiji K, Yoshimoto K, Ogata H, Okamura T, Sata M. Blastoid variant of mantle cell lymphoma with lactic acidosis: a case report. Int J Hematol. 2004;80(5):428–431. doi:10.1532/IJH97.04069.
- Chan FH, Carl D, Lyckholm LJ. Severe lactic acidosis in a patient with B-cell lymphoma: a case report and review of the literature. Case Rep Med. 2009;2009:534561. doi:10.1155/2009/534561.
- Friedenberg AS, Brandoff DE, Schiffman FJ. Type B lactic acidosis as a severe metabolic complication in lymphoma and leukemia: a case series from a single institution and literature review. Medicine (Baltimore). 2007;86(4):225–232. doi:10.1097/MD.0b013e318125759a.
