ABSTRACT
Significant progress has been made in the diagnosis and treatment of cancer; however, significant challenges remain. Conditionally replicating adenoviruses (CRAds), which not only kill cancer cells, but also serve as vectors to express therapeutic genes, are a novel and effective method to treat cancer. However, most adenoviruses are Ad5, which infect cells through the coxsackie and adenovirus receptor (CAR). The transduction efficacy of Ad5 is restricted because of the absent or low expression of CAR on several cancer cells. Ad serotype 35 has a different tropism pattern to Ad5. Ad35 attaches to cells via a non-CAR receptor, CD46, which is expressed widely on most tumor cells. Thus, chimeric adenoviral vectors consisting of the knob and shaft of Ad35 combined with Ad5 have been constructed. The chimeric fiber adenoviral vectors can transduce CAR-positive and CAR-negative cell lines. In this review, we explore the application of the novel fiber chimeric conditionally replicative adenovirus-Ad5/F35 in tumor therapy in terms of safety, mechanism, transduction efficacy, and antitumor effect.
Introduction
With advances in imaging and diagnostic techniques, we have obtained a more comprehensive understanding of the molecular processes leading to cancer. In addition, using a combination of chemotherapy and radiotherapy, overall survival rates have improved significantly in patients with cancer. However, cancer remains the second leading cause of death, mostly because of the limited efficacy of chemotherapeutic agents in killing tumors.Citation1,Citation2 Traditional research and development has focused on the development of a curative agent for each type of cancer; however, tolerance to chemotherapy drugs greatly reduces the effectiveness of cancer treatment. Combination therapy, as the most effective method to improve the survival rate, has attracted much attention.
An effective and promising approach to treat malignant tumors is the application of conditionally replicating adenoviruses (CRAds),Citation3 which can replicate in, and lyse, tumor cells, but do not affect normal cells. When infected cancer cells are lysed, CRAds are released, and then adjacent tumor cells are infected. This characteristic is superior to non-replicating adenoviruses (Ads).Citation4 In addition, CRAds may be used as a vehicle to deliver a therapeutic transgene to further mediate gene therapy by targeting tumor cells. CRAds not only selectively replicate in and kill cancer cells, but also enhance therapeutic gene expression and function in the tumor microenvironment.Citation5
Some laboratories have found that CRAds induce an enhanced antitumor activity when armed with antiproliferative and proapoptotic transgenes, for example, second mitochondria-derived activator of caspases (Smac).Citation6 tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL).Citation7 melanoma differentiation-associated gene-7/interleukin-24 (mda-7/IL-24).Citation8 a short interfering RNA (siRNA) against the antiapoptotic factor Apollon.Citation9 and the antioxidant enzyme, manganese superoxide dismutase (MnSOD).Citation10 In addition, CRAds armed with immunostimulatory cytokines, such as granulocyte-macrophage colony-stimulating factorCitation11 heat shock protein 21.Citation12 and interleukin-12 (IL-12)Citation13 can recruit effector cells and enhance host antitumor immunity. Furthermore, CRAds can also mediate suicide genes, such as the herpes simplex virus thymidine kinase suicide gene (HSV-TK).Citation14
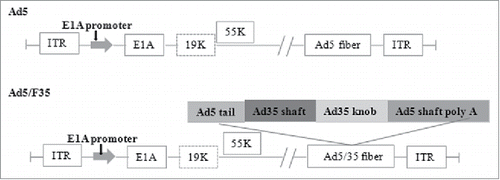
The antitumor efficacy of CRAds is associated with the ability of adenoviruses to infect tumor cells. However, many factors influence the infection capability of CRAds. One of the most critical factors is the expression level of the adenovirus receptor on the surface of tumor cells.Citation15,Citation16 Adenoviruses infect tumor cells by binding to their target receptor on the cell surface. Most of traditional adenoviral vectors are serotype 5 adenovirus (Ad5) of subgroup C, whose receptor on the cell membrane is coxsackie and adenovirus receptor (CAR).Citation17 However, the ability of Ad5 to infect a variety of primary tumor cells is often limited by the absent or low expression of CAR on several cancer cells.Citation18 To improve adenoviral infectivity, many researchers considered using other Ad serotypes that infect cells via non-CAR receptors. Certain subgroup B adenoviruses can infect tumor cells via the non-CAR receptor, CD46, which is a widely expressed membrane cofactor protein. To make CRAds that can exert an anti-tumor effect on various tumors, chimeric adenoviruses that can bind to CAR and CD46 have been constructed. Chimeric adenoviruses comprise the knob and shaft of adenovirus of serotype 35 (replacing the knob and shaft of Ad5), such that the chimeric adenovirus inherits the infection characteristics of Ad35, which can transduce many CAR-negative tumor cells.Citation19 Both Ad3 and Ad35 attach to tumor cells via the non-CAR receptor, CD46. However, compared with Ad3, Ad35 has more cell membrane receptors, closer integration with the CD46 molecules, and can infect a variety of tumor cells efficiently.Citation20 Thus, researchers have constructed a novel fiber chimeric adenovirus-Ad5/F35 that can transduce cell lines with absent or low expression of CAR more efficiently than Ad5 vectors. The novel fiber chimeric adenoviruses can infect both CAR-positive and CAR-negative tumor cells. shows a diagram comparing the fiber structures of Ad5 and Ad5/Ad35.
In this review, we provide an overview of the novel fiber chimeric adenoviruses for tumor therapy.
The safety of the novel fiber chimeric adenovirus
The anti-tumor effect of Ad5-based vectors on various tumor cells has been observed. Ad5-based vectors are well tolerated when injected directly into tumors; however, toxicity associated with non-oncolytic Ad5 administration has been observed at high systemic doses, and might induce complement activation, cytokine release, and consequent vascular damage, which leads to systemic inflammatory responses. The uptake of Ad5 by its target cells can induce elevation of liver transaminases, tissue damage, and release of cytokines and chemokines, which in turn lead to inflammation and the induction of an antiviral adaptive immune response.Citation21–Citation24 Thus, the safety of systemic adenovirus injection has received significant research attention.
The safety of Ad5/F35 vectors should be considered seriously if Ad5/F35 vectors are to be applied in clinical trials. Compared with animals injected with Ad5 vectors, animals injected with Ad5/F35 vectors had lower serum proinflammatory cytokine levels,Citation25–Citation27 lower toxicity,Citation28,Citation29 lower liver enzyme levels,Citation27,Citation30 a higher survival rate, and less weight loss.Citation27 Recently, a studyCitation31 evaluating the effects of Ad5/F35 vectors on the heart found that Ad5 vectors evoked more severe tissue damage, with large areas of interstitial inflammatory cell infiltration and myocyte necrosis, compared with Ad5/F35 vectors. Taken together, these studies suggested that the chimeric adenovirus Ad5/F35 has a better safety profile than Ad5.
Possible mechanisms of the antitumor effect of Ad5/F35 mediated therapy
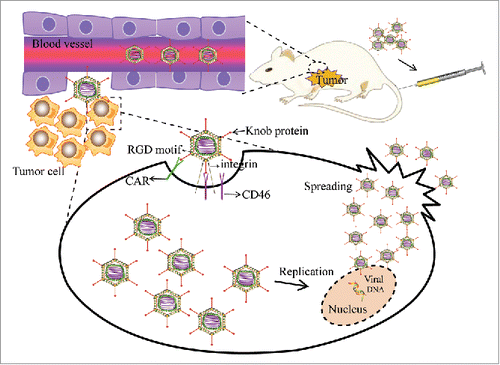
When CRAds are administrated into the body, they initially pass through the connective tissue and extracellular matrix (ECM), and then enter cells through a series of complex processes to kill tumor cells. A schematic diagram of these processes is shown in . The Ad35 fiber knob in Ad5/F35 attaches to CD46, and once attached, an Arg-Gly-Asp (RGD) motif in the penton base interacts with integrin which is a secondary receptor, resultly in promotion of endocytosis and internalization of the adenovirus.Citation32–Citation34 After cell internalization, viruses escape from the endosome into the cytosol. Viral escape is one of the most important steps of infection and one of the least understood. CRAds then traffic toward the nucleus along microtubules, via interactions with the motor protein dynein.Citation35 The viral genome is then delivered to the cell nucleus. Ad5/F35 then selectively replicates in tumor cells, and lyses then via apoptosis. The released adenovirus then infects adjacent tumor cells.
Figure 2. The mechanism of the antitumor effect of vectors incorporating the novel chimeric fiber Ad5/F35 adenovirus. Ad5/F35 first attaches to cells via CD46 or CAR. Once attached, the RGD motif in the penton base interacts with integrin to promote endocytosis and internalization of the adenovirus. Ad5/F35 then lyses the tumor cells via apoptosis. The released adenoviruses infect adjacent tumor cells.
Factors influencing the transduction efficacy of the novel fiber chimeric adenovirus Ad5/F35
The transduction efficacy of adenoviruses depends mainly on the capacity of the adenovirus to enter the targeted cells and whether they successfully transfer their genome into the host nucleus, which is largely dependent on the intracellular route by which the virus travels within the host cells. Hence, all factors that participate in this process can influence the transduction efficacy of adenoviruses ().
Table 1. Factors influencing the transduction efficacy of Ad5/F35 vectors.
When chimeric adenoviruses are injected into the body, they are first cleared by neutralizing antibodies (nAbs) in the humoral immune system. nAbs against type 5 adenovirus are commonly ecountered.Citation36 and greatly weaken the effect of Ad5.Citation37 However, less than 10% of patients can produce nAbs to Ad35Citation36,Citation38,Citation39 because people are exposed to Ad5 more frequently than to Ad35. However, nAbs are not the most critial factor influencng transduction efficiency. The adenovirus receptor is one of the most important factors that influence transduction efficiency.Citation15,Citation16 CAR and integrins are the main factors that influence the transduction efficacy of Ad5. CAR and integrins are low in most tumor cells. However, in contrast Ad5, the expression level of CD46 is a vital factor that influences the transduction efficacy of Ad5/F35. Ad5/F35 can improve the systemic gene delivery properties of the adenovirus vectors in human and rodent cells, especially in cells lacking in sufficient CAR expression.Citation40 Furthermore, Koizumia et al.Citation41 and Acharya et al.Citation42 found that the transduction efficacy of Ad5/F35 vector was significantly higher in trophoblast cell lines and renal carcinoma cells with high expression CD46, compared with that of Ad5 vectors. However, in both human dilated cardiomyopathy (DCM) hearts and non-DCM hearts with higher CAR expression and lower CD46 expression, the transduction efficacy of Ad5 was higher than that of Ad5/F35.Citation31,Citation43 Thus, the level of CD46, not CAR, mainly affects the transduction efficacy of Ad5/F35 vectors However, interestingly, Yu et al. found that in esophageal and oral tumor cellsCitation44 and pancreatic and breast cancer cells.Citation45 the transduction efficacy of Ad5/F35 was influenced by other receptors, and did not correlate with CD46.
Integrin is the second receptor for Ad5/F35 binding to targeted cells. The interaction between integrin and the RGD motif can increase the rate of adenovirus entry into cells and contributes to adenovirus escaping from endosomes, eventually improving the transduction efficacy. For example, Shayakhmetov et al.Citation46 found that deletion of RGD motify significantly reduced the rate of adenovirus internalization for Ad5/F35 vectors.
Binding to CD46 is significantly inhibited by inserting foreign peptides into HI, IJ, or FG loops in the fiber knob of Ad35, especially the HI loop, because the HI loop is the most crucial region for CD46 binding. However, in CD46-negative cells, insertion of foreign peptides into the HI, IJ, or FG loop can significantly increase the transduction efficacy of Ad5/F35 vectors.Citation47 Another studyCitation20 showed that insertion of foreign peptides into the HI or FG loops mediated the highest transduction efficacy in CD46-negative cells. In CD46-positive cells, the transduction efficacy of Ad5/F35 vectors containing two copies of the foreign peptide was higher than that of that of Ad5/F35 containing one copy of the foreign peptide, although lower than that of parent Ad5/F35.Citation20 These observations demonstrated that insertion of two copies of the foreign peptides would reduce the affinity to CD46, but not by as much as inserting one copy of the foreign peptide, because multimeric foreign peptides have a higher binding affinity for integrins than monomeric foreign peptides.Citation48–Citation50 Thus, the affinity of adenovirus for CD46 also influences the transduction efficacy of Ad5/F35.Citation51,Citation52 Wang et al.Citation51 found that the affinity of Ad5/F35 for CD46 produced different transduction efficiencies in vitro and in vivo. In vitro, regardless of the CD46 receptor density on the cells, the higher affinities of Ad5/35+ and Ad5/35++ to CD46 did not lead to correspondingly higher transduction efficiency. Whereas, in vivo, Ad5/F35 vectors with increased affinity for CD46 showed higher transduction efficiency. This is because Ad5/35 vectors with increased affinity to CD46 can be sequestered less efficiently by blood cells and tissue macrophages after intravenous injection. Similarly, Shayakhmetov et al.Citation52 found that the transduction efficiency of Ad5/F35 in vivo was not only dependent on the high affinity for CD46, but also was influenced strongly by the surrounding ECM.
Recent evidence has demonstrated that connective tissue and ECM components may play an important role in inhibiting viral spread following administration of adenovirus.Citation53–Citation55 Therefore, wider viral distribution is observed when the ECM is degraded. Viral spread can be improved by proteolytic enzymesCitation56 including hyaluronidase and matrix metalloproteinases. Ganesh et al.Citation57 observed that the transduction efficiency increased using a combination of Ad5/F35 and hyaluronidase, which degraded the hyaluronan-rich matrix and depolymerized viscoelastic ECM components.Citation58,Citation59 The peptide hormone Relaxin could upregulate matrix metalloproteinasesCitation58,Citation60–Citation63 and increase the spread of adenoviruses within tumors when combined with Ad5/F35.Citation64
The anti-tumor effect of CRAd-mediated therapy is significantly hindered by coagulation factor X (FX), which binds to CRAds, especially Ad5.Citation65,Citation66 However, transduction with an Ad5/F35 vector in epithelial ovarian cancer cells (EOCs) in vitro was not affected by FXCitation67 because binding of Ad35 to FX is either not detectable.Citation68 or much lower than that of Ad5.Citation69 X-binding protein (X-bp), a snake venom-derived protein that binds to FX with high affinity, can inhibit the binding of Ad5 to FX.Citation70 In addition, X-bp significantly reduced liver transduction after injection of Ad5 vectors.Citation69 Similarly, Liu et al.Citation71 and Greig et al.Citation72 showed that X-bp improved the transduction of Ad5/F35 in liver metastases and increased the antitumor efficacy of Ad5/35-based vectors by reducing hepatocyte transduction and increasing the circulation time after intravenous injection of Ad5/F35 vectors.
In addition, features of cells, such as endocytosis, trafficking, and sorting mechanisms for animal viruses, have a critical influence on the fate of the infecting virus.Citation73–78 Drouin et al.Citation77 found that the selection of intracellular trafficking routes is not only determined by the fiber knob domain and the cellular receptor, but also by the targeted cell type. For efficiently transduced cells, adenoviruses were localized in early endosomes or the cytosol. By contrast, in poorly transduced cells, they were localized within late endosomes/lysosomes. Phosphatase inhibitors (PSPs) can later the intracellular entry routes. PSPs are involved in cell cycle regulation and apoptosisCitation78 and treatment with PSPs, such as okadaic acid, can increase caveolae endocytosis.Citation79,Citation80 In addition, Cayer et al.Citation81 found that the only PSPs that could enhance the transduction efficacy of Ad5/F35 were those specific to protein phosphatase 2 phosphatase activator (PP2A). Thus, PSPs, especially those targeting PP2A, have a major effect on the transduction efficiency of Ad5/F35. Interestingly, Samson et al.Citation78 found that there was a link between virus endocytic trafficking and the metabolic activity of the host cell. Ad5/F35 showed higher transduction efficiency in cells displaying strong transcriptional activity, which results in differences in intracellular trafficking. Kim et al.Citation82 showed that the transduction efficiency of Ad5/F35 specifically correlates with the survival potential of the cell type. The transduction efficacy of Ad5/F35 was enhanced in certain cancer cells that were exposed to rapamycin, an autophagy inducer.
Thus, we concluded that many factors can influence the transduction of Ad5/F35, and that Ad5/F35-mediated therapy for cancers is a complex process.
The application of the fiber chimeric adenovirus Ad5/F35 in tumor therapy
Although CRAds can be used to treat cancer, they still cannot completely eradicate tumors. To achieve a more effective anti-tumor effect, combination therapies, such as CRAds with chemotherapy, CRAds with radiotherapy, CRAds-mediated gene therapy, and CRAds-mediated gene therapy in combination with chemotherapy or radiotherapy, have been developed. The following text comprises a review of Ad5/F35 vectors in cancer therapy.
Ad5/F35 alone for cancer treatment
The novel oncolytic adenovirus-Ad5/F35 can produce an oncolytic effect on most cancers (). For example, in head and neck cancer in vitro and in vivo,Citation83,Citation84 melanoma cells in vitro and in vivo,Citation83 glioblastoma in vitro and in vivo,Citation85 B-lymphocytic malignancies in vitro and in vivoCitation86 and renal cancer cells in vitro.Citation42 Ad5/F35 administration into tumors significantly inhibit growth and enhance apoptosis. These studies demonstrated that the anti-tumor effect of Ad5/F35 is higher than that of Ad5 in most tumors with high expression of CD46.
Table 2. Studies using Ad5/F35-based vectors in various cancers.
Ad5/F35-mediated gene therapy
As an oncolytic transgene delivery system, CRAds can amplify therapeutic gene expression and function in the tumor microenvironment.Citation5 This makes CRAds- mediated gene therapy a promising method for tumor therapy. To date, some laboratories have constructed CRAds armed with various genes, such as proapoptotic transgenes, immunostimulatory cytokines, and suicide genes. Several studies have demonstrated that Ad5/F35 vectors-mediated gene therapy has significant anti-tumor effect in a variety of cancers (), such as glioblastomaCitation87 and leukemia.Citation88 in vivo and in vitro via TRAIL; hepatocellular carcinoma in vivo and in vitro by mediating XAF1 (XIAP associated factor 1)Citation89 which induces apoptosis or p53Citation90 breast cancer in vivo and in vitro via p53Citation91 and renal cancer in vitro and in vivo by mediating Hep27 (encoding dehydrogenase/reductase 2)Citation92 which can inhibit the degradation of p53 by binding to Mdm2 (mouse double minute 2, human homolog of; p53-binding protein).
In addition, the replication capacity of CRAds can be expanded by including a promoter that controls E1 gene expression, which is the necessary for CRAd replication in cells. summarizes studies demonstrating the effect of Ad5/F35 vectors containing other promoters, such as TERT, which is the telomerase reverse transcriptase promoter in head and neck cancer in vivo and in vitro.Citation93 Other studies used MKp, which is a midkine minimal promoter, in lung cancer in vitro.Citation94 in mesothelioma in vitroCitation95 or both in vitro and in vivo,Citation96 in bladder cancer in vitro.Citation97 and in osteosarcoma in vivo and in vitro.Citation98
Those studies demonstrated that Ad5/F35-mediated gene therapy produce stronger anti-tumor effect than Ad5/F35 alone or Ad5-mediated gene therapy.Citation89–Citation91,Citation94,Citation97–Citation98 However, some studiesCitation95,Citation96 found Ad5/F35-mediated gene therapy produces a similar anti-tumor effect compared with Ad5-mediated gene therapy in vitro, mainly because the mesothelioma cells in the study expressed a high level of CAR. Interestingly, another studyCitation96 showed that in high CAR-expressing mesothelioma cell lines, Ad5/F35 mediated gene therapy resulted in similar or even higher levels compared with Ad5 mediated gene therapy.
Thus, Ad5/F35-mediated gene therapy may be a promising antitumor strategy, with dramatic antitumor effects on most cancers, especially those with low levels of CAR.
Ad5/F35 vectors with chemotherapy
Despite the emergence of many new and effective chemotherapeutic agents, a radical cure for cancer remains a great challenge. CRAds combined with chemotherapy have shown promising results, which are summarized in . Ad5/F35 vectors in combination with chemotherapy produce a more effective anti-tumor effect than Ad5/F35 vectors alone.Citation99,Citation100 In addition, Ad5/F35 vectors combined with chemotherapy are superior to Ad5 vectors combined with chemotherapy.Citation99–Citation101 These studies implied that Ad5/F35 vectors combined with chemotherapy are a promising method to treat cancer without increased toxicity because of the lack of cross-resistance between Ad5/F35 vectors and chemotherapeutic drugs.
Ad5/F35 vectors with radiotherapy
The mechanism by which CRAds kill cancer cells is different from that of radiotherapy. This has prompted researchers to study CRAds in combination with radiation. The results of studies on Ad5/F35 vectors combined with radiation are shown in . In head and neck cancer, Ad5/F35 vectors combined with radiation significantly delayed tumor progression and increased the survival of mice bearing highly aggressive tumors in vivo.Citation101 Another study demonstrated that a combination of Ad5/F35 expressing an APE1 (apurinic/apyrimidinic endodeoxyribonuclease 1) siRNA with radiation significantly enhanced the sensitivity of colorectal cancer cells to irradiation in vitro, and enhanced the inhibition of tumor growth by irradiation in vivo.Citation102 The above studies also showed that Ad5/F35 vectors combined with radiation produce an enhanced anti-tumor effect compared with than Ad5 vectors combined with radiation. Thus, Ad5/F35 vectors combined with radiation might be an effective method to overcome the resistance of tumor cells to radiation.
Conclusions and perspectives
Compared with traditional Ad5, the novel fiber chimeric adenovirus Ad5/F35 not only binds to CAR-positive cells, but also binds to CAR-negative cells by binding to the Ad35 components, CD46, which is expressed on the surface of most cancer cells. Importantly, Ad5/F35-based vectors have higher safety and lower toxicity compared with Ad5-based vectors. Therefore, Ad5/F35 could be considered as a better choice for tumor therapy compared with Ad5-based vectors. By analyzing the mechanism of the transfer of Ad5/F35-based vectors into cancer cells, we could eliminate the factors that influence the transduction efficacy negatively, thereby increasing the antitumor effect of Ad5/F35-based vectors.
CRAds are a novel anti-tumor approach with confirmed efficacy. As novel antitumor agents, CRAds not only selectively replicate in and lyse cancer cells, but also can enhance the expression and efficacy of therapeutic genes. A number of studies have demonstrated that Ad5/F35-mediated gene therapy has greater anti-tumor effects than Ad5/F35 alone. Chemotherapy agents and radiation are important therapies for cancers. However, resistance to chemotherapeutic agents or radiation restricts their effects. Emerging evidence from preclinical and clinical trials show that Ad5/F35-mediated gene therapy, combined with chemotherapy or radiation, might have complementary or synergistic effects, leading to a greater antitumor effect than either treatment alone.
Thus, considering the characteristics of Ad5/F35-based vectors, we believe that these vectors represent a better therapeutic approach to treat cancer.
Conflict of interest
The authors have no conflicts of interest to declare.
Funding
This work was supported by the National Natural Science Foundation of China [grant numbers 81372916, 81572976], the Science and Technology Department of Jiangsu Province [grant number BK20141142], the China Postdoctoral Science Foundation [grant numbers 2017T100407, 2016M590505], and the Jiangsu Provincial Medical Talent Foundation.
References
- Perez-Tomas R. Multidrug resistance: retrospect and prospects in anti-cancer drug treatment. Current medicinal chemistry. 2006;13: 1859–76.
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA: A cancer journal for clinicians. 2009;59:225–49.
- Kirn D, Martuza RL, Zwiebel J. Replication-selective virotherapy for cancer: Biological principles, risk management and future directions. Nature medicine. 2001;7:781–7.
- Qian W, Liu J, Tong Y, Yan S, Yang C, Yang M, Liu X. Enhanced antitumor activity by a selective conditionally replicating adenovirus combining with MDA-7/interleukin-24 for B-lymphoblastic leukemia via induction of apoptosis. Leukemia. 2008;22:361–9.
- Cody JJ, Douglas JT. Armed replicating adenoviruses for cancer virotherapy. Cancer gene therapy. 2009;16:473–88.
- Pei Z, Chu L, Zou W, Zhang Z, Qiu S, Qi R, Gu J, Qian C, Liu X. An oncolytic adenoviral vector of Smac increases antitumor activity of TRAIL against HCC in human cells and in mice. Hepatology. 2004;39:1371–81.
- Pan Q, Liu B, Liu J, Cai R, Liu X, Qian C. Synergistic antitumor activity of XIAP-shRNA and TRAIL expressed by oncolytic adenoviruses in experimental HCC. Acta oncologica. 2008;47:135–44.
- Zhao L, Gu J, Dong A, Zhang Y, Zhong L, He L, Wang Y, Zhang J, Zhang Z, Huiwang J, et al. Potent antitumor activity of oncolytic adenovirus expressing mda-7/IL-24 for colorectal cancer. Human gene therapy. 2005;16:845–58.
- Chu L, Gu J, Sun L, Qian Q, Qian C, Liu X. Oncolytic adenovirus-mediated shRNA against Apollon inhibits tumor cell growth and enhances antitumor effect of 5-fluorouracil. Gene therapy. 2008;15:484–94.
- Zhang Y, Gu J, Zhao L, He L, Qian W, Wang J, Wang Y, Qian Q, Qian C, Wu J, et al. Complete elimination of colorectal tumor xenograft by combined manganese superoxide dismutase with tumor necrosis factor-related apoptosis-inducing ligand gene virotherapy. Cancer research. 2006;66:4291–8.
- Bristol JA, Zhu M, Ji H, Mina M, Xie Y, Clarke L, et al. In vitro and in vivo activities of an oncolytic adenoviral vector designed to express GM-CSF. Molecular therapy: the journal of the American Society of Gene Therapy. 2003;7:755–64.
- Ren Z, Ye X, Fang C, Lu Q, Zhao Y, Liu F, Liang M, Hu F, Chen HZ, et al. Intratumor injection of oncolytic adenovirus expressing HSP70 prolonged survival in melanoma B16 bearing mice by enhanced immune response. Cancer biology & therapy. 2008;7:191–95.
- Bortolanza S, Bunuales M, Otano I, Gonzalez-Aseguinolaza G, Ortiz-de-Solorzano C, Perez D, Prieto J, Hernandez-Alcoceba R. Treatment of pancreatic cancer with an oncolytic adenovirus expressing interleukin-12 in Syrian hamsters. Molecular therapy: the journal of the American Society of Gene Therapy. 2009;17:614–22.
- Bailey SM, Knox RJ, Hobbs SM, Jenkins TC, Mauger AB, Melton RG, Burke PJ, Connors TA, Hart IR. Investigation of alternative prodrugs for use with E. coli nitroreductase in ‘suicide gene’ approaches to cancer therapy. Gene therapy. 1996;3:1143–50.
- Bauerschmitz GJ, Barker SD, Hemminki A. Adenoviral gene therapy for cancer: from vectors to targeted and replication competent agents (review). International journal of oncology. 2002;21:1161–74.
- Kanerva A, Hemminki A. Modified adenoviruses for cancer gene therapy. International journal of cancer. 2004;110:475–80.
- Hamilton MM, Byrnes GA, Gall JG, Brough DE, King CR, Wei LL. Alternate serotype adenovector provides long-term therapeutic gene expression in the eye. Molecular vision. 2008;14:2535–46.
- Ballard EN, Trinh VT, Hogg RT, Gerard RD. Peptide targeting of adenoviral vectors to augment tumor gene transfer. Cancer gene therapy. 2012;19:476–88.
- Kanerva A, Mikheeva GV, Krasnykh V, Coolidge CJ, Lam JT, Mahasreshti PJ, et al. Targeting adenovirus to the serotype 3 receptor increases gene transfer efficiency to ovarian cancer cells. Clinical cancer research: an official journal of the American Association for Cancer Research. 2002;8:275–80.
- Matsui H, Sakurai F, Katayama K, Kurachi S, Tashiro K, Sugio K, Kawabata K, Mizuguchi H. Enhanced transduction efficiency of fiber-substituted adenovirus vectors by the incorporation of RGD peptides in two distinct regions of the adenovirus serotype 35 fiber knob. Virus research. 2011;155:48–54.
- Lieber A, He CY, Meuse L, Schowalter D, Kirillova I, Winther B, Kay MA. The role of Kupffer cell activation and viral gene expression in early liver toxicity after infusion of recombinant adenovirus vectors. Journal of virology. 1997;71:8798–807.
- Muruve DA, Barnes MJ, Stillman IE, Libermann TA. Adenoviral gene therapy leads to rapid induction of multiple chemokines and acute neutrophil-dependent hepatic injury in vivo. Human gene therapy. 1999;10:965–76.
- Muruve DA. The innate immune response to adenoviral vectors. Hum Gene Ther. 2004;15:1157–66.
- Shayakhmetov DM, Li ZY, Ni S, Lieber A. Interference with the IL-1-signaling pathway improves the toxicity profile of systemically applied adenovirus vectors. Journal of immunology. 2005;174:7310–9.
- Ni S, Bernt K, Gaggar A, Li ZY, Kiem HP, Lieber A. Evaluation of biodistribution and safety of adenovirus vectors containing group B fibers after intravenous injection into baboons. Human gene therapy. 2005;16:664–77.
- DiPaolo N, Ni S, Gaggar A, Strauss R, Tuve S, Li ZY, et al. Evaluation of adenovirus vectors containing serotype 35 fibers for vaccination. Molecular therapy: the journal of the American Society of Gene Therapy. 2006;13:756–65.
- Ganesh S, Gonzalez-Edick M, Gibbons D, Waugh J, Van Roey M, Jooss K. Evaluation of biodistribution of a fiber-chimeric, conditionally replication-competent (oncolytic) adenovirus in CD46 receptor transgenic mice. Human gene therapy. 2009;20:1201–13.
- Yotnda P, Onishi H, Heslop HE, Shayakhmetov D, Lieber A, Brenner M, Davis A. Efficient infection of primitive hematopoietic stem cells by modified adenovirus. Gene therapy. 2001;8:930–7.
- Ni S, Gaggar A, Di Paolo N, Li ZY, Liu Y, Strauss R, Sova P, Morihara J, Feng Q, Kiviat N, et al. Evaluation of adenovirus vectors containing serotype 35 fibers for tumor targeting. Cancer gene therapy. 2006;13:1072–81.
- Shinozaki K, Suominen E, Carrick F, Sauter B, Kahari VM, Lieber A, Woo SL, Savontaus M. Efficient infection of tumor endothelial cells by a capsid-modified adenovirus. Gene therapy. 2006;13:52–9.
- Toivonen R, Koskenvuo J, Merentie M, Soderstrom M, Yla-Herttuala S, Savontaus M. Intracardiac injection of a capsid-modified Ad5/35 results in decreased heart toxicity when compared to standard Ad5. Virology journal. 2012;9:296.
- Lindert S, Silvestry M, Mullen TM, Nemerow GR, Stewart PL. Cryo-electron microscopy structure of an adenovirus-integrin complex indicates conformational changes in both penton base and integrin. Journal of virology. 2009;83:11491–501.
- Wickham TJ, Filardo EJ, Cheresh DA, Nemerow GR. Integrin alpha v beta 5 selectively promotes adenovirus mediated cell membrane permeabilization. The Journal of cell biology. 1994;127:257–64.
- Wickham TJ, Mathias P, Cheresh DA, Nemerow GR. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–19.
- Kelkar SA, Pfister KK, Crystal RG, Leopold PL. Cytoplasmic dynein mediates adenovirus binding to microtubules. Journal of virology. 2004;78:10122–32.
- D'Ambrosio E, Del Grosso N, Chicca A, Midulla M. Neutralizing antibodies against 33 human adenoviruses in normal children in Rome. The Journal of hygiene. 1982;89:155–61.
- Uusi-Kerttula H, Legut M, Davies J, Jones R, Hudson E, Hanna L, Stanton RJ, Chester JD, Parker AL. Incorporation of Peptides Targeting EGFR and FGFR1 into the Adenoviral Fiber Knob Domain and Their Evaluation as Targeted Cancer Therapies. Human gene therapy. 2015;26:320–9.
- Nwanegbo E, Vardas E, Gao W, Whittle H, Sun H, Rowe D, Robbins PD, Gambotto A. Prevalence of neutralizing antibodies to adenoviral serotypes 5 and 35 in the adult populations of The Gambia, South Africa, and the United States. Clinical and diagnostic laboratory immunology. 2004;11:351–7.
- Vogels R, Zuijdgeest D, van Rijnsoever R, Hartkoorn E, Damen I, de Bethune MP, et al. Replication-deficient human adenovirus type 35 vectors for gene transfer and vaccination:efficient human cell infection and bypass of preexisting adenovirus immunity. Journal of virology. 2003;77:8263–71.
- Mizuguchi H, Hayakawa T. Adenovirus vectors containing chimeric type 5 and type 35 fiber proteins exhibit altered and expanded tropism and increase the size limit of foreign genes. Gene. 2002;285:69–77.
- Koizumi N, Mizuguchi H, Kondoh M, Fujii M, Hayakawa T, Watanabe Y. Efficient gene transfer into human trophoblast cells with adenovirus vector containing chimeric type 5 and 35 fiber protein. Biological & pharmaceutical bulletin. 2004;27:2046–8.
- Acharya B, Terao S, Suzuki T, Naoe M, Hamada K, Mizuguchi H, Gotoh A. Improving gene transfer in human renal carcinoma cells: Utilization of adenovirus vectors containing chimeric type 5 and type 35 fiber proteins. Experimental and therapeutic medicine. 2010;1:537–40.
- Toivonen R, Mayranpaa MI, Kovanen PT, Savontaus M. Dilated cardiomyopathy alters the expression patterns of CAR and other adenoviral receptors in human heart. Histochemistry and cell biology. 2010;133:349–57.
- Yu L, Takenobu H, Shimozato O, Kawamura K, Nimura Y, Seki N, Uzawa K, Tanzawa H, Shimada H, Ochiai T, et al. Increased infectivity of adenovirus type 5 bearing type 11 or type 35 fibers to human esophageal and oral carcinoma cells. Oncology reports. 2005;14:831–5.
- Yu L, Shimozato O, Li Q, Kawamura K, Ma G, Namba M, Ogawa T, Kaiho I, Tagawa M. Adenovirus type 5 substituted with type 11 or 35 fiber structure increases its infectivity to human cells enabling dual gene transfer in CD46-dependent and -independent manners. Anticancer research. 2007;27:2311–6.
- Shayakhmetov DM, Eberly AM, Li ZY, Lieber A. Deletion of penton RGD motifs affects the efficiency of both the internalization and the endosome escape of viral particles containing adenovirus serotype 5 or 35 fiber knobs. Journal of virology. 2005;79:1053–61.
- Matsui H, Sakurai F, Kurachi S, Tashiro K, Sugio K, Kawabata K, Yamanishi K, Mizuguchi H. Development of fiber-substituted adenovirus vectors containing foreign peptides in the adenovirus serotype 35 fiber knob. Gene therapy. 2009;16:1050–7.
- Boturyn D, Coll JL, Garanger E, Favrot MC, Dumy P. Template assembled cyclopeptides as multimeric system for integrin targeting and endocytosis. Journal of the American Chemical Society. 2004;126:5730–9.
- Sancey L, Garanger E, Foillard S, Schoehn G, Hurbin A, Albiges-Rizo C, et al. Clustering and internalization of integrin alphavbeta3 with a tetrameric RGD-synthetic peptide. Molecular therapy: the journal of the American Society of Gene Therapy. 2009;17:837–43.
- Ye Y, Bloch S, Xu B, Achilefu S. Design, synthesis, and evaluation of near infrared fluorescent multimeric RGD peptides for targeting tumors. Journal of medicinal chemistry. 2006;49:2268–75.
- Wang H, Liu Y, Li Z, Tuve S, Stone D, Kalyushniy O, Shayakhmetov D, Verlinde CL, Stehle T, McVey J, et al. In vitro and in vivo properties of adenovirus vectors with increased affinity to CD46. Journal of virology. 2008;82:10567–79.
- Shayakhmetov DM, Li ZY, Ni S, Lieber A. Targeting of adenovirus vectors to tumor cells does not enable efficient transduction of breast cancer metastases. Cancer research. 2002;62:1063–8.
- Harrison D, Sauthoff H, Heitner S, Jagirdar J, Rom WN, Hay JG. Wild-type adenovirus decreases tumor xenograft growth, but despite viral persistence complete tumor responses are rarely achieved–deletion of the viral E1b-19-kD gene increases the viral oncolytic effect. Human gene therapy. 2001;12:1323–32.
- Sauthoff H, Hu J, Maca C, Goldman M, Heitner S, Yee H, Pipiya T, Rom WN, Hay JG. Intratumoral spread of wild-type adenovirus is limited after local injection of human xenograft tumors: virus persists and spreads systemically at late time points. Human gene therapy. 2003;14:425–33.
- Parato KA, Senger D, Forsyth PA, Bell JC. Recent progress in the battle between oncolytic viruses and tumours. Nature reviews Cancer. 2005;5:965–76.
- Kuriyama N, Kuriyama H, Julin CM, Lamborn K, Israel MA. Pretreatment with protease is a useful experimental strategy for enhancing adenovirus-mediated cancer gene therapy. Human gene therapy. 2000;11:2219–30.
- Ganesh S, Gonzalez-Edick M, Gibbons D, Van Roey M, Jooss K. Intratumoral coadministration of hyaluronidase enzyme and oncolytic adenoviruses enhances virus potency in metastatic tumor models. Clinical cancer research: an official journal of the American Association for Cancer Research. 2008;14:3933–41.
- Victor R, Chauzy C, Girard N, Gioanni J, d'Anjou J, Stora De Novion H, Delpech B. Human breast-cancer metastasis formation in a nude-mouse model: studies of hyaluronidase, hyaluronan and hyaluronan-binding sites in metastatic cells. International journal of cancer. 1999;82:77–83.
- Shuster S, Frost GI, Csoka AB, Formby B, Stern R. Hyaluronidase reduces human breast cancer xenografts in SCID mice. International journal of cancer. 2002;102:192–7.
- Unemori EN, Amento EP. Relaxin modulates synthesis and secretion of procollagenase and collagen by human dermal fibroblasts. The Journal of biological chemistry. 1990;265:10681–5.
- Binder C, Hagemann T, Husen B, Schulz M, Einspanier A. Relaxin enhances in-vitro invasiveness of breast cancer cell lines by up-regulation of matrix metalloproteases. Molecular human reproduction. 2002;8:789–96.
- Binder C, Simon A, Binder L, Hagemann T, Schulz M, Emons G, Trümper L, Einspanier A. Elevated concentrations of serum relaxin are associated with metastatic disease in breast cancer patients. Breast cancer research and treatment. 2004;87:157–66.
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nature reviews Cancer. 2002;2:161–74.
- Ganesh S, Gonzalez Edick M, Idamakanti N, Abramova M, Vanroey M, Robinson M, Yun CO, Jooss K. Relaxin-expressing, fiber chimeric oncolytic adenovirus prolongs survival of tumor-bearing mice. Cancer research. 2007;67:4399–407.
- Abbink P, Lemckert AA, Ewald BA, Lynch DM, Denholtz M, Smits S, Holterman L, Damen I, Vogels R, Thorner AR, et al. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. Journal of virology. 2007;81:4654–63.
- Parker AL, Waddington SN, Buckley SM, Custers J, Havenga MJ, van Rooijen N, Goudsmit J, McVey JH, Nicklin SA, Baker AH. Effect of neutralizing sera on factor x-mediated adenovirus serotype 5 gene transfer. Journal of virology. 2009;83:479–83.
- Hulin-Curtis SL, Uusi-Kerttula H, Jones R, Hanna L, Chester JD, Parker AL. Evaluation of CD46 re-targeted adenoviral vectors for clinical ovarian cancer intraperitoneal therapy. Cancer gene therapy. 2016;23:229–34.
- Kalyuzhniy O, Di Paolo NC, Silvestry M, Hofherr SE, Barry MA, Stewart PL, Shayakhmetov DM. Adenovirus serotype 5 hexon is critical for virus infection of hepatocytes in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:5483–8.
- Waddington SN, McVey JH, Bhella D, Parker AL, Barker K, Atoda H, Pink R, Buckley SM, Greig JA, Denby L, et al. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell. 2008;132:397–409.
- Atoda H, Ishikawa M, Mizuno H, Morita T. Coagulation factor X-binding protein from Deinagkistrodon acutus venom is a Gla domain-binding protein. Biochemistry. 1998;37:17361–70.
- Liu Y, Wang H, Yumul R, Gao W, Gambotto A, Morita T, Baker A, Shayakhmetov D, Lieber A. Transduction of liver metastases after intravenous injection of Ad5/35 or Ad35 vectors with and without factor X-binding protein pretreatment. Human gene therapy. 2009;20:621–9.
- Greig JA, Buckley SM, Waddington SN, Parker AL, Bhella D, Pink R, Rahim AA, Morita T, Nicklin SA, McVey JH, et al. Influence of coagulation factor x on in vitro and in vivo gene delivery by adenovirus (Ad) 5, Ad35, and chimeric Ad5/Ad35 vectors. Molecular therapy: the journal of the American Society of Gene Therapy. 2009;17:1683–91.
- Miyazawa N, Crystal RG, Leopold PL. Adenovirus serotype 7 retention in a late endosomal compartment prior to cytosol escape is modulated by fiber protein. Journal of virology. 2001;75:1387–400.
- Shayakhmetov DM, Li ZY, Ternovoi V, Gaggar A, Gharwan H, Lieber A. The interaction between the fiber knob domain and the cellular attachment receptor determines the intracellular trafficking route of adenoviruses. Journal of virology. 2003;77:3712–23.
- Colin M, Renaut L, Mailly L, D'Halluin JC. Factors involved in the sensitivity of different hematopoietic cell lines to infection by subgroup C adenovirus: implication for gene therapy of human lymphocytic malignancies. Virology. 2004;320:23–39.
- Leopold PL, Crystal RG. Intracellular trafficking of adenovirus: many means to many ends. Advanced drug delivery reviews. 2007;59:810–21.
- Drouin M, Cayer MP, Jung D. Adenovirus 5 and chimeric adenovirus 5/F35 employ distinct B-lymphocyte intracellular trafficking routes that are independent of their cognate cell surface receptor. Virology. 2010;401:305–13.
- Samson M, Jung D. Intracellular trafficking and fate of chimeric adenovirus 5/F35 in human B lymphocytes. The journal of gene medicine. 2011;13:451–61.
- Thomsen P, Roepstorff K, Stahlhut M, van Deurs B. Caveolae are highly immobile plasma membrane microdomains, which are not involved in constitutive endocytic trafficking. Molecular biology of the cell. 2002;13:238–50.
- Tagawa A, Mezzacasa A, Hayer A, Longatti A, Pelkmans L, Helenius A. Assembly and trafficking of caveolar domains in the cell: caveolae as stable, cargo-triggered, vesicular transporters. The Journal of cell biology. 2005;170:769–79.
- Cayer MP, Samson M, Bertrand C, Dumont N, Drouin M, Jung D. Suppression of protein phosphatase 2A activity enhances Ad5/F35 adenovirus transduction efficiency in normal human B lymphocytes and in Raji cells. Journal of immunological methods. 2012;376:113–24.
- Kim SY, Kang S, Song JJ, Kim JH. The effectiveness of the oncolytic activity induced by Ad5/F35 adenoviral vector is dependent on the cumulative cellular conditions of survival and autophagy. International journal of oncology. 2013;42:1337–48.
- Reddy PS, Ganesh S, Yu DC. Enhanced gene transfer and oncolysis of head and neck cancer and melanoma cells by fiber chimeric oncolytic adenoviruses. Clinical cancer research: an official journal of the American Association for Cancer Research. 2006;12:2869–78.
- Suominen E, Toivonen R, Grenman R, Savontaus M. Head and neck cancer cells are efficiently infected by Ad5/35 hybrid virus. The journal of gene medicine. 2006;8:1223–31.
- Hoffmann D, Meyer B, Wildner O. Improved glioblastoma treatment with Ad5/35 fiber chimeric conditionally replicating adenoviruses. The journal of gene medicine. 2007;9:764–78.
- Wang G, Li G, Liu H, Yang C, Yang X, Jin J, Liu X, Qian Q, Qian W. E1B 55-kDa deleted, Ad5/F35 fiber chimeric adenovirus, a potential oncolytic agent for B-lymphocytic malignancies. The journal of gene medicine. 2009;11:477–85.
- Wohlfahrt ME, Beard BC, Lieber A, Kiem HP. A capsid-modified, conditionally replicating oncolytic adenovirus vector expressing TRAIL Leads to enhanced cancer cell killing in human glioblastoma models. Cancer research. 2007;67:8783–90.
- Jin J, Liu H, Yang C, Li G, Liu X, Qian Q, Qian W. Effective gene-viral therapy of leukemia by a new fiber chimeric oncolytic adenovirus expressing TRAIL: in vitro and in vivo evaluation. Molecular cancer therapeutics. 2009;8:1387–97.
- Zhu LM, Shi DM, Dai Q, Cheng XJ, Yao WY, Sun PH, Ding Y, Qiao MM, Wu YL, Jiang SH, et al. Tumor suppressor XAF1 induces apoptosis, inhibits angiogenesis and inhibits tumor growth in hepatocellular carcinoma. Oncotarget. 2014;5:5403–15.
- Chen W, Wu Y, Liu W, Wang G, Wang X, Yang Y, Chen W, Tai Y, Lu M, Qian Q, et al. Enhanced antitumor efficacy of a novel fiber chimeric oncolytic adenovirus expressing p53 on hepatocellular carcinoma. Cancer letters. 2011;307:93–103.
- He X, Liu J, Yang C, Su C, Zhou C, Zhang Q, Li L, Wu H, Liu X, Wu M, et al. 5/35 fiber-modified conditionally replicative adenovirus armed with p53 shows increased tumor-suppressing capacity to breast cancer cells. Human gene therapy. 2011;22:283–92.
- Fang L, Cheng Q, Liu W, Zhang J, Ge Y, Zhang Q, Li L1, Liu J, Zheng J. Selective effects of a fiber chimeric conditionally replicative adenovirus armed with hep27 gene on renal cancer cell. Cancer biology & therapy. 2016;17:664–73.
- Toivonen R, Suominen E, Grenman R, Savontaus M. Retargeting improves the efficacy of a telomerase-dependent oncolytic adenovirus for head and neck cancer. Oncology reports. 2009;21:165–71.
- Kanno T, Gotoh A, Nakano T, Tagawa M, Nishizaki T. Beneficial oncolytic effect of fiber-substituted conditionally replicating adenovirus on human lung cancer. Anticancer research. 2012;32:4891–5.
- Gotoh A, Kanno T, Nagaya H, Nakano T, Tabata C, Fukuoka K, Tagawa M, Nishizaki T. Gene therapy using adenovirus against malignant mesothelioma. Anticancer research. 2012;32:3743–7.
- Takagi-Kimura M, Yamano T, Tamamoto A, Okamura N, Okamura H, Hashimoto-Tamaoki T, Tagawa M, Kasahara N, Kubo S. Enhanced antitumor efficacy of fiber-modified, midkine promoter-regulated oncolytic adenovirus in human malignant mesothelioma. Cancer science. 2013;104:1433–9.
- Gotoh A, Nagaya H, Kanno T, Tagawa M, Nishizaki T. Fiber-substituted conditionally replicating adenovirus Ad5F35 induces oncolysis of human bladder cancer cells in in vitro analysis. Urology. 2013;81:920 e7-11.
- Takagi-Kimura M, Yamano T, Tagawa M, Kubo S. Oncolytic virotherapy for osteosarcoma using midkine promoter-regulated adenoviruses. Cancer gene therapy. 2014;21:126–32.
- Hoffmann D, Bayer W, Heim A, Potthoff A, Nettelbeck DM, Wildner O. Evaluation of twenty-one human adenovirus types and one infectivity-enhanced adenovirus for the treatment of malignant melanoma. The Journal of investigative dermatology. 2008;128:988–98.
- Cho YS, Do MH, Kwon SY, Moon C, Kim K, Lee K, Lee SJ, Hemmi S, Joo YE, Kim MS. Efficacy of CD46-targeting chimeric Ad5/35 adenoviral gene therapy for colorectal cancers. Oncotarget. 2016.
- Ganesh S, Gonzalez-Edick M, Gibbons D, Ge Y, VanRoey M, Robinson M, et al. Combination therapy with radiation or cisplatin enhances the potency of Ad5/35 chimeric oncolytic adenovirus in a preclinical model of head and neck cancer. Cancer gene therapy. 2009;16:383–92.
- Xiang DB, Chen ZT, Wang D, Li MX, Xie JY, Zhang YS, Qing Y, Li ZP, Xie J. Chimeric adenoviral vector Ad5/F35-mediated APE1 siRNA enhances sensitivity of human colorectal cancer cells to radiotherapy in vitro and in vivo. Cancer gene therapy. 2008;15:625–35.