ABSTRACT
Recently, increasing evidence has indicated that the presence of tumor infiltrating immune cells has shown predictive significance for many solid tumors. Present study was performed to evaluate the predictive value of stromal tumor-infiltrating lymphocytes (TILs) for the presence of liver metastasis and overall survival in PDAC (pancreatic ductal adenocarcinoma) patients after complete resection and to explore the potential role of lymphocytes in PDAC. A total of 155 resectable patients with PDAC were enrolled in our study. Stromal TIL density was investigated in hematoxylin and eosin-stained sections of surgical specimens and scored. The effect and possible mechanism of lymphocytes on cancer cells was evaluated using co-culture techniques and ELISA test. Stromal TIL negative status (HR = 2.80, 95% CI 1.75-4.48, P < 0.01) was not only an independent predictor of worse OS (HR = 2.7, 95% CI 1.80-4.06, P = <0.01) but also a significant independent predictor of liver metastasis. Higher CEA (P = 0.01) or CA19-9 (P = 0.01) levels were associated with low stromal TIL density. Stromal TIL negative patients appeared to develop tumors with a higher CEA (P = 0.01), larger diameter (P = 0.05) and advanced stage (P = 0.02). The co-culture experiment suggests that lymphocytes can inhibit pancreatic cancer cell proliferation. Further ELISA and cell culture test indicate that lymphocytes may cause pancreatic cancer cells apoptosis through TNF-alpha secretion. Our data suggest a potential favorable role of stromal TILs in predicting liver metastasis and overall survival of patients with PDAC after complete resection. Lymphocytes may inhibit the growth of PDAC through TNF-alpha secretion, which suggest a potential therapeutic approach against PDAC.
Introduction
Pancreatic cancer (PC) is one of the most aggressive malignancies and represents a leading cause of cancer-related death.Citation1-Citation3 Pancreatic ductal adenocarcinoma (PDAC) is the most common histological type of PC. However, because of the lack of effective methods for early diagnosis, PDAC is often advanced at the time of diagnosis, and more than 38% PDAC patients are found to have metastasis, especially liver metastasis. Only a minority of patients can undergo surgical resection, and its 5-year survival rate is only 3–5%.Citation1-Citation4 Additionally, we have shown that approximately 70% of patients died of distant metastasis after complete resection, 84.3% of whom had liver metastasis.Citation5 Therefore, it is essential to identify additional prognostic factors to predict liver metastasis, which could be valuable for the development of therapeutic strategies and the selection of suitable treatment options for individual patients.
Recently, the presence of tumor infiltrating immune cells in the tumor microenvironment has shown predictive significance for many solid tumors.Citation6-Citation10 A study evaluating tumor infiltrating lymphocytes (TILs) in PDAC demonstrated that the presence of CD4+ TILs together with CD8+ TILs serves as a good indicator of the patient's outcome after surgical resection.Citation11,Citation12 However, no previous investigation has assessed the predictive significance of stromal lymphocytes in PDAC independently.
A standardized analysis of hematoxylin and eosin (HE)-stained sections has been recommended by an international TIL working group recently assessing stromal TILs in breast cancer as the main objective parameter.Citation10 However, patients with PDAC generally experience much worse outcomes postoperatively. The tumor microenvironment has been found to be inherently immunosuppressive with a vast array of mechanisms to escape immune surveillance.Citation13-Citation15 The density of TILs in PDAC may be very different from that in breast cancer. More stringent evaluation criteria may be more suitable for PDAC. Therefore, we performed an analysis of the predictive value of stromal TILs and clinical parameters on the liver metastasis and the overall survival of patients with PDAC after complete resection.
Moreover, the administration of lymphocytes for the treatment of some cancers has recently received increasing attention.Citation16-Citation18 A study found that TILs can respond to pancreatic tumor-associated antigens,Citation19 but whether lymphocytes can inhibit the progression of PDAC is still unclear. The role of lymphocytes against pancreatic cancer cells has seldom been studied in vitro. Thus, we also examined the effect of lymphocytes in a co-culture system.
Results
Patient characteristics
One hundred and fifty-five patients who had undergone a primary attempt of a complete resection for PDAC were enrolled, including 98 males and 57 females ranging in age between 23 and 86 years, with a mean age of 63.1 years. All ’patient diagnoses were ultimately confirmed both clinically and pathologically. At the end of the follow-up period, 129 patients had died, and 103 patients had a definite cause of death, including 68 (66.0%) patients with liver metastases. Among the 155 PDAC patients, 82 (52.9%) were from Beijing, 24 (15.5%) were from Hebei, 11(7.1%) were from Inner Mongolia, 7 (4.5%) were from Shandong, 5 (3.2%) were from Shanxi, and the remaining patents were from other cities. According to the scoring system (), we statistics all the patient's information, the quantitative clinical characteristics are shown in , and the categorical clinical factors are summarized in . Overall, 55 (35.5%) patients were found to be stromal TIL positive, and the remaining 100 (64.5%) patients were determined to be stromal TIL negative.
Figure 1. (A) Stromal TIL negative group (score 0): almost no lymphocytic infiltration into the stroma of surrounding cancer cell nests; (B) Stromal TIL negative group (score 1): low lymphocytic infiltration into the stroma of surrounding cancer cell nests; (C) Stromal TIL positive group (score 2): moderate lymphocytic infiltration into the stroma without tumor cell nest permeation and (D) Stromal TIL positive group (score 3): intense lymphocytic infiltration into the stroma and between tumor cells. (E) and (F): Representative figure of stromal TILs at modetate scores in the same tissue section verified by HE and the expression of CD45 by immunohistochemistry, respectively. (G) and (H): Representative figure of stromal TILs at low scores based on HE stainings in the same tissue section verified by the expression of CD45 by immunohistochemistry. [100 × magnification, H & E-stained sections or IHC sections. (A-E, G); 100 × magnification, IHC section (F, H)]. Abbreviations: TIL = tumor-infiltrating lymphocyte.
![Figure 1. (A) Stromal TIL negative group (score 0): almost no lymphocytic infiltration into the stroma of surrounding cancer cell nests; (B) Stromal TIL negative group (score 1): low lymphocytic infiltration into the stroma of surrounding cancer cell nests; (C) Stromal TIL positive group (score 2): moderate lymphocytic infiltration into the stroma without tumor cell nest permeation and (D) Stromal TIL positive group (score 3): intense lymphocytic infiltration into the stroma and between tumor cells. (E) and (F): Representative figure of stromal TILs at modetate scores in the same tissue section verified by HE and the expression of CD45 by immunohistochemistry, respectively. (G) and (H): Representative figure of stromal TILs at low scores based on HE stainings in the same tissue section verified by the expression of CD45 by immunohistochemistry. [100 × magnification, H & E-stained sections or IHC sections. (A-E, G); 100 × magnification, IHC section (F, H)]. Abbreviations: TIL = tumor-infiltrating lymphocyte.](/cms/asset/3dc54abb-b81d-4a01-b189-ee1dd43db89d/kcbt_a_1416932_f0001_oc.jpg)
Table 1. Comparison of quantitative clinical factors between according to stromal tumor-infiltrating lymphocytes negative group and positive group.
Table 2. Univariate analysis of clinical characteristics according to stromal tumor-infiltrating lymphocytes.
Comparison of the clinical variables related to OS after pancreatic resection
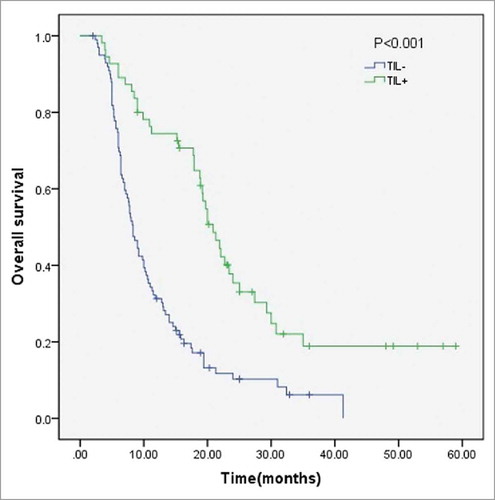
In a univariate analysis, CEA, CA19-9, tumor diameter, T staging, lymph node metastases, AJCC Staging, and stromal TILs were all significant prognostic factors for OS (all P < 0.01, ). In a multivariate analysis that also included CA125 (P = 0.051), higher levels of CEA (HR = 1.63, 95% CI 1.04-2.59, P = 0.04), a greater tumor diameter (HR = 1.50, 95% CI 1.02-2.22, P = 0.04), a higher T Staging (HR = 1.55, 95% CI 1.03-2.334, P = 0.04), lymph node metastasis (HR = 1.95, 95% CI 1.32-2.87, P < 0.01) and negative TILs (HR = 2.7, 95% CI 1.80-4.06, P < 0.01) were all significant independent predictors of a worse OS (). The TIL density was also correlated with OS ().
Table 3. Univariate and multivariate analysis of clinicopathologic variables in relation to liver metastasis and overall survival after curative operation.
Comparison of the clinical variables related to liver metastasis after pancreatic resection
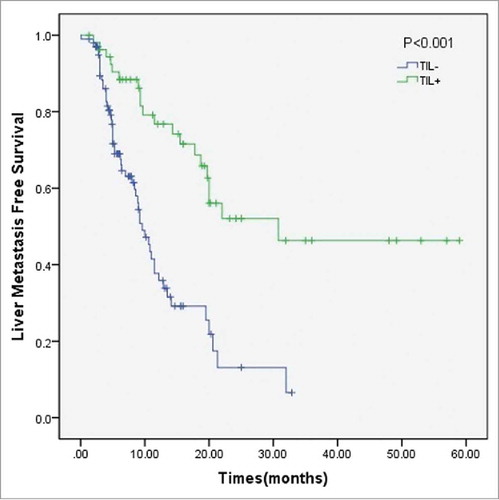
In a univariate analysis, T Staging and TIL status (both P < 0.001) were significant prognostic factors for liver metastasis after complete resection (, ). Including AJCC Staging, three variables examined with univariate analysis (P < 0.1) were selected as potential independent risk factors in multivariate analysis. The results showed that higher T Staging (HR = 1.67, 95% CI 1.06-2.62, P = 0.03) and stromal TIL negative status (HR = 2.80, 95% CI 1.75-4.48, P < 0.01) were significant independent predictors of early liver metastasis (). The predictive value of stromal TILs to liver metastasis was also determined in the liver metastasis curve ().
Clinical parameters associated with stromal TILs in negative and positive PDACs
As shown in , tumor markers CEA and CA19-9 were significantly different between the two groups (P = 0.01 and P < 0.01, respectively). Although not reaching statistical significance, TIL negative patients appeared to develop larger tumors (P = 0.09). With respect to the other quantitative clinical parameters including age, albumin, AFP, CA125, platelets, lymphocytes and neutrophils, no significant differences were observed between the two groups.
Comparisons of the categorical variables in the TIL negative and positive groups indicated that CEA (P = 0.01), tumor diameter (P = 0.05) and T Staging (P = 0.02) showed significant differences according to the stromal TIL density (). Moreover, the level of serum CA19-9 also appeared to be higher in TIL negative group, almost reaching statistical significance (P = 0.05). No significant association in the TIL negative and positive groups was observed with respect to gender, age, albumin, CA125, ABO blood type, tumor location, tumor differentiation, T Staging, lymph node metastasis or AJCC Staging ().
The effect of lymphocytes on pancreatic cancer cell lines in vitro
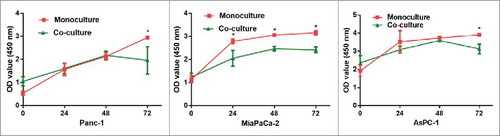
We compared the morphology of monocultures (only cancer cells) and co-cultures (cancer cells cultured with lymphocytes) by optical microscopy for the three cell lines after three days (), The co-culture (both indirect co-culture, and direct co-culture) with lymphocytes markedly limited the proliferation of pancreatic cancer cell lines, and many cancer cells showed evidence of apoptosis, such effect are much more evident in indirect co-culture (). The quantitative results of 6 parallel groups are shown in a histogram, cell counting of both the direct co-culture and the indirect co-culture are significantly lower than monoculture in all three pancreatic cancer cell lines (P < 0.05) (). As indicated in the CCK8 assay (), significant growth inhibition was also seen in all three cell lines co-cultured with lymphocytes. Taken together, these findings suggest that lymphocytes may exert an inhibitory effect on tumor growth in vitro, such effect does not require direct contact.
Figure 4. Comparison of the monoculture, indirect co-culture, and direct co-culture of the three cell lines (Panc-1, MiaPaCa-2 and AsPC-1) at 3 days. The co-culture (both indirect co-culture, and direct co-culture) with lymphocytes markedly limited the proliferation of pancreatic cancer cell lines, and many cancer cells showed evidence of apoptosis, such effect are much more evident in indirect co-culture. The quantitative results of 6 parallel groups are shown in a histogram, cell counting of both the direct co-culture and the indirect co-culture are significantly lower than monoculture in all three pancreatic cancer cell lines. (* P < 0.05, **P < 0.01).
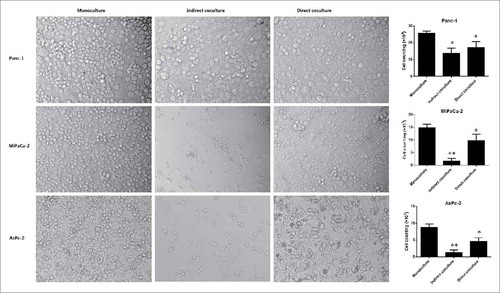
Figure 5. CCK8 assay to test the function of lymphocytes on pancreatic cancer cells (Panc-1, MiaPaCa-2 and AsPC-1). The co-culture with lymphocytes inhibited pancreatic cancer cell growth. (monoculture: pancreatic cancer cell culture alone, co-culture: pancreatic cancer cells culture with lymphocytes. * P < 0.05).
Cytokines involved in the effect between lymphocytes and pancreatic cancer cells
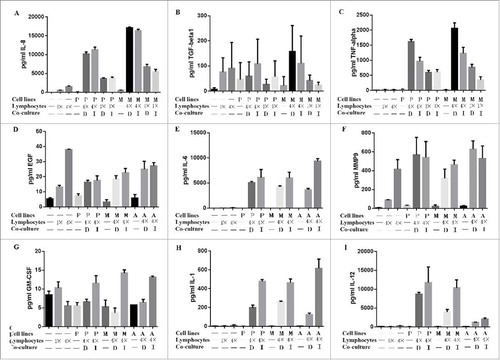
Paracrine stimulation of cancer and immune cells has been reported in several malignancies, including breast adenocarcinoma (27). We investigated the possible role of lymphocytes secreted cytokines that can inhibit cancer cells. show the results from an ELISArray experiment, where groups are tested for multiple cytokines simultaneously. shows that cocultures of lymphocytes and cancer cells at a 4:1 ratio increased the secretion of TNF-alpha in the culture media compared with at a ratio of 1:1, and much higher than lymphocytes or cancer cells alone. Further cell culture indicate that TNF-alpha can inhibit the growth of pancreatic cancer cells (). IL-8, IL-6, IL-1and IL-12 are also secreted significantly, but there were no significant additive effects in the coculture groups (, , and ). There was no significant change in the TGF-beta, EGF, MMP9 and GM-CSF concentration among the experimental groups (, , and ).
Figure 6. ELISA experiments to test the possible cytokines secreted from lymphocytes on pancreatic cancer cells (Panc-1, MiaPaCa-2 and AsPC-1). The co-culture with lymphocytes inhibited pancreatic cancer cell growth. Cocultures of lymphocytes and cancer cells at a 4:1 ratio increased the secretion of IL-8 and TNF-alpha in the culture media compared with at a ratio of 1:1, and much higher than lymphocytes or cancer cells alone(A and C). IL-6, IL-1and IL-12 are also secreted significantly, but there were no significant additive effects in the coculture groups (E, H and I). There was no significant change in the TGF-beta, EGF, MMP9 and GM-CSF concentration among the experimental groups (B, D, F and G). (P: Panc-1; M: MiaPaCa-2; A: AsPC-1: D: direct coculture; I: indirect coculture).
Discussion
Our study used routine histopathological analysis to examine the prognostic value of stromal TILs in patients after complete resection of PDAC. Our results implied that a low level of stromal TILs is a poor prognostic indicator for overall survival of PDAC patients (P < 0.01), which is consistent with many other solid tumors including breast cancer, lung cancer, and colorectal cancer.Citation6-Citation10 Such results may imply that lymphocytes may restrict the development of tumors, as also suggested by our cell experiments. Our data also showed that 64.5% (100/155) of PDAC patients were found to be stromal TIL negative, which indicates that most PDAC patients may have a poor prognosis after complete resection. Such results may contribute to the devastating prognosis of PDAC patients. As PDAC consists of a poorly vascularized tumor tissue, lymphocyte infiltration is limited, which may account for the unexpectedly poor prognosis of PDAC patients.
On the other hand, liver metastasis is the main cause (66% of cases) of death of PDAC patients after complete resection. Therefore, identifying an effective predictor of liver metastasis after complete resection is of great clinical importance. Our data showed that a low level of stromal TILs is also closely related to liver metastasis (P < 0.001). Stromal TIL density reflects the environment of tumor immunogenicity. A lack of an effective immunoenvironment will be beneficial to metastasis. It has been shown that cancer cells could develop an immunosuppressive environment for immune escape by employing a set of mechanisms to suppress TIL infiltration, such as suppressing IFN-I signaling.Citation20 By comparing stromal TILs in primary and metastatic tumors in breast cancer patients, Ogiya's study indicated that immune escape plays a role in tumor progression.Citation21
Because the above analysis indicated that stromal TILs were significantly associated with both OS and liver metastasis after complete resection, we further compared the TIL negative and TIL positive patients. Our results showed that a lack of stromal TILs appeared to be associated with a higher level of serum CEA and CA19-9, larger tumor diameter and higher T Staging. All of these can be accounted for by the lack of an effective immune environment as unconstrained tumor progression tends to promote a higher level of serum tumor markers, a larger tumor burden and higher T Staging.
Moreover, our data also indicated that T Staging is an independent predictive indicator for both liver metastasis (P = 0.026) and poor overall survival (P = 0.038) of PDAC patients. This conclusion is in agreement with a previous study.Citation22 It has also been shown that T staging is among the top 3 variables associated with long-term survival of PDAC patients in a large cohort of long-term (10 years) survivors.Citation23
Other clinical parameters such as CEA (P = 0.04), tumor diameter (P = 0.04) and lymph node metastasis (P < 0.01) were also independent predictive indicators for worse overall survival of PDAC patients. CEA is a common serum marker for tumors, that is widely used in the clinic, and its prognostic value in PDAC has been established.Citation24,Citation25 Lymph node metastasis has been shown to be an effective independent predictive indicator for worse overall survival of PDAC patients in our previous study.Citation26 As a parameter for T staging, which has been found to be a predictor for PDAC, it is self-evident that the larger tumor diameter contributes to a worse OS of PDAC patients.
Using co-culture techniques, our cell experiments suggested that lymphocytes can inhibit the proliferation of pancreatic cancer cells. Such effect may even much more evident in indirect co-culture, which may agree with the clinical value of stromal TILs that are not direct contact cancer cells. Therefore, we speculate that by the main way TILs inhibit PDAC is secreting certain cytokines rather than direct contact. This result may not only account for the observation that TILs positive patients have a better prognosis but also point to a new therapeutic approach for PDAC. Recently, by mincing tumor specimens and culturing tumor fragments in media supplemented with a high dose of IL-2 for up to 6 weeks, TILs expanded from pancreatic tumors were functional and capable of responding to pancreatic tumor-associated antigens, which supported the development of adoptive cell therapy strategies using TILs for the treatment of pancreatic cancer.Citation19 Moreover, as an application of lymphocytes for cancer treatment, ACRT may overcome many limitations of conventional therapies and has shown promise in the treatment of CLL and glioblastoma.Citation16-Citation18 Therefore, we hypothesize that if the endogenous immune system is capable of recognizing the growing tumor in tissue, the tumor specific antigens may trigger the lymphocytic response, promote the inflammation, and slow the progression of PDAC. Like the ACRT, some lymphocytes activated by a specific response may be capable of killing tumor cells, leading to the anti-tumor effect.
Our ELISA results indicate that lymphocytes secrete TNF-alpha by paracrine toward pancreatic cancer cells. A significant increase was observed in TNF-alpha concentration in supernatants of lymphocytes and pancreatic cancer cells cocultures. TNFa has been shown to induce apoptosis in gastric cancer cell through crosstalk between Smad and JNK signaling pathways.Citation27 TNF-α-induced apoptosis has been reported widely in colorectal cancer,Citation28,Citation29 breast cancer,Citation30 as well as pancreatic ductal adenocarcinoma.Citation31,Citation32 We propose that lymphocytes may cause pancreatic cancer cells apoptosis through TNF-alpha secretion. Although some other cytokines may also increased during lymphocytes and pancreatic cancer cells cocultures, there were no significant cancer cell inhibiting effect. We further evaluated the cytokines in tissue sections-through ICH, and found that G-CSF had a significant relationship with poor prognosis (data not show). G-CSF has been proved to be released by cancer cells when stimulated by TNF-alpha.Citation33 Such finding and results in vitro are in line with the expression of G-CSF in patients, therefore, the behavior in vitro may show what happens in patients to some extent.
Strengths and limitations
The retrospective nature and the use of intra-tumoral heterogeneity are potential limitations of our study. However, as a simple, inexpensive, convenient and effective prognostic indicator, our study demonstrate the potential favorable role of stromal TILs in predicting liver metastasis and overall survival of patients with PDAC after complete resection. We also show that Lymphocytes may inhibit the growth of PDAC through TNF-alpha secretion, which suggest a potential therapeutic approach against PDAC.
Conclusions
The present study showed that stromal TIL status, which may secrete TNF-alpha by paracrine in tumor immunoenvironment suppressing cancer cells, is an effective indicator of overall survival of PDAC patients, as well as an indicator of liver metastasis. Lymphocytes may suggest a potential therapeutic approach against PDAC. However, the present study did not evaluate the role of certain lymphocytes in pancreatic cancer, further studies are needed to clarify the specific mechanism underlying the certain lymphocytes effects on the pancreatic cancer cells.
Methods
Ethics statement
The study was approved by the Clinical Ethics Committee of Peking University Third Hospital. Because this study is a retrospective study, written consent was not obtained from all participants, and the patients’ data were analyzed anonymously. All methods were performed in accordance with the relevant guidelines and regulations.
Study population and design
All participants were enrolled at Peking University Third Hospital (Beijing, China) between February 2005 and January 2015. PDAC patients who underwent a complete resection and whose diagnoses were confirmed by pathological examination were enrolled in this study. Pre-operative cytological examination was not performed regularly. Patients whose pre-operative examination showed tumor invasion of the celiac artery or superior mesenteric artery or distant metastasis (liver, lung or bone metastases) may not receive complete resection whether they have regional lymph node metastasis or not. However, four patients found to have liver metastasis during surgery but not at the pre-operative examination were also enrolled. Eight PDAC patients were excluded from the study because of neoadjuvant chemotherapy or chemoradiation therapy. Tumor specimens from 169 patients were evaluated for TILs. Permanent HE-stained sections of 14 patients were not available, which precluded assessing their TILs. All surgical specimens underwent pathological evaluation to determine the extent of tumor differentiation, LN metastases, and surgical margins following surgery. The pathological Staging of PDAC was determined according to the American Joint Committee on Cancer (AJCC), 7th Edition.Citation34
Diagnosis of postoperative liver metastasis
During the postoperative follow-up, laboratory examinations including CA19-9, ultrasound or CT scanning were performed regularly. If newly developed lesions were identified in the liver by CT scan or ultrasound with no definite evidence of metastasis from other cancers or recurrence elsewhere, we consider these patients liver metastasis patients. Some of them were characterized by PET-CT. Metastasis could not be routinely confirmed by needle biopsy for fear of spreading tumor cells along the needle tract.
HE-staining and immunohistochemistry (IHC)
For the staining of paraffin sections, tissue was fixed overnight in 10% formalin, embedded with paraffin and sectioned at a thickness of 3 μm. CD45, which is expressed in all cells of hematopoietic origin, was used to identify TILs from cancer cells. Hence, sections were deparaffinized and rehydrated before they were stained with HE as well as with rabbit anti-CD45 antibody (1:200; Abcam). IHC was visualized with an avidin biotin-peroxidase complex (ABC Elite; Vector Laboratories).
Stromal TIL evaluation
Surgical specimens were collected and evaluated using HE. Stromal TILs in each case were evaluated by examination of permanent full-face HE-stained sections from surgical specimens, which were retrieved from the pathology archives. The area for stromal TIL evaluation was defined as within the borders of the invasive tumors, which did not include immune infiltrates in adjacent normal tissue or ductal carcinoma in situ. TILs in the tumor area with crush artifacts, necrosis, or regressive hyalinization were also excluded. All stromal mononuclear cells not directly in contact with cancer cells were considered as stromal TILs, which may also include plasma cells. The level of stromal TILs was reported as none, low, moderate or high (which were scored as 0, 1, 2 and 3, respectively). Representative images of different stromal TIL levels are shown in . For the further evaluation of stromal TILs, some sections were also stained with rabbit anti-CD45 antibody, compared to the same section of HE (). Most lymphocytes are small lymphocytes with diameters of 5 to 8μm, whose nucleus is round, with a shallow concave on one side and dense clumpy of chromatin, and less cytoplasm. lymphocytes are significantly different with DC (10 to 20μm) and other mononuclear cells (14 to 20μm), which is very easy to to distinguish, especially for experienced pathologist. A more granular view on the nature of lymphocytic cells are also provided (supplementary figure 1). The scattered presence of lymphocytes within the stroma of surrounding cancer nests were categorized as low lymphocyte infiltration. The modest presence of lymphocytes in the stroma without tumor nest permeation were defined as moderate lymphocyte infiltration and high lymphocyte infiltration, corresponding to an intense or marked presence of lymphocytes in the stroma as well as the insertion of lymphocytes between tumor cells. Patients were further stratified into the stromal TIL negative (none to low infiltration) or TIL positive groups (moderate to high infiltration) based on the pathologic evaluation. For patients whose stromal TIL density was heterogeneous in a single tumor section, we evaluated different regions and reported the average of five highest levels. A consensus evaluation of all surgical specimens was reached by reexamination. Two pathologists who were blinded to the clinical outcomes evaluated stromal TILs independently. The Spearman correlation coefficient between the two pathologists was 0.86 (P <0.001). If there were discrepancies between percentage of stromal TIL, the higher grade was chosen to represent the grade.
Cell culture
Lymphocytes were isolated from fresh blood samples collected from 6 healthy donors using lymphocyte separation media (HLSM1077, Multi Sciences) according to the hospital manual. Three human pancreatic cancer cell lines, Panc-1, MiaPaCa-2 and AsPC-1 (American Type Culture Collection, ATCC, Manassas, VA, 5 × 106/L), were cultured in Dulbecco's Modified Eagle Medium (DMEM) medium containing 10% fetal bovine serum at 24-well culture plates (Costar, Corning, NY, USA) over night for 24 h. The following day, isolated lymphocytes (3 × 106/L in DMEM medium) were added. Each cells lines were divided into three groups (monoculture, direct co-culture and indirect co-culture). As direct co-culture of cancer cells with lymphocytes group, lymphocytes were resuspended in DMEM and added to each well. As the indirect co-culture group, 12-well transwell inserts (membrane pore size: 0.4 μm, Costar, Corning, NY, USA) were used to separate cancer cells (at lower chambers) from DMEM (at upper chambers). As control (defined as monoculture), cancer cells were cultured in the culture plate alone in equal amount of DMEM. Cells (1 × 105) were grown at 37°C in a humidified incubator in the presence of 5% CO2.
Cell proliferation assay
Proliferation assays were performed using CCK8 (Dojindo). Cells were plated at approximately 1000 cells per well in 96-well plates in triplicate and cultured in the growth medium. Cells were then co-cultured with lymphocytes or not, and the numbers of cells per well were measured based upon the absorbance (450 nm) of reduced water-soluble tetracycline salt (WST) at the indicated time points.
ELISA
ELISArray kit was custom made from Life technologies (Multi Sciences) and included the human EGF, MMP9, IL6, IL-1beta, IL8, IL12, GM-CSF, TNF-alpha, and TGF-beta1. For the ELISA experiments, 5,000 cancer cells (Panc-1, MiaPaCa-2 and AsPC-1) were cultured in 1 mL of culture media in 24 well plate. Where indicated, lymphocytes were added at a 1:1 ratio or 4:1 ratio. The cells were cultured for 24 hours and the supernatants were collected and centrifuged at 400 g for 5 minutes to remove cells and stored at 80°C until use. The ELISA experiments followed the manufacturer's protocol for each cytokine tested.
Cytokine effect detection
Three human pancreatic cancer cell lines, Panc-1, MiaPaCa-2 and AsPC-1, were also cultured at the same condition over night for 24 h. The following day, each cells lines were divided into two groups (TNF-alpha positive and TNF-alpha negative), Human TNF-alpha (Pepro Tech) was add in TNF-alpha positive group at a final level of 1000pg/ml.
Statistical analysis
The Mann–Whitney U-test was used to describe the normality of each continuous parameter's distribution. χ2-test was used for the analysis of categorical variables in the stromal TIL negative and positive groups. Quantitative results are reported as the means ± standard deviations. Associations between clinical and histopathological parameters with overall survival (OS) were analyzed using Kaplan-Meier curves and compared with the log-rank test. Multivariable Cox proportional hazard models (backward conditional stepwise) were used to adjust for different risk factor distributions between the groups. Based upon the univariate analysis, those variables with P value less than 0.1 were included in the multivariate logistic regression analysis to confirm independent variables. Hazard ratios (HRs) of each independent variable were estimated from the Cox analysis and shown as relative risks with corresponding 95% confidence intervals (CIs). All analyses were performed using SPSS 22.0 statistical software (SPSS, IL, USA). P < 0.05 was considered statistically significant.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Authors contributions
Dr. Xiu Dianrong and Tao Lianyuan conceived and designed this study; Tao Lianyuan performed the HE, IHC and cell culture; Tao Lianyuan, Xiu Dianrong, Yuan Chunhui, Ma Zhaolai and Jian Bing performed the statistical analysis and interpreted the data; Xiu Dianrong and Tao Lianyuan wrote the manuscript. All authors reviewed and accepted the final manuscript for submission.
2017CBT10609R-f08-z-4c.jpg
Download JPEG Image (267.2 KB)Additional information
Funding
References
- Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362(17):1605–1617. doi:10.1056/NEJMra0901557.
- Zhang Q, Zeng L, Chen Y, Lian G, Qian C, Chen S, Li J, Huang K. Pancreatic Cancer Epidemiology, Detection, and Management. Gastroenterol Res Pract. 2016;2016:8962321. doi:10.1155/2016/8962321
- Long J, Luo GP, Xiao ZW, Liu ZQ, Guo M, Liu L, Liu C, Xu J, Gao YT, Zheng Y, et al. Cancer statistics: current diagnosis and treatment of pancreatic cancer in Shanghai, China. Cancer Lett. 2014;346(2):273–277. doi:10.1016/j.canlet.2014.01.004.
- Dimastromatteo J, Houghton JL, Lewis JS, Kelly KA. Challenges of Pancreatic Cancer. Cancer J. 2015;21(3):188–193. doi:10.1097/PPO.0000000000000109.
- Tao L, Zhang L, Peng Y, Tao M, Li L, Xiu D, Yuan C, Ma Z, Jiang B. Neutrophils assist the metastasis of circulating tumor cells in pancreatic ductal adenocarcinoma: A new hypothesis and a new predictor for distant metastasis. Medicine. 2016;95(39):e4932. doi:10.1097/MD.0000000000004932.
- Criscitiello C, Esposito A, Trapani D, Curigliano G. Prognostic and predictive value of tumor infiltrating lymphocytes in early breast cancer. Cancer Treat Rev. 2016;50:205–207. doi:10.1016/j.ctrv.2016.09.019.
- Gasparri ML, Attar R, Palaia I, Perniola G, Marchetti C, Di Donato V, Farooqi AA, Papadia A, Panici PB. Tumor infiltrating lymphocytes in ovarian cancer. Asian Pac J Cancer Prev: APJCP. 2015;16(9):3635–3638. doi:10.7314/APJCP.2015.16.9.3635.
- Geng Y, Shao Y, He W, Hu W, Xu Y, Chen J, Wu C, Jiang J. Prognostic Role of Tumor-Infiltrating Lymphocytes in Lung Cancer: a Meta-Analysis. Cell Physiol Biochem: International Journal Of Experimental Cellular Physiology, Biochemistry, and Pharmacology. 2015;37(4):1560–1571. doi:10.1159/000438523.
- Rozek LS, Schmit SL, Greenson JK, Tomsho LP, Rennert HS, Rennert G, Gruber SB. Tumor-Infiltrating Lymphocytes, Crohn's-Like Lymphoid Reaction, and Survival From Colorectal Cancer. J Natl Cancer Inst. 2016;108(8). doi:10.1093/jnci/djw027.
- Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, Wienert S, Van den Eynden G, Baehner FL, Penault-Llorca F, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol: Official Journal of the European Society For Medical Oncology 2015;26(2):259–271. doi:10.1093/annonc/mdu450.
- Lohneis P, Sinn M, Bischoff S, Juhling A, Pelzer U, Wislocka L, Bahra M, Sinn BV, Denkert C, Oettle H, et al. Cytotoxic tumour-infiltrating T lymphocytes influence outcome in resected pancreatic ductal adenocarcinoma. Eur J Cancer. 2017;83:290–301. doi:10.1016/j.ejca.2017.06.016.
- Fukunaga A, Miyamoto M, Cho Y, Murakami S, Kawarada Y, Oshikiri T, Kato K, Kurokawa T, Suzuoki M, Nakakubo Y, et al. CD8+ tumor-infiltrating lymphocytes together with CD4+ tumor-infiltrating lymphocytes and dendritic cells improve the prognosis of patients with pancreatic adenocarcinoma. Pancreas. 2004;28(1):e26–31. doi:10.1097/00006676-200401000-00023.
- Apte MV, Xu Z, Pothula S, Goldstein D, Pirola RC, Wilson JS. Pancreatic cancer: The microenvironment needs attention too! Pancreatology. 2015;15(4 Suppl):S32–38. doi:10.1016/j.pan.2015.02.013.
- Carr RM, Fernandez-Zapico ME. Pancreatic cancer microenvironment, to target or not to target? EMBO Mol Med. 2016;8(2):80–82. doi:10.15252/emmm.201505948.
- Sun XJ, Jiang TH, Zhang XP, Mao AW. Role of the tumor microenvironment in pancreatic adenocarcinoma. Front Biosci (Landmark Ed). 2016;21:31–41. doi:10.2741/4374.
- Brown CE, Alizadeh D, Starr R, Weng L, Wagner JR, Naranjo A, Ostberg JR, Blanchard MS, Kilpatrick J, Simpson J, et al. Regression of Glioblastoma after Chimeric Antigen Receptor T-Cell Therapy. N Engl J Med. 2016;375(26):2561–2569. doi:10.1056/NEJMoa1610497.
- Garfall AL, Maus MV, Hwang WT, Lacey SF, Mahnke YD, Melenhorst JJ, Zheng Z, Vogl DT, Cohen AD, Weiss BM, et al. Chimeric Antigen Receptor T Cells against CD19 for Multiple Myeloma. N Engl J Med. 2015;373(11):1040–1047. doi:10.1056/NEJMoa1504542.
- Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–1517. doi:10.1056/NEJMoa1407222.
- Hall M, Liu H, Malafa M, Centeno B, Hodul PJ, Pimiento J, Pilon-Thomas S, Sarnaik AA. Expansion of tumor-infiltrating lymphocytes (TIL) from human pancreatic tumors. J Immunother Cancer. 2016;4:61. doi:10.1186/s40425-016-0164-7.
- Lei Y, Xie Y, Tan YS, Prince ME, Moyer JS, Nor J, Wolf GT. Telltale tumor infiltrating lymphocytes (TIL) in oral, head & neck cancer. Oral Oncol. 2016;61:159–165. doi:10.1016/j.oraloncology.2016.08.003.
- Ogiya R, Niikura N, Kumaki N, Bianchini G, Kitano S, Iwamoto T, Hayashi N, Yokoyama K, Oshitanai R, Terao M, et al. Comparison of tumor-infiltrating lymphocytes between primary and metastatic tumors in breast cancer patients. Cancer Sci. 2016;107(12):1730–1735. doi:10.1111/cas.13101.
- Allen PJ, Kuk D, Castillo CF, Basturk O, Wolfgang CL, Cameron JL, Lillemoe KD, Ferrone CR, Morales-Oyarvide V, He J, et al. Multi-institutional Validation Study of the American Joint Commission on Cancer (8th Edition) Changes for T and N Staging in Patients With Pancreatic Adenocarcinoma. Ann Surg. 2017;265(1):185–191. doi:10.1097/SLA.0000000000001763.
- Paniccia A, Hosokawa P, Henderson W, Schulick RD, Edil BH, McCarter MD, Gajdos C. Characteristics of 10-Year Survivors of Pancreatic Ductal Adenocarcinoma. JAMA Surg. 2015, 150(8):701–710. doi:10.1001/jamasurg.2015.0668.
- Chen Y, Gao SG, Chen JM, Wang GP, Wang ZF, Zhou B, Jin CH, Yang YT, Feng XS. Serum CA242, CA199, CA125, CEA, and TSGF are Biomarkers for the Efficacy and Prognosis of Cryoablation in Pancreatic Cancer Patients. Cell Biochem Biophys. 2015;71(3):1287–1291. doi:10.1007/s12013-014-0345-2.
- Liu L, Xu H, Wang W, Wu C, Chen Y, Yang J, Cen P, Xu J, Liu C, Long J, et al. A preoperative serum signature of CEA+/CA125+/CA19-9 >/ = 1000 U/mL indicates poor outcome to pancreatectomy for pancreatic cancer. Int J Cancer. 2015;136(9):2216–2227. doi:10.1002/ijc.29242.
- Tao L, Zhang L, Peng Y, Tao M, Li G, Xiu D, Yuan C, Ma C, Jiang B. Preoperative neutrophil-to-lymphocyte ratio and tumor-related factors to predict lymph node metastasis in patients with pancreatic ductal adenocarcinoma (PDAC). Oncotarget. 2016;7(45):74314–74324.
- Wu ZQ, Zhang R, Chao C, Zhang JF, Zhang YQ: Histone deacetylase inhibitor trichostatin A induced caspase-independent apoptosis in human gastric cancer cell. Chin Med J (Engl). 2007;120(23):2112–2118.
- Benderska N, Ivanovska J, Rau TT, Schulze-Luehrmann J, Mohan S, Chakilam S, Gandesiri M, Ziesche E, Fischer T, Soder S, et al. DAPK-HSF1 interaction as a positive-feedback mechanism stimulating TNF-induced apoptosis in colorectal cancer cells. J Cell Sci. 2014;127(Pt 24):5273–5287. doi:10.1242/jcs.157024.
- Han J, Soletti RC, Sadarangani A, Sridevi P, Ramirez ME, Eckmann L, Borges HL, Wang JY. Nuclear expression of beta-catenin promotes RB stability and resistance to TNF-induced apoptosis in colon cancer cells. Mol Cancer Res: MCR. 2013;11(3):207–218. doi:10.1158/1541-7786.MCR-12-0670.
- Wang Y, Wang X, Zhao H, Liang B, Du Q. Clusterin confers resistance to TNF-alpha-induced apoptosis in breast cancer cells through NF-kappaB activation and Bcl-2 overexpression. J Chemother. 2012;24(6):348–357. doi:10.1179/1973947812Y.0000000049.
- Jamieson NB, Mohamed M, Oien KA, Foulis AK, Dickson EJ, Imrie CW, Carter CR, McKay CJ, McMillan DC. The relationship between tumor inflammatory cell infiltrate and outcome in patients with pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2012;19(11):3581–3590. doi:10.1245/s10434-012-2370-y.
- McDade TP, Perugini RA, Vittimberga FJ, Jr., Carrigan RC, Callery MP. Salicylates inhibit NF-kappaB activation and enhance TNF-alpha-induced apoptosis in human pancreatic cancer cells. J Surg Res. 1999;83(1):56–61. doi:10.1006/jsre.1998.5560.
- Bharadwaj U, Li M, Zhang R, Chen C, Yao Q. Elevated Interleukin-6 and G-CSF in Human Pancreatic Cancer Cell Conditioned Medium Suppress Dendritic Cell Differentiation and Activation. Cancer Res. 2007;67:5479–88. doi:10.1158/0008-5472.CAN-06-3963.
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–1474. doi:10.1245/s10434-010-0985-4.