ABSTRACT
Breast cancer (BC) is the most frequent malignancy in both pre- and postmenopausal women. However, it is exceedingly rare in very young patients, and especially in adolescents. Herein, we report a case of an 18-year-old female diagnosed with invasive BC. The proband had been found to be negative for BC in close family members. A common BC genetic screening test for the Polish population did not detect any known founder mutations in the BRCA1 gene. Further evaluation identified a p.Ile157Thr (I157T) mutation in the CHEK2 gene, a p.Ala1991Val (A1991V) variant of unknown significance in the BRCA2 gene, p.Lys751Gln (K751Q) variant in the XPD (ERCC2) gene, and a homozygous p.Glu1008Ter (E1008*) mutation in the NOD2 gene. No other mutation had been found by next generation sequencing in major BC high-risk susceptibility genes BRCA1, BRCA2, as well as 92 other genes. To date, all these found alterations have been considered as low to moderate risk factors in the general population and moderate risk factors in younger women (<35 years of age). There are no previous articles relating low and moderate risk gene mutations to very young onset (below 20 years) BC with a fatal outcome. In our patient, a possible cumulative or synergistic risk effect for these 4 alterations, and a mutation in the NOD2 gene in particular, of which both presumably healthy parents were found to be carriers, is suggested.
Introduction
Breast cancer (BC) is the most frequent cancer in women worldwide. BC lifetime risk is estimated to be 10% in the general population. It is known that the incidence of developing BC rises with age. The majority of BCs are diagnosed in women older than 40 years. Approximately 7% of all BCs are detected in women under 40, 2.4% under 35, 0.7% under 30, and less than 0.2% below 20.Citation1-5 Other statistics regarding the youngest patient populations are scarce. According to the Polish Cancer Registry, between 2000 and 2010 approximately 150 000 new BC cases were recorded with only 23 being under the age of 20, an incidence of approximately 0.015%.Citation6
BC is a heterogeneous disease where many genes are known to be responsible. Among them are BRCA1 and BRCA2 which are of major significance due to a high lifetime risk of developing BC ranging from 46 to 87%.Citation7-9 Three founder mutations (c.181T>G in exon 5, c.4035delA in exon 11, and c.5266dupC in exon 20) have been identified in Polish families with breast and ovarian cancers.Citation10 This panel has been extended by three new mutations c.3819del5, c.185delAG, and c.5370C>T.Citation11 Recently, next generation sequencing (NGS) is the most challenging technique for the identification of rare mutations.Citation12 There is also a wide variety of other genes of low to moderate penetrance which should be considered for testing in women with BC, especially those at a young age and with a positive family history. They include, among others, CHEK2, NOD2, NBS1, ATM, p53, PALB2, CDKN2A, CYP1P1, and RECQL.Citation13-16
The CHEK2 encodes for a cell cycle checkpoint kinase 2 protein, or CHK2, which acts as a tumor suppressor in response to DNA damage. CHK2 plays an important role in cell-cycle regulation through interactions with other cancer susceptibility genes (e.g. ATM, p53, BRCA1, BRCA2). CHEK2 is known as a risk modifier of other cancer susceptibility genes and vice versa.Citation17,Citation18 Four CHEK2 mutations (c.470T>C p.Ile157Thr, deletion of 5395bp, c.1100delC, and IVS2+1G>A) have been commonly described in women of Slavic origin (from Poland, the Czech Republic, Belarus, and Russia) diagnosed with BC.Citation14,Citation19-22 Interesting findings were recently reported that a CHEK2 mutation in young (aged 20–30) women had an incidence of 3.6% compared to 1.7% in non-affected individuals.Citation21 Furthermore, protein truncating mutations (deletion of 5395bp, c.1100delC, and IVS2+1G>A) of CHEK2 were associated with a rather high (1.5-3.0-fold) risk of BC among both young and older women. Invasive ductal carcinoma, grade 2, and estrogen receptor (ER) and progesterone receptor (PGR) positivity were abundant in the carriers of these mutations.Citation20 In contrast, carriers of missense mutation p.Ile157Thr had a 1.4-fold increased risk.Citation23
No founder mutations in the BRCA2 have been identified so far in the Central European population. Similarly, the role of a c.5972C>T variant of the BRCA2 as a BC risk enhancer has not been evaluated worldwide. Górski et al. genotyped for a BRCA2 c.5972C>T variant in 3241 cases of BC in women <51 years of age, unselected for family history.Citation24 This alteration was found to be present in approximately 6% of the general population, in 8.3% of patients diagnosed for BC below 40, and in 5.7% of patients diagnosed between 41 and 50 years of age. The overall odds ratio (OR) for BC in women with a single copy of the BRCA2 c.5972C>T variant was 1.1. However, this effect was statistically significant for both patients diagnosed under 40 (OR = 1.4; P = 0.04) and women who had ductal carcinoma in situ (DCIS) with microinvasion (OR = 2.8; P < 0.0001).Citation24
A report by Serrano-Fernández et al. confirmed the hypothesis that truncating mutations in the CHEK2 have a synergistic effect of interaction with a BRCA2 polymorphic variant c.5972C>T.Citation18 In their study, the low-penetrance missence variant of BRCA2 alone was associated with a reduced risk of BC (OR = 0.62; P = 0.0007), and four common mutations of the CHEK2 gave a modest predisposition (OR = 2.2; P = 0.0001). However, the BC risk was substantially higher (OR = 5.7; P = 0.006) in carriers for both alterations.
The XPD (Xeroderma pigmentosum complementation group D; ERCC2) is one of the most important low-penetrance genes for BC development. Two variants, p.Asp312Asn and p.Lys751Gln in the coding region for XPD, have been extensively studied. The many reports upon the possible role of these XPD variants were reviewed by Pabalan et al.,Citation25 yielding unequivocal results. In contrast, a recent meta-analysis by Yan et al. confirmed a significant role of a p.Lys751Gln variant for BC risk.Citation26
The NOD2 was initially known as a predisposing factor for Crohn's disease, later as a colon cancer susceptibility gene, and finally as a multiple organ (lung, larynx, breast, thyroid, ovarian, and colon) cancer susceptibility gene.Citation27-30 A frameshift mutation, c.3020insC p.Glu1008Ter in NOD2, was found to correlate with an increased risk (OR = 1.9) for BC especially in the younger patient population (<50 years). DCIS was more prevalent in young NOD2 mutation carriers.Citation27
Clinical characteristics for breast malignancies in younger women differ from those arisen in later life. Such tumors in patients younger than 35 are more frequently of a higher histological gradeCitation31-33 and are more frequently classified as a triple-negative BC (TNBC), i.e. ERs, PGRs, and human epidermal growth factor receptor-2 (HER2) negative.Citation34-37 In addition, young women are more likely to have local recurrences, be diagnosed at a more advanced stage, and have a worse 5-year survival as compared to their older premenopausal counterparts.Citation38,Citation39 This more aggressive clinical phenotype reflects an increased incidence of more aggressive molecular subtypes in younger women with BC.Citation36,Citation40 Consequently, BC at an early age (35 years or less) has a rather fatal prognosis.Citation9,Citation33,Citation35,Citation36,Citation41-43
Herein we report a case of an 18-year-old female diagnosed initially with DCIS and later with invasive lobular BC who was found to be a carrier for a heterozygous c.5972C>T polymorphic variant of the BRCA2, a heterozygous p.Ile157Thr mutation for the CHEK2, a heterozygous p.Lys751Gln variant for the XPD, and a homozygous c.3020insC mutation for the NOD2, and died after a fulminant course of disease at the age of 22 years.
Case report
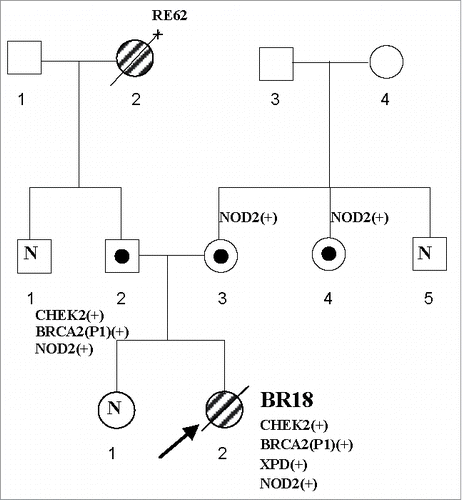
In June 2008, a slim otherwise healthy 18-year-old woman (weight 50 kg, height 160 cm, Body Mass Index = 19.5) was referred by her mother for a periodic green watery discharge from her left breast nipple over the past two months, refractory to treatment with oral bromocriptine and quinagolidum. Her gynecologic history was unremarkable: menarche occurred at 12 and subsequent menses were normal and regular. There was no family history of cancer in first-degree relatives. Among second-degree relatives, the paternal grandmother was diagnosed for renal cancer at 62 ().
Figure 1. Pedigree of the very young breast cancer patient under study. Arrow - proband. White circle with a black dot – healthy female carrier. Black circle – woman affected with cancer. White square with black dot – healthy male carrier. White circle or square with N inside – a non-carrier woman or man, respectively, tested for any family mutations. Age at cancer diagnosis or age at mutation identification are given in parentheses. BR – breast cancer, RE – renal cancer, age at cancer diagnosis follows.
Physical examination of the breasts revealed no palpable mass, asymmetry, pain, or discomfort. No axillary lymphadenopathies were present. Upon slight compression of the left areolar region, a few droplets of greenish watery discharge appeared and were sent for cytological examination. The cytology result read: “Presence of glandular cells suspected of atypia.” A fine needle aspiration biopsy indicated a diagnosis of atypical papillomatous epithelial proliferation and the patient was referred for diagnostic imaging. Breast ultrasound revealed several hypoechoic masses (21 × 10 mm, 10 × 9 mm, 9 × 7 mm, and 5 × 5 mm). A subsequent fine needle aspiration biopsy came back with a “suspicion of breast carcinoma”.
During her first surgery in August 2008, an intraoperative anatomopathological examination revealed an invasive carcinoma and a subsequent tumorectomy was performed. Precise postoperative examination of the excised specimen revealed multifocal DCIS (grading G1) in an area of abundant papillomatosis; staining highly positive for E-cadherin: (+++), cytokeratin 5/6: (−), smooth muscle actin: (+); ERs positive in 70%, PGRs and HER2 were negative. Total amputation of the breast with lymph node dissection were performed a month later. Anatomopathological evaluation revealed invasive lobular carcinoma: G1; pT3, N1 (lymph node involvement – 1/13), MX; ERs positive in 40%, PGRs negative, HER2: (+).
From October 2008 to February 2009, the patient underwent chemotherapy treatment consisting of 6 courses of TAC: docetaxel (Taxotere, Sanofi-Aventis, Paris, France) + doxorubicin (Adriamycin, of various manufacturers) + cyclophosphamide (Endoxan, Baxter Poland, Warsaw, Poland), relative to body surface area. This was followed by radical radiotherapy of the thorax and subcutaneous hormonal therapy with a gonadotropin releasing hormone analog of goserelin (Zoladex, AstraZeneca, Macclesfield, United Kingdom). After an initial good response and remission, sudden progression with metastases to the lungs and liver were detected in April 2011. Docetaxel and capecitabine (Xeloda, Roche, Welwyn Garden City, United Kingdom) were administered. Profound neutropenia was noted during chemotherapy. In spite of the introduction of everolimus (Afinitor, Novartis Europharm, Camberley, United Kingdom), the patient died of multiple organ failure in October 2012.
Informed written consent for both further testing and publishing was obtained from the patient's parents.
Methods
Peripheral blood samples were collected for DNA analyses from the proband, parents, a 20-year-old sister, and aunt (mother's sister). DNA was extracted from lymphocytes using a rapid non-enzymatic method by Lahiri and Schnabel.Citation44
BRCA1 analysis
The first step involved testing for three common founder mutations (c.181T>G in exon 5, c.4035delA in exon 11, and c.5266dupC in exon 20), representing 80% of all high-risk BRCA1 changes in the Polish population,Citation10 using a multiplex polymerase chain reaction (PCR) by a patent pending method (Number PL 185957). All these tests were performed at the Department of Genetics and Pathology, International Hereditary Cancer Center, Pomeranian Medical University (the patent holder).
Analysis of c.5972C>T p.Ala1991Val variant in BRCA2
Restriction fragment length polymorphism (RFLP)-PCR using b5972F (5′-CTC TCT AGA TAA TGA TGA ATG ATG CA) and b5972R (5′-CCA AAC TAA CAT CAC AAG GTG) primers detected 2 BRCA2 variants. Sequence analysis detected a variant of unknown significance: heterozygous C to T base substitution at nucleotide position 5972 (c.5972C>T) resulting in the substitution of the amino acid Alanine for Valine at codon 1991 (p.Ala1991Val). The forward primer introduces an artificial restriction site for the Mph1103I enzyme (Fermentas GmbH, St. Leon-Rot, Germany) and PCR products are digested in mutation-positive cases. PCR products were separated in 3% agarose gel and visualized in ultraviolet light. For positive cases with RFLP-PCR (cleaved by Mph1103I), a DNA sample was sequenced to confirm the presence of the mutation. Additionally, 72 randomly selected samples negative for the c.5972C>T variant with RFLP-PCR were sequenced. The results of RFLP-PCR and of direct DNA sequencing were 100% concordant.
A fragment of BRCA2 exon 11 was amplified by PCR using b5972F/b5972R primers. The purified PCR products were sequenced directly with a BigDye Terminator v3.0 DNA Sequencing Kit (PerkinElmer, Foster City, CA, USA) and the same reverse primer used previously for the amplification of exon 11 of the BRCA2. Products of sequencing reactions were separated and analyzed on an ABI Prism® 377 DNA Sequencer (PerkinElmer). The procedure was performed according to the patent number: PL 2006/000062.Citation24
Analysis of CHEK2
Four common mutations (5,395 bp, c.470T>C, c.1100delC, p.Ile157Thr) in CHEK2 were genotyped. These procedures were performed according to the pending patents numbers PL 215152, PL 395482, and PL 405774.Citation20,Citation21
Analysis of XPD (ERCC2)
The XPD was assessed by molecular analysis performed using a combination of real-time PCR (LightCycler 480, Roche Diagnostics GmbH, Penzberg, Germany) and MassARRAY MALDI-TOF mass spectrometry analysis (Sequenom Inc., San Diego, CA, USA). Sequence analysis detected a heterozygous A to C base substitution at nucleotide position 2251 (c.2251A>C) resulting in the substitution of the amino acid Lysine for Glutamine at codon 751 p.Lys751Gln. TaqMan probes were used for real-time PCR (Applied Biosystems, Foster City, CA). MALDI-TOF analysis was based on a primer extension reaction to determine the single nucleotide polymorphism (SNP) allele. Reactions were performed according to the manufacturer's instructions. Random DNA samples were sequenced to verify the results of the MassARRAY genotyping and real-time PCR analysis (data not shown). Sequencing was conducted using universal primers in combination with an ABI PRISM BigDye Terminator Cycle kit (Applied Biosystems). The sequencing was performed in a 3130 Genetic Analyzer (Applied Biosystems).Citation45
Analysis of NOD2
Using an allele-specific PCR assay, we detected a single nucleotide C base insertion at nucleotide position 3020 (c.3020insC) resulting in termination at codon 1008 p.Glu1008Ter. The assay was performed using a kit produced by the Molecular Laboratory of the Pomeranian Medical University (patents numbers PL 202116 and PL 202119).Citation27
NGS testing
This stage was conducted at the DNA Research Center, Poznań. The quality and purity of isolated DNA were determined by electrophoresis and spectrophotometry, respectively. DNA concentration was assessed by a fluorescence based method (Qubit). For library preparation of NGS, we used a commercially available targeted resequencing kit, the TruSight Cancer Sequencing Panel (Illumina, San Diego, CA, USA) according to the manufacturer's protocol. In brief, 50 ng of genomic DNA measured with the Qubit dsDNA BR Assay Kit (ThermoFisher Scientific) was used for library preparation. For this purpose a TruSight Cancer kit was used. Each sample was processed separately as the first step of library preparation. This involved gDNA fragmentation and adding Illumina adapters and two index tags. Biotinylated DNA probes were then hybridized to the targeted regions of interest. The biotinylated hybrids were next physically separated using streptavidin beads and the non-target DNA was washed away. These libraries were then quantified and diluted prior to sequencing. Direct sequencing was performed on an Illumina MiSeq genome sequencer with 2 × 150bp reads using a MiSeq Reagent Kit, 300-cycles (Illumina). The sample was mapped to human genome reference GRCh37/hg19. All reportable sequence variants were confirmed by visual inspection of the alignment. Pathogenic mutations were additionally confirmed by PCR and Sanger sequencing.
The panel targets 94 genes and 284 SNPs associated with a predisposition towards cancer. These are: AIP, ALK, APC, ATM, BAP1, BLM, BMPR1A, BRCA1, BRCA2, BRIP1, BUB1B, CDC73, CDH1, CDK4, CDKN1C, CDKN2A, CEBPA, CEP57, CHEK2, CYLD, DDB2, DICER1, DIS3L2, EGFR, EPCAM, ERCC2, ERCC3, ERCC4, ERCC5, EXT1, EXT2, EZH2, FANCA, FANCB, FANCC, FANCD2, FANCE, FANCF, FANCG, FANCI, FANCL, FANCM, FH, FLCN, GATA2, GPC3, HNF1A, HRAS, KIT, MAX, MEN1, MET, MLH1, MSH2, MSH6, MUTYH, NBN, NF1, NF2, NSD1, PALB2, PHOX2B, PMS1, PMS2, PRF1, PRKAR1A, PTCH1, PTEN, RAD51C, RAD51D, RB1, RECQL4, RET, RHBDF2, RUNX1, SBDS, SDHAF2, SDHB, SDHC, SDHD, SLX4, SMAD4, SMARCB1, STK11, SUFU, TMEM127, TP53, TSC1, TSC2, VHL, WRN, WT1, XPA, and XPC genes.
Alamut mutation interpretation software (Interactive Biosoftware, Rouen, France) was used for the interpretation and classification of the variants.
Results
In the analyzed DNA samples of the proband, none of 3 common Polish founder mutations in BRCA1 nor other rare variants were found. A variant of unknown significance c.5972C>T p.Ala1991Val (A1991V) in BRCA2, mutation c.470T>C p.Ile157Thr (I157T) in CHEK2, variant c.2251A>C p.Lys751Gln (K751Q) variant in XPD, and homozygous mutation c.3020insC p.Glu1008* (E1008*) in NOD2 were detected. All 4 alterations were present in heterozygous form in the presumably healthy father. A heterozygous mutation c.3020insC p.Glu1008Ter (E1008*) in NOD2 was also found in the mother and mother's sister. No familial mutations were seen in the patient's elder sister ().
Discussion
BC diagnosed in very young women is a rare finding, especially under 20 years of age, even in patients with high risk mutations such as those of BRCA1 or BRCA2 genes. Other risk modifiers have been suggested by many authors. There are several reports documenting early diagnoses for BC as a result of hereditary cancer syndromes such as Li-Fraumeni,Citation13 Lynch, Cowden, or Peutz-Jeghers, but these are relatively uncommon.
Delude et al. argue that chemotherapy and radiotherapy used for the treatment of malignancies can increase the risk of developing secondary malignancies, including BC.Citation46 These authors presented one of the youngest BC cancer cases ever reported in the literature: a nephroblastoma survivor (diagnosed at age of 6) who was found to have a familial BRCA1 mutation and diagnosed for DCIS at 21.
The lifetime risk for BC in carriers of CHEK2 truncating mutations is strictly correlated with positive family history. It was estimated to be as high as 20% for women with non-affected relatives, 28% for women with one second-degree relative affected, 34% for those with one first-degree relative, and 44% for women with both first- and second-degree relatives.Citation21 In our patient, neither first- nor second-degree relatives were known to have been diagnosed with BC. Her father, a carrier for all 4 mutations present in the patient, had no sister but a healthy brother.
Interestingly, BRCA1 or BRCA2 positive TNBCs are found most often in young women.Citation7,Citation9,Citation34,Citation37,Citation47 Our above-described very young patient was ERs positive, but mutations in the BRCA1 and BRCA2 genes were excluded by NGS. However, she harbored 4 different genetic alterations in moderate and low risk genes, including a homozygous c.3020insC in NOD2. Huzarski et al. established that this NOD2 founder mutation is relatively common in Poland, present in 7.3% of the general population and may be responsible for a significant proportion of breast, colon, lung, larynx, and ovarian cancers.Citation27 The NOD2 c.3020insC allele was found in approximately 8% of all BCsCitation29,Citation30 and in 13.2% of cases diagnosed before the age of 50.Citation27,Citation28 A c.3020insC in NOD2 increases the risk of DCIS-bearing BC below age 50 about 5-fold.Citation30 In the presented case, there was no strong association for the NOD2 mutation with a family history for BC. Others calculated that the mutation's frequency (11.4%) was two times higher in women of families with a single case of BC.Citation29
A c.5972C>T polymorphic variant in the BRCA2 is present in 8.3% of unselected BC patients diagnosed at 40 or less, and especially in those with DCIS with microinvasion, as was the case of our patient. Heterozygotes (C/T) for c.5972C>T in BRCA2 are at a 3-fold risk for DCIS BC before 50 compared with a 5-fold risk for homozygous (T/T) carriers.Citation24 The presented case supports the role of the BRCA2 c.5972C>T allele as a quite deleterious missense variant.
The CHEK2 is a risk modifier for other cancer susceptibility genes. BCs in women with a CHEK2 mutation were more commonly of lobular histology (21.5% versus 15.8%; P = 0.05), size > 2 cm (54.8% versus 43.5%; P = 0.01), and multifocal origin (28.7% versus 19.5%; P = 0.01) than cancers from women with no mutation.Citation22
In a recent meta-analysis,Citation26 it was concluded that only the XPD p.Lys751Gln variant significantly increases BC risk, especially in Caucasian and mixed races. A different modest association with BC risk was observed when the p.Lys751Gln_CC/Asp312Asn_AA genotype (OR = 1.5, P < 0.05) segregated together.Citation48
The current polygenic model for the development of cancer assumes that the co-occurrence of several factors that are otherwise associated with a low or moderate risk when taken separately, may turn to a high risk when present in combination. Penetrance of many genetic abnormalities is frequently determined by mutations in several genes. Sokolenko et al. detected double heterozygosity in BRCA1, CHEK2, NBS1, ATM, or BLM in BC patients.Citation22 Therefore, a panel of genetic markers covering 92% of consecutive BCs in Poland has been proposed.Citation49
All 4 gene alterations under study were located on different chromosomes (BRCA2 on chromosome 13, CHEK2 on chromosome 22, NOD2 on chromosome 16, and XPD on chromosome 19) and are known in the literature as maternal, rather, than paternal disomies. These genes are not imprinted genes, although still little is known about the effect of genomic imprinting in complex diseases, including cancer. We found no other report of such combination of genetic alterations in young women with BC or other solid malignancies.
In conclusion, this report draws attention to the observation that the accumulation of mutations in low and moderate BC risk genes, such as a homozygous mutation in NOD2 and heterozygous mutation in CHEK2 together with a missense variant c.5972C>T in BRCA2 and variant p.Lys751Gln in XPD, in a very young woman, was associated with a clinically highly aggressive course of BC. This constellation of genetic alterations may exert a strong cumulative or synergistic effect and seems worthy of verification in young BC patients.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thank Professor Jan Lubiński and his team from the International Hereditary Cancer Center, Pomeranian Medical University, Szczecin for providing the initial results of the genetic analyses. The work at the Medical Genetics Laboratory, DNA Research Center, Poznań was supported by the European Union from resources of the European Regional Development Fund, agreement number UDA-POIG.01.04.00-082/11-00.
Additional information
Funding
References
- Mintzer D, Glassburn J, Mason BA, Sataloff D. Breast cancer in the very young patient: a multidisciplinary case presentation. Oncologist. 2002;7:547–54. doi:10.1634/theoncologist.7-6-547.
- Brinton LA, Sherman ME, Carreon JD, Anderson WF. Recent trends in breast cancer among younger women in the United States. J Natl Cancer Inst. 2008;100:1643–8. doi:10.1093/jnci/djn344.
- Fredholm H, Eaker S, Frisell J, Holmberg L, Fredriksson I, Lindman H. Breast cancer in young women: Poor survival despite intensive treatment. PLoS ONE. 2009;4:e7695. doi:10.1371/journal.pone.0007695.
- Hickey M, Peate M, Saunders CM, Friedlander M. Breast cancer in young women and its impact on reproductive function. Hum Reprod Update. 2009;15:323–39. doi:10.1093/humupd/dmn064.
- Tichy JR, Lim E, Anders CK. Breast cancer in adolescents and young adults: a review with a focus on biology. J Natl Compr Canc Netw. 2013;11:1060–9. doi:10.6004/jnccn.2013.0128.
- Polish National Cancer Registry website. www.onkologia.org.pl; accessed on July 04, 2017.
- Evans JP, Skrzynia C, Susswein L, Harlan M. Genetics and the young woman with breast cancer. Breast Dis. 2005-2006;23:17–29. doi:10.3233/BD-2006-23104.
- Levy-Lahad E, Friedman E. Cancer risks among BRCA1 and BRCA2 mutation carriers. Br J Cancer. 2007;96:11–5. doi:10.1038/sj.bjc.6603535.
- Lubiński J, Górski B, Huzarski T, Byrski T, Gronwald J, Serrano-Fernández P, Domagała W, Chosia M, Uciński M, Grzybowska E, et al. BRCA1-positive breast cancers in young women from Poland. Breast Cancer Res Treat. 2006;99:71–6. doi:10.1007/s10549-006-9182-3.
- Górski B, Byrski T, Huzarski T, Jakubowska A, Menkiszak J, Gronwald J, Płużańska A, Bębenek M, Fischer-Maliszewska Ł, Grzybowska E, et al. Founder mutations in the BRCA1 gene in Polish families with breast-ovarian cancer. Am J Hum Genet. 2000;66:1963–8. doi:10.1086/302922.
- Szwiec M, Jakubowska A, Górski B, Huzarski T, Tomiczek-Szwiec J, Gronwald J, Dębniak T, Byrski T, Kluźniak W, Wokołorczyk D, et al. Recurrent mutations of BRCA1 and BRCA2 in Poland: an update. Clin Genet. 2015;87:288–92. doi:10.1111/cge.12360.
- Kraus C, Hoyer J, Vasileiou G, Wunderle M, Lux MP, Fasching PA, Krumbiegel M, Uebe S, Reuter M, Beckmann MW, et al. Gene panel sequencing in familial breast/ovarian cancer patients identifies multiple novel mutations also in genes others than BRCA1/2. Int J Cancer. 2017;140:95–102. doi:10.1002/ijc.30428.
- Gonzalez KD, Noltner KA, Buzin CH, Gu D, Wen-Fong CY, Nguyen VQ, Han JH, Lowstuter K, Longmate J, Sommer SS, et al. Beyond Li Fraumeni syndrome: clinical characteristics of families with p53 germline mutations. J Clin Oncol. 2009;27:1250–6. doi:10.1200/JCO.2008.16.6959.
- Cybulski C, Lubiński J, Wokołorczyk D, Kuźniak W, Kashyap A, Sopik V, Huzarski T, Gronwald J, Byrski T, Szwiec M, et al. Mutations predisposing to breast cancer in 12 candidate genes in breast cancer patients from Poland. Clin Genet. 2015;88:366–70. doi:10.1111/cge.12524.
- Cybulski C, Kluźniak W, Huzarski T, Wokołorczyk D, Kashyap A, Jakubowska A, Szwiec M, Byrski T, Dębniak T, Górski B, et al. and the Polish Hereditary Breast Cancer Consortium. Clinical outcomes in women with breast cancer and a PALB2 mutation: a prospective cohort analysis. Lancet Oncol. 2015;16:638–44. doi:10.1016/S1470-2045(15)70142-7.
- Cybulski C, Carrot-Zhang J, Kluźniak W, Rivera B, Kashyap A, Wokołorczyk D, Giroux S, Nadaf J, Hamel N, Zhang S, et al. Germline RECQL mutations are associated with breast cancer susceptibility. Nat Genet. 2015;47:643–6. Erratum in: Nat Genet 2016;48:473. doi:10.1038/ng.3284.
- Nevanlinna H, Bartek J. The CHEK2 gene and inherited breast cancer susceptibility. Oncogene. 2006;25:5912–9. doi:10.1038/sj.onc.1209877.
- Serrano-Fernández P, Dębniak T, Górski B, Bogdanova N, Dörk T, Cybulski C, Huzarski T, Byrski T, Gronwald J, Wokołorczyk D, et al. Synergistic interaction of variants in CHEK2 and BRCA2 on breast cancer risk. Breast Cancer Res Treat. 2009;117:161–5. doi:10.1007/s10549-008-0249-1.
- Bogdanova N, Enβen-Dubrowinskaja N, Feshchenko S, Lazjuk GI, Rogov YI, Dammann O, Bremer M, Karstens JH, Sohn C, Dörk T. Association of two mutations in the CHEK2 gene with breast cancer. Int J Cancer. 2005;116:263–6. doi:10.1002/ijc.21022.
- Cybulski C, Wokołorczyk D, Huzarski T, Byrski T, Gronwald J, Górski B, Dębniak T, Masojć B, Jakubowska A, van de Wetering T, et al. A deletion in CHEK2 of 5,395 bp predisposes to breast cancer in Poland. Breast Cancer Res Treat. 2007;102:119–22. doi:10.1007/s10549-006-9320-y.
- Cybulski C, Wokołorczyk D, Jakubowska A, Huzarski T, Byrski T, Gronwald J, Masojć B, Dębniak T, Górski B, Blecharz P, et al. Risk of breast cancer in women with a CHEK2 mutation with and without a family history of breast cancer. J Clin Oncol. 2011;29:3747–52. doi:10.1200/JCO.2010.34.0778.
- Sokolenko AP, Bogdanova N, Kluzniak W, Preobrazhenskaya EV, Kuligina ES, Iyevleva AG, Aleksakhina SN, Mitiushkina NV, Gorodnova TV, Bessonov AA, et al. Double heterozygotes among breast cancer patients analyzed for BRCA1, CHEK2, ATM, NBN/NBS1, and BLM germ-line mutations. Breast Cancer Res Treat. 2014;145:553–62. doi:10.1007/s10549-014-2971-1.
- Bąk A, Janiszewska H, Junkiert-Czarnecka A, Heise M, Pilarska-Deltow M, Laskowski R, Pasińska M, Haus O. A risk of breast cancer in women – carriers of constitutional CHEK2 gene mutations, originating from the North – Central Poland. Hered Cancer Clin Pract. 2014;12:10. doi:10.1186/1897-4287-12-10.
- Górski B, Narod SA, Lubiński J. A common missense variant in BRCA2 predisposes to early onset breast cancer. Breast Cancer Res. 2005;7:R1023–7. doi:10.1186/bcr1338.
- Pabalan N, Francisco-Pabalan O, Sung L, Jarjanazi H, Ozcelik H. Meta-analysis of two ERCC2 (XPD) polymorphisms, Asp312Asn and Lys751Gln, in breast cancer. Breast Cancer Res Treat. 2010;124:531–41. doi:10.1007/s10549-010-0863-6.
- Yan Y, Liang H, Light M, Li T, Deng Y, Li M, Li S, Qin X. XPD Asp312Asn and Lys751Gln polymorphisms and breast cancer susceptibility: A meta-analysis. Tumour Biol. 2014;35:1907–15. doi:10.1007/s13277-013-1256-3.
- Huzarski T, Lener M, Domagała W, Gronwald J, Byrski T, Kurzawski G, Suchy J, Chosia M, Woyton J, Ucinski M, et al. The 3020insC allele of NOD2 predisposes to early-onset breast cancer. Breast Cancer Res Treat. 2005;89:91–3. doi:10.1007/s10549-004-1250-y.
- Lubiński J, Huzarski T, Kurzawski G, Suchy J, Masojć B, Mierzejewski M, Lener M, Domagała W, Chosia M, Teodorczyk U, et al. The 3020insC allele of NOD2 predisposes to cancer of multiple organs. Hered Cancer Clin Practice. 2005;3:59–63. doi:10.1186/1897-4287-3-2-59.
- Janiszewska H, Haus O, Lauda-Świeciak A, Bąk A, Mierzwa T, Sir J, Laskowski R. The NOD2 3020insC mutation in women with breast cancer from the Bydgoszcz Region in Poland. First results. Hered Cancer Clin Pract. 2006;4:15–9. doi:10.1186/1897-4287-4-1-15.
- Kurzawski G, Suchy J, Cybulski C, Matyjasik J, Dębniak T, Górski B, Huzarski T, Janicka A, Szymańska-Pasternak J, Lubiński J. DNA testing for variants conferring low or moderate increase in the risk of cancer. Hered Cancer Clin Pract. 2008;6:84–7. doi:10.1186/1897-4287-6-2-84.
- Fernandopulle SM, Cher-Siang Ang P, Tan PH. Breast carcinoma in women 35 years and younger: a pathological study. Pathology. 2006;38:219–22. doi:10.1080/00313020600699268.
- Collins LC, Marotti JD, Gelber S, Cole K, Ruddy K, Kereakoglow S, Brachtel EF, Schapira L, Come SE, Winer EP, et al. Pathologic features and molecular phenotype by patient age in a large cohort of young women with breast cancer. Breast Cancer Res Treat. 2012;131:1061–6. doi:10.1007/s10549-011-1872-9.
- Keegan TH, Press DJ, Tao L, DeRouen MC, Kurian AW, Clarke CA, Gomez SL. Impact of breast cancer subtypes on 3-year survival among adolescent and young adult women. Breast Cancer Res. 2013;15:R95. doi:10.1186/bcr3556.
- Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P, Narod SA. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–34. doi:10.1158/1078-0432.CCR-06-3045.
- Anders CK, Hsu DS, Broadwater G, Acharya CR, Foekens JA, Zhang Y, Wang Y, Marcom PK, Marks JR, Febbo PG, et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol. 2008;26:3324–30. Erratum in: J Clin Oncol 2011;29:3721. doi:10.1200/JCO.2007.14.2471.
- Anders CK, Fan C, Parker JS, Carey LA, Blackwell KL, Klauber-DeMore N, Perou CM. Breast carcinomas arising at a young age: unique biology or a surrogate for aggressive intrinsic subtypes? J Clin Oncol. 2011;29:e18–20. doi:10.1200/JCO.2010.28.9199.
- Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363:1938–48. doi:10.1056/NEJMra1001389.
- Nixon AJ, Neuberg D, Hayes DF, Gelman R, Connolly JL, Schnitt S, Abner A, Recht A, Vicini F, Harris JR. Relationship of patient age to pathologic features of the tumor and prognosis for patients with stage I or II breast cancer. J Clin Oncol. 1994;12:888–94. doi:10.1200/JCO.1994.12.5.888.
- Colleoni M, Rotmensz N, Robertson C, Orlando L, Viale G, Renne G, Luini A, Veronesi P, Intra M, Orecchia R, et al. Very young women (<35 years) with operable breast cancer: features of disease at presentation. Ann Oncol. 2002;13:273–9. doi:10.1093/annonc/mdf039.
- Colleoni M, Anders CK. Debate: The biology of breast cancer in young women is unique. Oncologist. 2013;18:e13–5. doi:10.1634/theoncologist.2013-0118.
- Xiong Q, Valero V, Kau V, Kau SW, Taylor S, Smith TL, Buzdar AU, Hortobagyi GN, Theriault RL. Female patients with breast carcinoma age 30 years and younger have a poor prognosis. The M.D. Anderson Cancer Center experience. Cancer. 2001;92:2523–8. doi:10.1002/1097-0142(20011115)92:10%3c2523::AID-CNCR1603%3e3.0.CO;2-6.
- Azim HA Jr, Michiels S, Bedard PL, Singhal SK, Criscitiello C, Ignatiadis M, Haibe-Kains B, Piccart MJ, Sotiriou C, Loi S. Elucidating prognosis and biology of breast cancer arising in young women using gene expression profiling. Clin Cancer Res. 2012;18:1341–51. doi:10.1158/1078-0432.CCR-11-2599.
- Freedman RA, Partridge AH. Emerging data and current challenges for young, old, obese, or male patients with breast cancer. Clin Cancer Res. 2017;23:2647–54. doi:10.1158/1078-0432.CCR-16-2552.
- Lahiri DK, Schnabel B. DNA isolation by rapid method from human blood samples: effects of MgCl2, EDTA, storage time, and temperature on DNA yield and quality. Biochem Genet. 1993;31:321–8. doi:10.1007/BF00553174.
- Paszkowska-Szczur K, Scott RJ, Górski B, Cybulski C, Kurzawski G, Dymerska D, Gupta S, van de Wetering T, Masojć B, Kashyap A, et al. Polymorphisms in nucleotide excision repair genes and susceptibility to colorectal cancer in the Polish population. Mol Biol Rep. 2015;42:755–64. doi:10.1007/s11033-014-3824-z.
- Dulude AM, D'Souza J, Harrison N, Ramanathan RK. Development of breast cancer in a 21-year-old childhood Wilms' tumor survivor with a BRCA1 2634delC mutation. Clin Breast Cancer. 2011;11:268–9. doi:10.1016/j.clbc.2010.11.001.
- Atchley DP, Albarracin CT, Lopez A, Valero V, Amos CI, Gonzalez-Angulo AM, Hortobagyi GN, Arun BK. Clinical and pathologic characteristics of patients with BRCA-positive and BRCA-negative breast cancer. J Clin Oncol. 2008;26:4282–8. doi:10.1200/JCO.2008.16.6231.
- Dębniak T, Scott RJ, Huzarski T, Byrski T, Masojć B, van de Wetering T, Serrano-Fernandez P, Górski B, Cybulski C, Gronwald J, et al. XPD common variants and their association with melanoma and breast cancer risk. Breast Cancer Res Treat. 2006;98:209–15. doi:10.1007/s10549-005-9151-2.
- Lubinski J, Korzen M, Gorski B, Cybulski C, Debniak T, Jakubowska A, Medrek K, Matyjasik J, Huzarski T, Byrski T, et al. Breast cancer susceptibility genes. J BUON. 2007;12(Suppl 1):S23–9.
