ABSTRACT
Melanoma is the deadliest form of commonly encountered skin cancer, and has fast propagating and highly invasive characteristics. Pim-3, a highly expressed oncogene in melanoma, is a highly conserved serine/threonine kinase with various biological activities, such as proliferation-accelerating and anti-apoptosis effects on cancer progression. However, whether Pim-3 regulates melanoma metastasis has not been determined. Here, we constructed a Pim-3–silencing short hairpin RNA (sh-Pim-3), a TLR7-stimulating ssRNA and a dual-function vector containing a sh-Pim-3 and a ssRNA, and transfected them into the B16F10 melanoma cell line to investigate the effects of Pim-3 on migration and invasion in melanoma. We found that sh-Pim-3 inhibited B16F10 cell migration and invasion in vitro. In a tumor-bearing mouse model, sh-Pim-3 significantly downregulated pulmonary metastasis of B16F10 melanoma cell in vivo. Mechanistically, sh-Pim-3 inhibited metastasis by regulating the expression of genes related to epithelial–mesenchymal transition (EMT). Further study revealed that by promoting the phosphorylation of STAT3 (signal transducer and activator of transcription 3), Pim-3 induced the expression of Slug, Snail, and ZEB1, which enhanced EMT-related changes and induced melanoma migration and invasion. Our study suggests that Pim-3 is a potential effective target for melanoma therapy.
KEYWORDS:
| Abbreviations | ||
| bFGF | = | basic fibroblast growth factor |
| control | = | ctrl |
| EMT | = | epithelial–mesenchymal transition |
| ELISA | = | enzyme-linked immunosorbent assay |
| FBS | = | fetal bovine serum |
| IFN | = | interferon |
| IL-6 | = | interleukin 6 |
| JAK | = | Janus kinase |
| MMP | = | matrix metalloproteinase |
| NF-κB | = | nuclear factor-κB |
| PPAR-γ | = | peroxisome proliferator–activated receptor γ |
| shRNA | = | small hairpin RNA |
| SOCS1 | = | suppressor of cytokine signaling 1 |
| ssRNA | = | single-stranded RNA |
| STAT3 | = | signal transducer and activator of transcription 3 |
| TNF-α | = | tumor necrosis factor-α |
| TLR7 | = | Toll-like receptor 7 |
| VEGF | = | vascular endothelial growth factor. |
Introduction
Melanoma is the most serious skin cancer and had highly metastatic and invasive characteristics.Citation1 Metastasis is a complex, multi-factor, and multi-step process by which primary tumor cells invade the adjacent tissue, enter the systemic circulation, translocate through the vasculature, arrest in distant capillaries, extravasate into the surrounding tissue parenchyma, and finally proliferate from microscopic growths into macroscopic secondary tumors.Citation2 The initial and pivotal step of tumor migration and invasion is epithelial–mesenchymal transition (EMT), which is accompanied by dramatic changes in cellular morphology, the loss and remodeling of cell–cell and cell–matrix adhesions, and the gain of migratory and invasive capabilities.Citation3 Various factors (e.g., transfection factors, microRNAs, epigenetic modifications, long non-coding RNAs) can activate signaling cascades that mediate EMT progression. Signaling pathways, such as that of Wnt, Notch, Hedgehog, JAK-STAT (Janus kinase–signal transducer and activator of transcription 3), ERK, and nuclear factor-κB (NF-κB), are involved in the induction or modulation of EMT.Citation3-5 EMT is also a rate-limiting step in melanoma metastasis and is associated with poor prognosis of patients with melanoma.Citation6
Pim-3, a highly conserved serine/threonine kinase, is an oncogene with various biological activities. It prevents apoptosis and promotes cell survival by phosphorylating and inactivating the proapoptotic BH3-only protein BAD.Citation7 Pim-3 is highly expressed in several endoderm-derived tumors such as hepatocellular carcinoma, adenocarcinoma, and pancreatic cancer, but is barely detected in the normal organs.Citation8-10 It is reported that the higher expression of Pim-3 is associated with tumor overgrowth or oversurvival, and Pim3 knockdown or inhibition prevents or suppresses tumorigenesis.Citation11-14 Previously, we reported that a dual-function small hairpin RNA (shRNA) vector containing a Pim-3–silencing shRNA (sh-Pim-3) and a Toll-like receptor 7 (TLR7)-stimulating ssRNA (single-stranded RNA) significantly inhibited murine hepatoma growth by promoting tumor cell apoptosis and inducing the secretion of type I interferons (IFNs).Citation11 Interestingly, in the present study, we found that therapy with this dual-functional vector not only inhibited melanoma cell growth by promoting apoptosis, but also significantly reduced pulmonary metastasis in a B16F10 tumor–bearing mouse model. Exploration of the mechanism revealed that Pim-3 bound directly to STAT3 and promoted its phosphorylation. The activated STAT3 further augmented Slug, Snail, and ZEB1 expression, which enhanced EMT-related changes and induced melanoma invasion and metastasis. Consequently, Pim-3 may be a candidate molecular target for inhibiting melanoma metastasis and invasion.
Results
Function verification of ssRNA–sh-Pim-3 dual-function vector in B16F10 cells
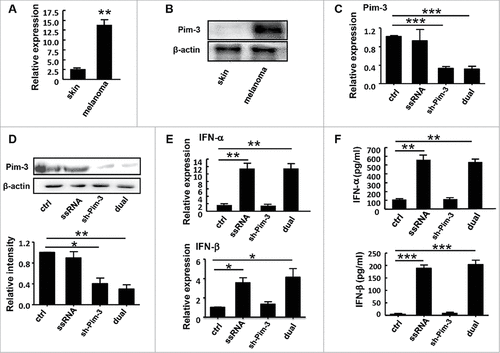
First, we compared the Pim-3 expression and metastatic capacity of two murine melanoma cell lines: B16 and B16F10. The results showed that B16F10 has a higher expression of Pim-3 and stronger ability to migration compared to B16 cells (Fig. S1). Importantly, B16F10 cell line has been usually used as a model system for melanoma metastasis studies,Citation15-17 so we used B16F10 as melanoma cell model to explore the role of Pim-3 on metastasis of melanoma. Next, we detected Pim-3 expression in mouse normal skin tissue and subcutaneously implanted B16F10 melanoma tissue by qRT-PCR and western blotting. Pim-3 expression in B16F10 melanoma tissue was significantly higher than that in normal skin tissue at both mRNA and protein level (, ). Transfection with sh-Pim-3 and dual-function vector significantly reduced Pim-3 expression in B16F10 cells at both mRNA and protein level (, ). It is known that, ssRNA acts as a ligand of TLR7 to activate the TLR7 signal pathway, leading to the production of type I IFNs and inflammatory cytokines.Citation18-20 As expected, transfection with the ssRNA and dual-function vector induced the mRNA expression of IFN-α and IFN-β in the B16F10 cells and increased the concentration of IFN-α and IFN-β in the supernatants (, ). Collectively, these results indicate that sh-Pim-3 and the dual-function vector downregulate Pim-3 expression; ssRNA and the dual-function vector stimulated the production of type I IFNs in B16F10 melanoma. These results suggested that the functional dual-function vector with both Pim-3 silencing and innate immune stimulation effects has been successfully constructed.
Figure 1. Dual-function vector silences Pim-3 expression and stimulates the production of type I IFN. Pim-3 expression in normal skin tissue and B16F10 melanoma was measured by qRT-PCR (A) and western blotting (B) B16F10 cells were transfected with pSIREN (ctrl), ssRNA, sh-Pim-3, or dual-function vector. (C) After 24 h, the gene expression of Pim-3 was assessed by qRT-PCR. (D) The protein levels of Pim-3 measured by western blotting. (E) The gene expression of IFN-α and IFN-β analyzed by qRT-PCR. (F) Supernatant levels of IFN-α and IFN-β detected by ELISA. Data are representative of three independent experiments. #P < 0.05, ##P < 0.01, ###P < 0.001 versus ctrl group.
Silencing Pim-3 reduces pulmonary metastasis of melanoma in vivo
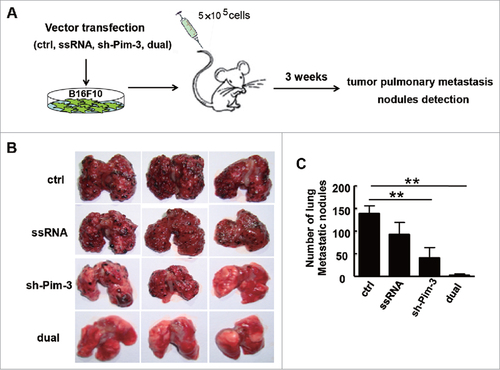
To explore the potential role of Pim-3 in melanoma metastasis, we injected C57BL/6 mice intravenously with B16F10 melanoma cells transfected with control, ssRNA, sh-Pim-3, or dual-function vector to establish the mouse model of melanoma pulmonary metastasis (). There were significantly fewer tumor nodules in the lungs in the sh-Pim-3 and dual-function vector transfection groups than in the ctrl and ssRNA groups (, ). Transfection with ssRNA also suppressed pulmonary metastasis, possibly due to the induction of type I IFNs and inflammatory cytokines as compared with the ctrl group. As expected, the dual-function vector caused the most significant inhibition (, ). These results indicate that Pim-3 might be involved in promoting melanoma metastasis.
Figure 2. In vivo inhibition of melanoma pulmonary metastasis by the therapeutic immunostimulatory sh-Pim-3 vector. (A) B16F10 cells were transfected with the vectors and injected intravenously into C57BL/6 mice (5 × 105 cells/mice). The mice were sacrificed after 3 weeks. (B) Metastatic nodules on the surface of the lungs were counted. (C) The average numbers of metastatic nodules on the lung surface. Data are representative of three independent experiments with three mice per group. ##P < 0.01 versus ctrl group.
Silencing Pim-3 inhibits B16F10 cell migration and invasion in vitro
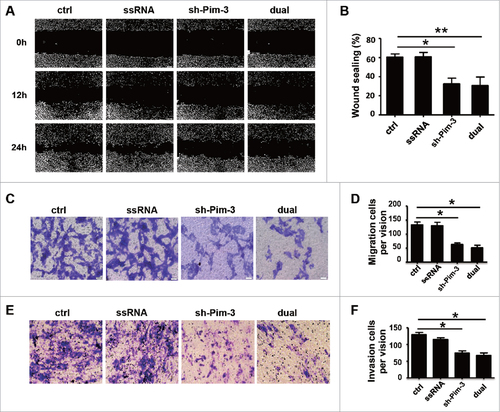
To confirm the effects of Pim-3 on B16F10 cell migration and invasion, we performed wound healing and Transwell migration assays in vitro. The wound healing assay showed an obvious decrease in the migration capability of Pim-3-silenced B16F10 cells (both sh-Pim-3 and dual-function vector group) as compared with ctrl cells (, ). Such changes were not associated with the proliferative capability of the cells, as the B16F10 cell proliferation rates did not change after transfection (Fig. S2). In addition, silencing Pim-3 significantly reduced B16F10 cell migration ability (, ). Boyden chamber membranes were pre-coated with Matrigel to test the effect of Pim-3 on B16F10 cell invasive capability. Consistent with the migration assay, silencing Pim-3 strongly decreased the number of cells that passed through the membrane (, ). These data indicate that Pim-3 plays an important role in promoting melanoma migration and invasion.
Figure 3. sh-Pim-3 inhibits B16F10 cell migration and invasion in vitro. (A, B) Wound healing assay assessment of B16F10 cell migration at 0, 12, and 24 h after transfection. (B) The wound healing assay data were averaged and summarized as the width ratio of migratory inhibition. Boyden chamber evaluation of the migration (C, D) and invasive (E, F) ability of transfected B16F10 cells. Data are representative of three independent experiments. #P < 0.05, ##P < 0.01 versus ctrl group.
Silencing Pim-3 inhibits EMT
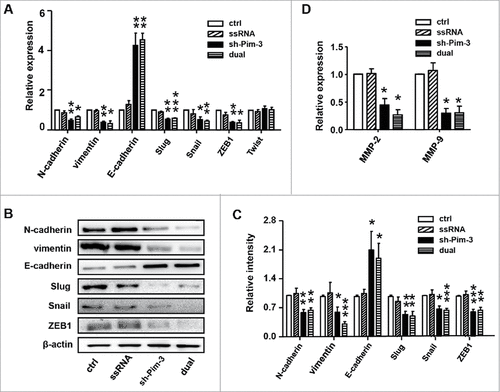
We explored the mechanisms of Pim-3 regulation of cell migration and invasion. EMT is a vital step during the early stage of metastasis. The typical characteristics in tumor cells during EMT include altered cell morphology, cytoskeleton rearrangement, and reciprocal change of several adhesion molecules such as E-cadherin, N-cadherin, and vimentin.Citation3 To determine whether silencing Pim-3 inhibited B16F10 cell invasiveness through EMT, we detected E-cadherin, N-cadherin, and vimentin expression through real-time PCR and western blotting in B16F10 cells transfected with ctrl, ssRNA, sh-Pim-3, or dual-function vector. Inhibiting Pim-3 significantly enhanced E-cadherin but reduced N-cadherin and vimentin expression (, ). We detected the expression of Snail, Slug, ZEB1, and Twist, as they are important transcription factors contributing to early EMT.Citation3,Citation21,Citation22 Transfection with shRNA and dual-function vector markedly decreased Snail, Slug, and ZEB1 expression at both mRNA and protein level, but did not impair the mRNA level of Twist (). Transfection with ssRNA did not affect the expression of these EMT markers and the related transcription factors (). We also detected the invasion-related indicators MMP-2 and MMP-9 post-transfection via real-time PCR. Silencing Pim-3 significantly inhibited the gene expression of MMP-2 and MMP-9, while ssRNA had no effect (). We conclude that silencing Pim-3 inhibits B16 cell EMT.
Figure 4. Pim-3 silencing inhibits EMT of B16F10 cells. (A) qRT-PCR detection of E-cadherin, N-cadherin, vimentin, Snail, Slug, Zeb1, and Twist expression in transfected B16F10 cells. (B) Western blot measurement of E-cadherin, N-cadherin, vimentin, Snail, Slug, and ZEB1 protein levels. (C) Statistical analysis of the relative intensity of the EMT markers. (D) qRT-PCR assessment of MMP-2 and MMP-9 expression. Data are representative of three independent experiments. #P < 0.05, ##P < 0.01, ###P < 0.001 versus ctrl group.
Pim-3 enhances B16F10 cell metastasis and invasion by phosphorylating STAT3
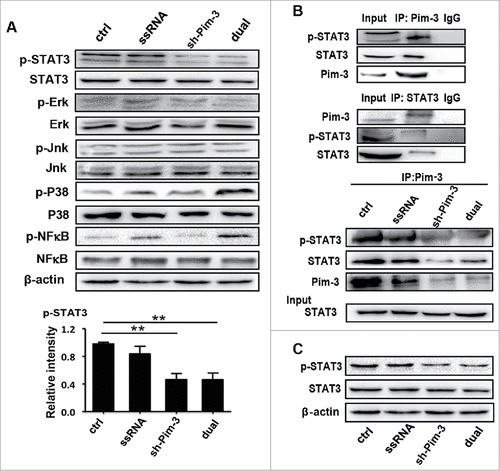
To further explore the exact molecular mechanism of Pim-3 regulation of invasion and metastasis, we examined the changes of the related signal pathways, including that of STAT3, ERK, JNK, P38, and NF-κB. B16F10 cells were transfected with ctrl, ssRNA, shRNA, or dual-function vector for 24 h, and then the phosphorylation of the indicated signal proteins was detected by western blotting. The expression of p-STAT3 was significantly lower in the cells transfected with sh-Pim-3 and dual-function vector than in the cells transfected with ctrl or ssRNA (), while the phosphorylation of the other signal proteins was not affected. Transfection with ssRNA and dual-function vector induced NF-κB and P38 activation, while silencing Pim-3 had no effect (). Next, the relationship between Pim-3 and STAT3 was detected by co-IP. Pim-3 bound to both STAT3 and p-STAT3 in the B16F10 cells, while silencing Pim-3 significantly decreased Pim-3 binding to STAT3 and p-STAT3 (). Furthermore, we detected the expression of p-STAT3 and STAT3 in metastatic nodules of the lung by Western blotting. The results showed that both treatment with the shRNA and dual-function vector significantly decreased the phosphorylation STAT3 compared with ctrl and ssRNA treatment (). These results further confirmed that Pim-3 promotes STAT3 phosphorylation.
Figure 5. Pim-3 silencing inhibits STAT3 phosphorylation in B16F10 cells. (A) Western blot detection of p-STAT3, STAT-3, p-NF-κB, NF-κB, p-P38, P38, p-JNK, JNK, p-ERK, ERK, and β-actin protein expression in B16F10 cells transfected with pSIREN (ctrl), ssRNA, sh-Pim-3, or dual-function vector for 24 h. Right: The relative intensity of p-STAT3 was analyzed. (B) Co-IP detection of the binding of endogenous Pim-3 and STAT-3 or p-STAT3 in B16F10 cells. Co-IP detection of binding of Pim-3 and STAT-3 or p-STAT3 in B16F10 cells transfected with pSIREN (ctrl), ssRNA, sh-Pim-3, or dual-function vector for 24 h. (C) The protein expression of p-STAT-3 and STAT-3 was detected by Western blotting in metastatic nodules on the lung surface. Data are representative of three independent experiments. ##P < 0.01 versus ctrl group. IgG, Immunoglobulin G.
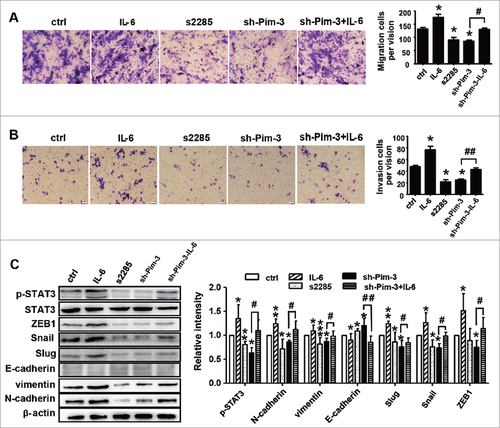
We then treated B16F10 cells with interleukin 6 (IL-6, a STAT3 stimulator) or the STAT3 inhibitor cryptotanshinone (S2285) to further confirm whether silencing Pim-3 inhibits B16F10 cell migration and invasion by downregulating STAT3 phosphorylation. First, the appropriate concentrations of IL-6 and S2285 were determined by western blotting, we found that 20 μM of S2285 and 1600 U/ml of IL-6 were appropriate (Fig. S3A/B), while and stimulating with IL-6 or s2285 had no influence on the expression of Pim-3 in B16F10 cells (Fig. S3C). S2285 (20 µM) and sh-Pim-3 significantly inhibited B16F10 cell migration and invasion, while IL-6 (1600 U/ml) promoted it. Furthermore, STAT3 activation by IL-6 significantly reversed the Pim-3 silencing-induced suppression of B16F10 cell migration and invasion (, ). STAT3 mediates EMT and is associated with the Snail and ZEB1 pathways.Citation23-25 As shown in , Pim-3 silencing regulated Slug, Snail, and ZEB1 expression, thereby inhibiting B16F10 cell EMT. Consequently, we explored whether silencing Pim-3 suppressed Slug, Snail, and ZEB1 expression and reduced EMT by inhibiting p-STAT3. Compared to sh-Pim-3-transfected B16F10 cells, IL-6-stimulated STAT3 activation significantly reversed the decrease in E-cadherin, N-cadherin, vimentin, Snail, Slug and ZEB1 expression caused by Pim-3 silencing (). These results strongly suggest that, by promoting STAT3 phosphorylation, Pim-3 augments Slug, Snail, and ZEB1 expression, which further enhances EMT-related changes and induces melanoma migration and invasion.
Figure 6. STAT3 activation reverses Pim-3 silencing suppression of B16F10 cell invasion and migration. Transwell migration assay evaluation of cell migration (A) and invasive (B) ability of B16F10 cells treated with S2285 (20 µM) or IL-6 (1600 U/ml), or transfected with sh-Pim-3 for 24 h, or pretreated with sh-Pim-3 for 6 h and then stimulated with 1600 U/ml IL-6. (C) Western blot determination of p-STAT3, STAT3, E-cadherin, N-cadherin, vimentin, Snail, Slug and ZEB1 protein expression. Data are representative of three independent experiments. #P < 0.05, ##P < 0.01 versus ctrl group; #P < 0.05, ##P < 0.01 versus shRNA group.
Discussion
The Pim-3 oncogene is frequently overexpressed in a wide range of tumors, including both hematological malignancy and solid cancer. A serine/threonine kinase, Pim-3 was originally demonstrated to promote survival signaling through the phosphorylation of BAD, which induces the release of the anti-apoptotic BCL-2 and BCL-X proteins, thereby preventing apoptosis. In the present study, we found higher Pim-3 expression in melanoma tissue than in normal skin tissue. Using our previously established ssRNA–sh-Pim-3 dual-function vector,Citation11 we found that silencing Pim-3 not only inhibited melanoma cell growth by promoting apoptosis, but also significantly reduced pulmonary metastasis in a B16F10 tumor-bearing mouse model. We found that Pim-3 bound directly to the transcription factor STAT3, and promoted STAT3 phosphorylation. The activated STAT3 further augmented the expression of Slug, Snail, and ZEB1, which enhanced EMT-related changes and induced melanoma cell invasion and metastasis (Fig. S4). As far as we know, our results provide a novel description of Pim-3 promotion of melanoma cell migration and invasion by phosphorylating STAT3. Therefore, Pim-3 may be a candidate molecular target for suppressing the metastasis and invasion of melanoma and other related tumors.
It is well-established that aberrant Pim-3 expression contributes to tumorigenesis and progression by preventing apoptosis and maintaining cell survival and the regulation of cell cycle progression.Citation7 Recently, several reports found that Pim-3 might also be involved in promoting cell invasion. For example, Pim-3 plays a role in endothelial cell spreading, migration, and tumor necrosis factor-α (TNF-α)-induced angiogenesis.Citation26 Pim-3 overexpression significantly accelerates ovarian cancer cell proliferation and migration.Citation27 Pim-3 enhances migration and metastatic growth in prostate cancer by phosphorylating the CXCR4 chemokine receptor, which might enable tumor cell migration towards tissues such as the lungs, which express the CXCL12 chemokine ligand.Citation28 In the present study, our data show that Pim-3 is highly expressed by melanoma cells (, ) and that sh-Pim-3 inhibits the migration and metastasis of murine melanoma B16F10 cells both in vitro and in vivo (, ; ). Importantly, silencing Pim-3 significantly inhibited EMT and the expression of MMP-2 and MMP-9 (). Our results indicated that Pim-3 promotes melanoma metastasis by regulating the expression of EMT-related genes and MMPs.
However, very little is known of the mechanism of Pim-3 promotion of tumor metastasis. Pim proteins mediate their physiological activities by phosphorylating a wide range of cellular substrates, such as SOCS1 (suppressor of cytokine signaling 1), BAD, and c-MYC. Recently, it was reported that the Pim-3-selective inhibitor M-110 or Pim-3-specific small interfering RNA significantly downregulate STAT3Tyr705 phosphorylation.Citation29 SGI-1776, a Pim inhibitor, specifically inhibits adipogenesis by downregulating the expression and/or phosphorylation levels of STAT3, C/EBP-α, PPAR-γ (peroxisome proliferator–activated receptor γ), and FAS.Citation30 Moreover, Pim-3 overexpression upregulated the intratumoral levels of p-STAT3Try705, p-survivinThr34, and vascular endothelial growth factor (VEGF) in human pancreatic cancer, while the increases were markedly diminished when Pim-3 was inactivated.Citation31 In the present study, we demonstrate for the first time that Pim-3 binds directly to STAT3 in B16F10 cells (), thereby promoting STAT3 phosphorylation. Indeed, silencing Pim-3 significantly decreased p-STAT3 levels and the binding of Pim-3 to STAT3 and p-STAT3 ().
Numerous studies have demonstrated the constitutive activation of STAT in a wide variety of tumors, including breast, colon, gastric, lung, head and neck, skin, prostate cancer, and melanoma.Citation32-38 Increasing evidence suggests that the STAT3 signaling pathway promotes tumor EMT,Citation39 a crucial process involved in the initiation of metastasis in melanoma and other cancers. Snail, Slug, and ZEB1 are important components of the metastatic program in melanoma cells.Citation39,Citation40 For example, STAT3 activation induced EMT through Snail activation in head and neck tumor, breast cancer, and hepatocellular carcinoma.Citation23-25 Furthermore, STAT3 activation in human melanoma promotes brain metastasis by regulating the expression of bFGF (basic fibroblast growth factor), VEGF, and MMP-2.Citation41 Our present data clearly show that STAT3 activation by IL-6 augmented the invasion, migration, and EMT changes in B16F10 melanoma, while both the STAT3 inhibitor S2285 and sh-Pim-3 significantly inhibited these changes. More importantly, IL-6 stimulation markedly attenuated sh-Pim-3–mediated suppression of invasion, migration, and EMT changes ().
It is known that, ssRNA acts as a ligand of TLR7 to activate the TLR7, comprises recruitment of MyD88, activation of the NF-kB and IRF7 pathway, and production of type I IFN and inflammatory cytokines.Citation18-20 Our data show that transfection with the ssRNA and dual-function vector induced the expression of IFN-α and IFN-β in the B16F10 cells (). We also observed the profiles of ssRNA to inhibit melanoma pulmonary metastasis in vivo (). However, transfection with ssRNA did not affect the migration and invasion of melanoma in vitro. It was reported that TLR7 activation can induced immunostimulation was concurrent with the activation of NK and T cells directly or activated antigenpresenting cells (APC) and leading to enhanced antitumor immune responses and suppression of tumor growth.Citation18-20,Citation42 Therefore, we speculate the reason why ssRNA has a role in vivo and doesn't work in vitro is likely to be that it can activate the TLR7, which may further enhances innate and adactive immune responses and inhibit melanoma pulmonary metastasis in vivo. The exact mechanism of these effects needs to be further investigated.
Collectively, our results strongly demonstrate that Pim-3 promotes melanoma cell metastasis by activating the STAT3 pathway, revealing a novel molecular mechanism of Pim-3–induced melanoma cell metastasis. Our data increase knowledge of the multiple roles of Pim-3 in melanoma and provide evidence for Pim-3 as a promising therapeutic target in melanoma and other related cancers with aberrant Pim-3 expression.
Materials and methods
Cell culture and animal model
B16F10 cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) at 37°C in a 5% CO2 incubator. C57BL/6 mice were purchased from HFK Bioscience (Beijing, China) and maintained under specific pathogen–free conditions. All mice were used according to the guidelines approved by the Shandong University Committee on the Ethics of Animal Experiments. B16F10 cells (5 × 105) transfected with pSIREN (Clontech, California, USA; control, ctrl), ssRNA, sh-Pim-3, and dual-function vector were intravenously inoculated into the C57BL/6 mice in 200 µl phosphate-buffered saline. The mice were sacrificed after 3 weeks to evaluate the pulmonary metastatic ability by counting the number of tumor nodules in the lungs.
Vector transfection
We designed the ssRNA, sh-Pim-3, and dual-function vector as described previously.Citation11 The sh-Pim-3 and dual-function vector were transfected into B16F10 cells to suppress Pim-3 expression. The ssRNA and pSIREN (empty vector) were transfected into B16F10 cells as control. Briefly, B16F10 cells were seeded in 6-well or 12-well plates in complete RPMI 1640 medium overnight and then transfected with respective vector using jet-PRIME transfection reagent (Polyplus Transfection Inc., New York, NY, USA) according to the manufacturer's instructions. After 24 h, the protein or RNA was extracted for subsequent experiments.
Wound healing assay
B16F10 cells were transfected with ssRNA, sh-Pim-3, or dual-function vector when they had grown to about 80% confluence, the cell monolayer was scraped with sterile 0.01-ml pipette tips, and then fresh medium was added. The gaps, i.e., wounds, were observed at 0, 12, and 24 h and digitally photographed under × 200 magnification.
Migration and invasion assays
The Transwell migration assay was performed using 6.5-mm diameter Transwell cell culture chamber units (Corning Costar, Cambridge, MA, USA) with 8-µm pore polycarbonate membranes. Briefly, B16F10 cells were transfected with ssRNA, sh-Pim-3, or dual-function vector for 24 h, and then 1 × 105 cells in 100 µl serum-free medium were seeded in the top chamber, while 600 µl medium containing 20% FBS was added to the bottom chamber. In the invasion assay, the membrane of the cell culture insert was covered with a film of Matrigel (BD Biosciences, San Jose, CA, USA). Cells that had migrated or invaded to the underside of the membranes were fixed in methanol and stained with crystal violet, and finally, the cells in three separate fields were counted under × 200 magnification.
Western blotting and co-immunoprecipitation (co-IP)
Western blotting was performed according to standard procedures using antibodies against Pim-3, E-cadherin, N-cadherin, vimentin, Snail, Slug, ZEB1, STAT3, phosphorylated (p)-STAT3, p–NF-κB, NF-κB, p-P38, P38, p-JNK, JNK, p-ERK, ERK, and β-actin. All antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA), except N-cadherin, which was purchased from EMD Millipore (Billerica, MA, USA). For co-IP, total cell lysates were incubated with anti–Pim-3 antibody (Cell Signaling Technology) or anti-STAT3 antibody (Cell Signaling Technology) at 4°C overnight. Then, the antibody–antigen complex was captured by Protein G agarose beads (Cell Signaling Technology). After centrifugation, the beads were collected and western blotting was performed.
Quantitative real-time PCR (qRT-PCR)
Total cellular RNA was extracted using TRIzol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol. Reverse transcription–PCR was performed using M-MLV reverse transcriptase (TianGen, Beijing, China). Samples were subjected to real-time PCR analysis using a SYBR Green kit (Roche, Indianapolis, IN, USA) on an iCycleriQ Real-Time PCR System (Bio-Rad, Hercules, CA, USA) under standard conditions. Supplementary Table I lists the primers for mouse Pim-3, IFN-α, IFN-β, E-cadherin, N-cadherin, vimentin, Snail, Slug, ZEB1, Twist, matrix metalloproteinase 2 (MMP-2), MMP-9, and β-actin. Relative gene expression was determined in comparison with β-actin.
Enzyme-linked immunosorbent assay (ELISA)
Cells were transfected with ssRNA, shRNA, or dual-function vector for 24 h and the levels of IFN-α (ExCellBiology, Shanghai, China) and IFN-β (CUSABIO, Wuhan, China) in the culture supernatants were quantified by ELISA.
Statistical analyses
All values are presented as the mean ± SEM of three independent experiments. Statistical analysis was performed using a paired Student's t-test. P values of <0.05 were determined to be statistically significant.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Sup_mat_1414756_KCBT.doc
Download ()Acknowledgments
This work was supported by the National Natural Science Foundation of China [grant numbers 81771686, 91442114, 81273220, 81472646].
Additional information
Funding
References
- Uong A, Zon LI. Melanocytes in development and cancer. J Cell Physiol. 2010;222(1):38-41. doi:10.1002/jcp.21935.
- Rinderknecht M, Detmar M. Tumor lymphangiogenesis and melanoma metastasis. J Cell Physiol. 2008;216(2):347-54. doi:10.1002/jcp.21494.
- Diepenbruck M, Christofori G. Epithelial-mesenchymal transition (EMT) and metastasis: yes, no, maybe? Curr Opin Cell Biol. 2016;43:7-13. doi:10.1016/j.ceb.2016.06.002.
- Nieto MA, Huang RY, Jackson RA, Thiery JP. Emt: 2016. Cell. 2016;166(1):21-45. doi:10.1016/j.cell.2016.06.028.
- Li S, Lu J, Chen Y, Xiong N, Li L, Zhang J, Yang H, Wu C, Zeng H, Liu Y. MCP-1-induced ERK/GSK-3beta/Snail signaling facilitates the epithelial-mesenchymal transition and promotes the migration of MCF-7 human breast carcinoma cells. Cell Mol Immunol. 2016;14(7):621-30. doi:10.1038/cmi.2015.106.
- Caramel J, Papadogeorgakis E, Hill L, Browne GJ, Richard G, Wierinckx A, Saldanha G, Osborne J, Hutchinson P, Tse G, et al. A switch in the expression of embryonic EMT-inducers drives the development of malignant melanoma. Cancer Cell. 2013;24(4):466-80 doi:10.1016/j.ccr.2013.08.018.
- Mukaida N, Wang YY, Li YY. Roles of Pim-3, a novel survival kinase, in tumorigenesis. Cancer Sci. 2011;102(8):1437-42. doi:10.1111/j.1349-7006.2011.01966.x.
- Fujii C, Nakamoto Y, Lu P, Tsuneyama K, Popivanova BK, Kaneko S, Mukaida N. Aberrant expression of serine/threonine kinase Pim-3 in hepatocellular carcinoma development and its role in the proliferation of human hepatoma cell lines. Int J Cancer. 2005;114(2):209-18. doi:10.1002/ijc.20719.
- Li YY, Popivanova BK, Nagai Y, Ishikura H, Fujii C, Mukaida N. Pim-3, a proto-oncogene with serine/threonine kinase activity, is aberrantly expressed in human pancreatic cancer and phosphorylates bad to block bad-mediated apoptosis in human pancreatic cancer cell lines. Cancer Res. 2006;66(13):6741-47. doi:10.1158/0008-5472.CAN-05-4272.
- Popivanova BK, Li YY, Zheng H, Omura K, Fujii C, Tsuneyama K, Mukaida N. Proto-oncogene, Pim-3 with serine/threonine kinase activity, is aberrantly expressed in human colon cancer cells and can prevent Bad-mediated apoptosis. Cancer Sci. 2007;98(3):321-28. doi:10.1111/j.1349-7006.2007.00390.x.
- Guo Q, Lan P, Yu X, Han Q, Zhang J, Tian Z, Zhang C. Immunotherapy for hepatoma using a dual-function vector with both immunostimulatory and pim-3-silencing effects. Mol Cancer Ther. 2014;13(6):1503-13. doi:10.1158/1535-7163.MCT-13-0722.
- Guo Q, Sui ZG, Xu W, Quan XH, Sun JL, Li X, Ji HY, Jing FB. Ubenimex suppresses Pim-3 kinase expression by targeting CD13 to reverse MDR in HCC cells. Oncotarget. 2017;8(42):72652-65. doi:10.18632/oncotarget.20194
- Wang YY, Taniguchi T, Baba T, Li YY, Ishibashi H, Mukaida N. Identification of a phenanthrene derivative as a potent anticancer drug with Pim kinase inhibitory activity. Cancer Sci. 2012;103(1):107-15. doi:10.1111/j.1349-7006.2011.02117.x.
- Xu D, Cobb MG, Gavilano L, Witherspoon SM, Williams D, White CD, Taverna P, Bednarski BK, Kim HJ, Baldwin AS, et al. Inhibition of oncogenic Pim-3 kinase modulates transformed growth and chemosensitizes pancreatic cancer cells to gemcitabine. Cancer Biol Ther. 2013;14(6):492-501. doi:10.4161/cbt.24343.
- Kim KJ, Kwon SH, Yun JH, Jeong HS, Kim HR, Lee EH, Ye SK, Cho CH. STAT3 activation in endothelial cells is important for tumor metastasis via increased cell adhesion molecule expression. Oncogene. 2017;36(39):5445-59. doi:10.1038/onc.2017.148.
- Putz EM, Guillerey C, Kos K, Stannard K, Miles K, Delconte RB, Takeda K, Nicholson SE, Huntington ND, Smyth MJ. Targeting cytokine signaling checkpoint CIS activates NK cells to protect from tumor initiation and metastasis. Oncoimmunology. 2017;6(2):e1267892. doi:10.1080/2162402X.2016.1267892.
- Purwar R, Schlapbach C, Xiao S, Kang HS, Elyaman W, Jiang X, Jetten AM, Khoury SJ, Fuhlbrigge RC, Kuchroo VK, et al. Robust tumor immunity to melanoma mediated by interleukin-9-producing T cells. Nat Med. 2012;18(8):1248-53. doi:10.1038/nm.2856.
- Meylan E, Tschopp J. Toll-like receptors and RNA helicases: two parallel ways to trigger antiviral responses. Mol Cell. 2006;22(5):561-9. doi:10.1016/j.molcel.2006.05.012.
- Ma Z, Zhang E, Yang D, Lu M. Contribution of Toll-like receptors to the control of hepatitis B virus infection by initiating antiviral innate responses and promoting specific adaptive immune responses. Cell Mol Immunol. 2015;12(3):273-82. doi:10.1038/cmi.2014.112.
- Ren D, Liu F, Dong G, You M, Ji J, Huang Y, Hou Y, Fan H. Activation of TLR7 increases CCND3 expression via the downregulation of miR-15b in B cells of systemic lupus erythematosus. Cell Mol Immunol. 2016;13(6):764-75. doi:10.1038/cmi.2015.48.
- Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7(6):415-28. doi:10.1038/nrc2131.
- Li S, Lu J, Chen Y, Xiong N, Li L, Zhang J, Yang H, Wu CH, Zeng HJ, Liu YY. MCP-1-induced ERK/GSK-3β/Snail signaling facilitates the epithelial–mesenchymal transition and promotes the migration of MCF-7 human breast carcinoma cells. Cell Mol Immunol. 2017;14:621-30. doi:10.1038/cmi.2015.106.
- Yadav A, Kumar B, Datta J, Teknos TN, Kumar P. IL-6 promotes head and neck tumor metastasis by inducing epithelial-mesenchymal transition via the JAK-STAT3-SNAIL signaling pathway. Mol Cancer Res. 2011;9(12):1658-67. doi:10.1158/1541-7786.MCR-11-0271.
- Kim MJ, Lim J, Yang Y, Lee MS, Lim JS. N-myc downstream-regulated gene 2 (NDRG2) suppresses the epithelial-mesenchymal transition (EMT) in breast cancer cells via STAT3/Snail signaling. Cancer Lett. 2014;354(1):33-42. doi:10.1016/j.canlet.2014.06.023.
- Yin X, Zhang BH, Zheng SS, Gao DM, Qiu SJ, Wu WZ, Ren ZG. Coexpression of gene Oct4 and Nanog initiates stem cell characteristics in hepatocellular carcinoma and promotes epithelial-mesenchymal transition through activation of Stat3/Snail signaling. J Hematol Oncol. 2015;8:23. doi:10.1186/s13045-015-0119-3.
- Yang H, Wang Y, Qian H, Zhang P, Huang C. Pim protein kinase-3 is regulated by TNF-alpha and promotes endothelial cell sprouting. Mol Cells. 2011;32(2):235–41. doi:10.1007/s10059-011-1026-z.
- Zhuang H, Zhao MY, Hei KW, Yang BC, Sun L, Du X, Li YM. Aberrant expression of pim-3 promotes proliferation and migration of ovarian cancer cells. Asian Pac J Cancer Prev. 2015;16(8):3325-31. doi:10.7314/APJCP.2015.16.8.3325.
- Santio NM, Eerola SK, Paatero I, Yli-Kauhaluoma J, Anizon F, Moreau P, Tuomela J, Harkonen P, Koskinen PJ. Pim Kinases Promote Migration and Metastatic Growth of Prostate Cancer Xenografts. PLoS One. 2015;10 (6):e0130340. doi:10.1371/journal.pone.0130340.
- Chang M, Kanwar N, Feng E, Siu A, Liu X, Ma D, Jongstra J. PIM kinase inhibitors downregulate STAT3(Tyr705) phosphorylation. Mol Cancer Ther. 2010;9(9):2478-87. doi:10.1158/1535-7163.MCT-10-0321.
- Park YK, Hong VS, Lee TY, Lee J, Choi JS, Park DS, Park GY, Jang BC. The novel anti-adipogenic effect and mechanisms of action of SGI-1776, a Pim-specific inhibitor, in 3T3-L1 adipocytes. Int J Mol Med. 2016;37(1):157-64. doi:10.3892/ijmm.2015.2415.
- Liu B, Wang Z, Li HY, Zhang B, Ping B, Li YY. Pim-3 promotes human pancreatic cancer growth by regulating tumor vasculogenesis. Oncol Rep. 2014;31(6):2625-34. doi:10.3892/or.2014.3158.
- Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9(11):798–809. doi:10.1038/nrc2734.
- Turkson J, Jove R. STAT proteins: novel molecular targets for cancer drug discovery. Oncogene. 2000;19(56):6613-26. doi:10.1038/sj.onc.1204086.
- Yoshimura A, Naka T, Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol. 2007;7(6):454-65. doi:10.1038/nri2093.
- Sansone P, Storci G, Tavolari S, Guarnieri T, Giovannini C, Taffurelli M, Ceccarelli C, Santini D, Paterini P, Marcu KB, et al. IL-6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland. J Clin Invest. 2007;117(12):3988-4002. doi:10.1172/JCI32533.
- Yin W, Cheepala S, Roberts JN, Syson-Chan K, DiGiovanni J, Clifford JL. Active Stat3 is required for survival of human squamous cell carcinoma cells in serum-free conditions. Mol Cancer. 2006;5:15. doi:10.1186/1476-4598-5-15.
- Xu Q, Briggs J, Park S, Niu G, Kortylewski M, Zhang S, Gritsko T, Turkson J, Kay H, Semenza GL, et al. Targeting Stat3 blocks both HIF-1 and VEGF expression induced by multiple oncogenic growth signaling pathways. Oncogene. 2005;24(36):5552-60. doi:10.1038/sj.onc.1208719.
- Cao HH, Chu JH, Kwan H.Y, Su T, Yu H, Cheng CY, Fu XQ, Guo H, Li T, Tse AK, et al. Inhibition of the STAT3 signaling pathway contributes to apigenin-mediated anti-metastatic effect in melanoma. Sci Rep. 2016;6:21731. doi:10.1038/srep21731.
- Nakamura M, Tokura Y. Epithelial-mesenchymal transition in the skin. J Dermatol Sci. 2011;61(1):7-13. doi:10.1016/j.jdermsci.2010.11.015.
- Denecker G, Vandamme N, Akay O, Koludrovic D, Taminau J, Lemeire K, Gheldof A, De Craene B, Van Gele M, Brochez L, et al. Identification of a ZEB2-MITF-ZEB1 transcriptional network that controls melanogenesis and melanoma progression. Cell Death Differ. 2014;21(8):1250-61. doi:10.1038/cdd.2014.44.
- Xie TX, Huang FJ, Aldape KD, Kang SH, Liu M, Gershenwald JE, Xie K, Sawaya R, Huang S. Activation of stat3 in human melanoma promotes brain metastasis. Cancer Res. 2006;66(6):3188-96. doi:10.1158/0008-5472.CAN-05-2674.
- Xiao TS. Innate immunity and inflammation. Cell Mol Immunol. 2017;14:1-3. doi:10.1038/cmi.2016.45.
