ABSTRACT
Purpose: DNA hypermethylation in gene promoter regions (CpG islands) is emerging as an important pathway in colorectal cancer tumourigenesis. Whilst genetic mutations have been associated with extramural vascular invasion (EMVI) in rectal cancer, no such association has yet been made with epigenetic factors.
Methods: 100 consecutive neoadjuvant-naïve patients undergoing curative surgery for rectal were classified according to the presence or absence of EMVI on histopathological examination. DNA was extracted from tumours and subjected to bisulfite conversion and methylation-specific PCR to determine CIMP status (high, intermediate, or low; according to a validated panel of 8 genes). CIMP status was correlated with EMVI status, histopathological, clinical, and demographic variables, in addition to overall (OS) and disease free (DFS) survival.
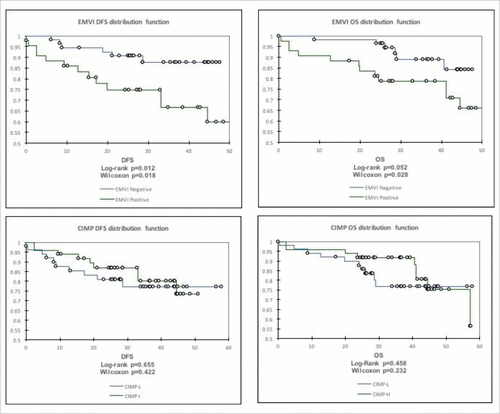
Results: 51 patients were characterised as CIMP-low, 48 CIMP-intermediate, and one patient CIMP-high. EMVI-positivity was associated with CIMP-intermediate epigenotype (p < 0.001). Patients with EMVI-positive tumours were found to have significantly more advanced disease by pT, pN, and pAJCC categorisation (p = 0.002, p < 0.001, and = p < 0.001, respectively). EMVI-positivity was significantly associated with the requirement for adjuvant chemotherapy (p < 0.001), and worse DFS but not OS (p = 0.012 and p = 0.052).
Conclusions: Given the association between CIMP-intermediate epigenotype and EMVI-positivity, and the subsequent disadvantage in pathological stage, requirement for adjuvant therapy and worse survival, tumour epigenotyping could potentially play an important role in personalising patients’ cancer care. Further work is required to understand the mechanisms that underlie the observed effect, with the hope that they may provide novel opportunities for intervention and inform treatment decisions in rectal cancer.
Introduction
Colorectal cancer (CRC) is the third most frequent cancer and the second leading cause of cancer death worldwide.Citation1 Each year, one million people will develop CRC, and 40–50% will die within five years.Citation2 Furthermore, rectal and distal sigmoid cancers are known to present at a later stage, and have a poorer prognosis than other colonic cancers.Citation3 It has long been known that patients with rectal cancer that have pathological extramural vascular invasion (EMVI-positive) are known to have a poorer prognosis than those with EMVI-negative tumours,Citation4,Citation5 and EMVI has been demonstrated to be an important risk factor for systemic recurrence,Citation6 local recurrenceCitation7 and death.Citation6-9 Additionally, EMVI status influences the need for neoadjuvant chemoradiotherapy and adjuvant chemotherapy,Citation10 as it has been demonstrated that chemoradiation (CRT) can cause vessel fibrosis in EMVI-positive tumours; which may influence survival outcomes.Citation11
Current best-practice for local staging of rectal cancer is based upon high-resolution MRI and judicious use of endorectal ultrasound, especially in low tumour where the sphincters may be threatened.Citation10,Citation12,Citation13 The ability of MRI to accurately predict the circumferential resection margin has been the most rigorously interrogated component of this investigation; the results from the MERCURY trials indicating that MRI is accurate, specific, and has a high negative-predictive value for preoperative assessment of tumour involvement at the circumferential resection margin (CRM).Citation11,Citation14 These studies, however, do not address the specific issue of EMVI in rectal cancers as separate from primary tumour or nodal involvement at the CRM. Previous studies of MRI-detected EMVI have suffered from low sensitivity and specificity,Citation15 especially for small vessels,Citation16 thus the individual prognostic significance of EMVI has been hard to extrapolate, although the presence of MRI-detected EMVI has been shown to greatly increase the relative risk of systemic recurrence.Citation17 A recent review of MRI-detected EMVI in rectal cancers suggested that some of the limitation in previous studies of is related to variable radiological reporting, but that despite this MRI-detected EMVI is a consistent predictor of recurrence and poor outcomes.Citation18 This conclusion is supported by the recent results from the GEMCAD 0801 trial, suggesting that MRI detected EMVI at baseline in CRM-threatened rectal cancer patients is an independent risk factor for poor prognosis,Citation19 and may be prognostically important in assessing response to neoadjuvant therapy.Citation20 These shortcomings have led researchers to seek biomarkers that may act as an adjunct or alternative to radiological staging in rectal cancer.
The process of carcinogenesis is now accepted to be an imbalance between the cell-cycle control mechanism and the development of mutations. Evolving from the Vogelstein model,Citation21 three overlapping genomic and epigenomic pathways are implicated in the carcinogenesis process; chromosomal instability (CIN), microsatellite instability (MSI), and an epigenetic mechanism resulting from DNA hypermethylation called CpG island methylator phenotype (CIMP).Citation22 The relationship between these mechanisms and the influence of the tumour inflammatory and immunological microenvironment is complex; and as a greater understanding of the transcriptomic pathways that influence the cancer cell phenotype is developed, the true complexity of CRC tumorigenesis is emerging.Citation23 Current consensus suggests four identifiable molecular subtypes based on transcriptomic analysis, the first of which is based upon a high degree of underlying MSI and hypermethylation (including CIMP), as opposed to CIN. Previous work has demonstrated EMVI has an inverse relationship to KRAS mutation, indicating a CIN dominated pathway in this tumour phenotype more typical of the classical adenoma-to-carcinoma pathway often found the distal colon and rectum.Citation24 MSI-high tumours on the other hand have been associated with serrated polyps and a high prevalence of BRAF mutations in the proximal colon,Citation25-27 although a significant percentage affects the rectum and may represent a pathologically worse molecular subtype.Citation28,Citation29 There is however no current biomarker available to accurately predict the presence of EMVI, although is suspected that MSI hypermutation and hypermethylation may be the predominating tumourigenic processes in this phenotype.
There are some significant challenges to addressing the question of whether CIMP can be utilised as a prognostic biomarker; the primary hurdle being the standards by which CIMP status is defined. CIMP was first defined by Toyota based upon a subset of colorectal cancers displaying methylated in tumour (MINT) CpG islands at multiple loci.Citation30 As a greater understanding of the role of methylation in CRC has developed, more specific gene loci have been implicated leading to a greater diversity of CIMP panels, implicating a wider array of tumour suppressor and housekeeping genes.Citation31,Citation32 Divergence in the panels used by individual research groups contributes to the muddy waters of the CIMP-EMVI relationship; as does the inclusion of both colon and rectal tumours, different disease stages different, CRT regimens, and clinical endpoints. Our own CIMP gene panel is based upon the two-panel approach described by Kaneda,Citation33 as it increased the fidelity of classification into CIMP-high, -intermediate, and -low; and has been shown to correlate directly with KRAS and BRAF status. The aim of this study was to determine if there is an association between CIMP status and EMVI in patients with rectal cancer.
Materials and methods
One hundred consecutive patients undergoing curative surgery for primary rectal cancer without neoadjuvant therapy were included in this study. All patients had the index surgery between January 2010 and May 2013 in a single centre (Swansea Colorectal Unit, Swansea, UK) serving a local population of 500,000 with tertiary services to 1 million people. Demographic data was extracted from a prospectively maintained database supplemented with additional data from the national (Welsh) cancer registry. Patients were stratified into those who had pathologically verified evidence of extramural vascular invasion and those who did not. Tissue blocks were retrieved from pathology department archives and the EMVI status confirmed by a consultant pathologist according to current standard definitions and in accordance with the validated Royal College of Pathologists colorectal cancer minimum reporting datasheet.Citation34,Citation35 Patients with hereditary tumours (such as familial adenomatous polyposis, Lynch syndrome, etc) identified by cross-correlation with a local hereditary cancer database or clinical history were excluded, as were patients who received neoadjuvant chemotherapy and/or radiotherapy as this could potentially change genetic or epigenetic characteristics of the samples. Patients who had also received chemo- or local radiotherapy, such as to the prostate, were also excluded. Ethical approval for this study was granted by South West Wales REC (Project Ref No.:11/WA/0256) and the study was sponsored by the R&D Department, Abertawe Bro Morgannwg University LHB, Morriston Hospital, Swansea. Consent was not required in accordance with the Human Tissue Act 2004.
DNA extraction
Tissue samples, typically two or three adjacent 8μm sections from formalin-fixed paraffin-embedded (FFPE) tissue blocks were used for each patient. Haematoxylin and Eosin (H&E) stained sections were examined by a consultant histopathologist to ensure they contained greater than 60 per cent tumour cells. In a small number of cases where the tumour sample contained less than 60 per cent tumour, the tumour cells were marked with indelible marker on H&E slides and the normal tissue was dissected manually from the slide using a scalpel blade, using the corresponding H&E slide as a guide.
DNA from tissues was obtained using the MasterPure Complete DNA and RNA purification kit (Epicentre, Illumina, Wisconsin, USA). The quantity and quality of DNA was measured at absorbance between 230nm and 320nm using spectrophotometry (Nanodrop ND-1000, Software v 3.1.2, Thermoscientific, Delaware, USA). DNA quantity was calculated by multiplying the measured concentration following spectrophotometry at 260nm with the dilution factor. DNA was diluted to a working concentration of 20ng μl−1. Purity was further assured by calculating the absorbance at 260nm to absorbance at 280nm ratio.
Bisulfite conversion and methylation specific PCR (MSP)
Methylation-specific polymerase chain reaction (MSP) was accomplished after bisulfite conversion of genomic DNA (Imprint DNA Modification Kit, Sigma Aldrich, USA). The PCR products were resolved using gel electrophoresis on a 30% polyacrylamide gel. Depending on the methylation status of each CpG island, each patient could be classified as one of three epigenotypes; CIMP-high (CIMP-H), intermediate (CIMP-I), or low (CIMP-L); using a two panel approach.Citation33,Citation36 The first panel consists of SOCS1, MINT-1 and hMLH, which are associated strongly with CIMP-H. The second panel consist of NEUROG1, THBD, HAND1, ADAMTS1, and IGFBP3. All patients had PCR performed for all eight loci. CIMP status could then be determined using the following system.
| · | CIMP-H if ≥2/3 group 1 markers methylated | ||||
| · | CIMP-I if <2/3 group 1 but ≥3/5 group 2 methylated | ||||
| · | CIMP-L if <2/3 group 1 and <3/5 group 2 methylated | ||||
Statistical analysis
Statistical analysis was performed using Microsoft Excel v.15.27 with Addinsoft XLSTAT 19.01 plug-in statistics software, and SPSS v.18 Chicago: SPSS Inc. Data was tested for normality using a Kolomogorov-Smirnov Test, and Student's T Test was for analysis of normally distributed continuous data. Categorical variables were compared using Chi-square test. Relationship between independent variables and time to event was compared using Kaplan Meier methodology. Multivariable analysis was performed using bivariate logistical regression and Cox Proportional Hazards modelling. Statistical significance was assumed at the 5% level.
Results
Patient and tumour characteristics
There were 100 patients included in this study (44 patients with an EMVI-positive tumour and 56 with no evidence of EMVI). When differences between both groups were analysed (), patients with EMVI-positive tumours had more advanced pathological staging by pT, pN, and AJCC classifications (p = 0.002, p = <0.0001, and p-<0.0001, respectively). There was a corresponding association between EMVI-positive tumours and the need for adjuvant chemotherapy (p = <0.0001). There was, however, no statistically significant difference in CRM positivity (p = 0.86), tumour perforation (p = 0.07) or tumour differentiation (p = 0.52). Patients were of a similar age in both groups, but there was a greater male predominance in the EMVI-positive group (p = 0.022).
Table 1a. Patient & Tumour Characteristics.
EMVI and CIMP status
There were a total of 51 patients with CIMP-L, 48 CIMP-I, and one patient with CIMP-H phenotype (). Chi-square (Pearson) demonstrated a positive correlation between EMVI-positive tumours and CIMP-intermediate epigenotype (p = <0.001). This effect was preserved if the single CIMP-high sample was amalgamated with the CIMP-intermediate group (p = 0.00014), and did not significantly affect any of the regression analysis performed henceforth. CIMP-intermediate tumours were associated with a more advanced pAJCC grade (p = 0.03), but was not found to be significantly associated with any other demographic or histopathological feature ().
Table 1b. CIMP and EMVI Status.
Table 1c. CIMP-I vs. CIMP-L.
Multivariable analysis
Using a multivariable logistic regression model (), CIMP status remained a highly significant predictor of EMVI status (p = 0.001), independent of pAJCC staging (p = <0.001). When considering the development of systemic recurrence, only the presence of a perforated tumour was significantly associated (p = 0.038).
Table 1d. Multivariable Logistic Regression analysis of factors associated with EMVI, Metastatic Disease, and Overall & Disease Free Survival: Standard Coefficients (SC) & Hazard Ratios (HR).
Survival
Kaplan-Meier analysis () demonstrated that overall survival (OS) and disease-free survival (DFS) were greater in the EMVI-negative group, but only reached statistical significance for DFS, although strong trend was observed for OS (p = 0.012 and p = 0.52 respectively, by log-rank). This effect was however overcome by Gehan-Breslow-Wilcoxon method (which weighs positively for early deaths (p = 0.018 and p = 0.028 for DFS and OS, respectively). CIMP status was not statistically related to DFS or OS. There was a total of 20 patients with systemic metastases (2 of these had synchronous metastases) and six had local recurrence. Although a significantly greater number of patients with EMVI-positive tumours received adjuvant chemotherapy (p < 0.0001), this relationship was not observed for CIMP-intermediate tumours, although trend was observed (p = 0.055). Significance was not achieved through a re-sampling (bootstrapping) technique.
Discussion
The findings of this study demonstrate the important relationship between extramural vascular invasion and the epigenotype of rectal tumours. EMVI is an independent risk factor for systemic and local recurrence, and death; and is an indication for adjuvant chemotherapy.Citation6-9 Currently, neoadjuvant therapy from rectal cancers is advised on the basis of threatened circumferential resection margins as demonstrated on preoperative radiological imaging,Citation10 and tumour genetic and epigenetic analysis is not routinely part of pre-therapeutic assessment. By associating CIMP epigenotype with EMVI, the possibility of utilising neoadjuvant therapies in patients who are not demonstrated to have locally advanced disease by radiological standards, but who may be judged to be at higher risk based on epigenetic tumour profiling, may improve outcomes. For this reason, it is important to understand the role that epigenetic phenotypes may have on tumour behaviour so that they may be harnessed to therapeutic effect and inform clinical decision making.
Although a significant relationship was demonstrated between CIMP-intermediate status and EMVI positivity, this was not translated in to a disease-free or overall survival disadvantage for CIMP-intermediate patients based on Kaplan-Meier analysis, despite EMVI-positivity being an independent risk-factor for disease recurrence (Cox Proportional Hazard r = 5.98 (1.10-32.50), p = 0.038). These findings are in keeping with prior results in a similar cohort from our own unit,Citation37 which indicate a positive correlation between CIMP-high status and EMVI in a series of 160 rectal cancers undergoing neoadjuvant treatment; although this did not translate into a significant relationship to survival. There was also no significant relationship between KRAS or BRAF and CIMP. More broadly, results recently published by Kim who also demonstrated that a higher CIMP status was associated with worse DFS, but only for colonic tumours as opposed to rectal tumours based on a mixed cohort of 157 patients.Citation38 In another study, high CIMP epigenotype was also found to be associated with worse overall- and progression-free survival in patients with metastatic colorectal cancer,Citation39 although this study also included both colonic and rectal tumours. Each of these studies are contributory to the hypothesis that higher CIMP is associated with more locally advanced tumour types and poorer clinical outcomes, although the relationship is not straightforward. In a review of 20 heterogeneous studies, Gallois discusses the prognostic value of EMVI in mixed cohorts of colorectal cancers, concluding that currently there is not sufficient evidence to support EMVI as a prognostic indicator.Citation40 Despite the differences between the panels used and mixed cohorts, what is clear is that the relationship between CIMP status and clinical outcomes is not solely limited to the relationship to EMVI, else DFS and OS outcomes would be more closely aligned, pointing towards additional roles for methylation in processes such as response to CRT, cell death, and tumour-immunological interaction. Early investigation of a possible mechanisms, based on quantitative real-time PCR array for genes associated with tumour invasion and metastasis, has indicated a significant change in expression of metalloproteinases (MMPs) and their inhibitors (data not shown) following demethylation. Clearly this line of enquiry needs further exploration, but the role of metalloproteinases in cancer pathogenesis is already well appreciated,Citation41 although associations between MMPs and methylation in pathological processes are only recently being realized.Citation42,Citation43
Although patients receiving neoadjuvant therapy were excluded in our study, the relationship between methylation and response to CRT (neoadjuvant and adjuvant) is likely to be significant, as has been identified in previous studies.Citation37,Citation44 The benefits of neoadjuvant therapy in rectal cancer are clear, but it is acknowledged that a tumour's response to neoadjuvant therapy is currently not predictable,Citation45 and identifying significant factors that affect response may be beneficial in managing patients. Currently, cN+ status, mucinous tumours, and poorly differentiated tumours have all been associated with poor response to neoadjuvant therapy,Citation46 but the prognostic values of these measures is limited and has no clinical utility in restricting access to pre-surgical therapies. Unfortunately this means that a proportion of patients who undergo neoadjuvant therapy will gain no benefit, and may potentially come to harm as a result of systemic chemotherapy and/or local radiotherapy,Citation47 and/or miss the opportunity to have a surgical intervention. For these reasons there is an urgency in identifying reliable molecular markers of tumour response, including exploration of the methylome for significant relationships.Citation48-50 One successful example of this approach is the utilisation of a single nucleotide mutation in the KRAS gene in predicting the response of patients to adjuvant therapy using epidermal growth factor receptor (EGFR) inhibitors; which are increasingly utilised in order to increase the effectiveness of treatment.Citation51 With regards methylation, Yokoi recently reported that DNA methylation may play an important role in affecting response to radiotherapy in an in vitro colorectal cell line model.Citation52 This process was dependent on methylation controlled expression of cellular retinol binding protein 1; cellular response to radiotherapy being strongly related to expression. In our study, CIMP-intermediate was associated with strong trend towards requirement for adjuvant chemotherapy (p = 0.055), although it did not reach significance and was not an independent risk factor for DFS or OS. Studies examining the correlation between CIMP status and response to CRT have shown some promise, although the results have been inconsistent and are plagued by methodological inconsistencies.Citation53-55 Further studies have identified methylation to be important in the response of tumours to chemoradiotherapy in other organ systems, including lung,Citation56,Citation57 breast,Citation58 glioma,Citation59 and othersCitation60,Citation61; and is a field that warrants further investigation in the context of rectal cancers. The response of tumours to neoadjuvant therapy is especially important when considering the challenges of predicting EMVI on pre-operative imaging. MRI has been shown to be accurate in the local staging rectal tumours and indicating where circumferential resection margins are threatened, thus indicating the need for neoadjuvant therapy,Citation12,Citation14 but there is an acknowledged shortcoming in the sensitivity and specificity of MRI-detected EMVI,Citation11 especially where EMVI is present in vessels smaller than 3 mm or when EMVI volume is low.Citation62 If the prognostic validity of CIMP status could be established based on biopsy, it may open the opportunity to intervene on radiologically EMVI-negative but CIMP-intermediate/-high and pathologically unfavourable tumours before surgery.
The authors acknowledge the limitations of this study, including a relatively small sample group and a lack of blinding on behalf of the researchers regarding the identity of patient samples. Although the follow-up time may be considered short (some patients will not have reached 60 months at time of analysis), the majority of systemic and local recurrences develop within 3 years of surgery. In addition to standard log-rank analysis, a Wilcoxon analysis that gives weight to early events was also used to emphasise early differences in DFS and OS between groups, demonstrating the increased significance of EMVI in OS by this method. Further research is needed to better understand this relationship, and to investigate whether CIMP status may be used to determine appropriateness for neoadjuvant CRT.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors would like to thank Mr U Khot, Mr M Davies, Mr MD Evans and Mr TV Chandrasekaran for contributing their patients to the series. We would also like to thank Miss Samantha Spencer-Harty for providing the tumour samples.
References
- Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN, Jemal A, Schymura MJ, Lansdorp-Vogelaar I, Seeff LC, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116(3):544–73. doi:10.1002/cncr.24760.
- Antelo M, Balaguer F, Shia J, Shen Y, Hur K, Moreira L, Cuatrecasas M, Bujanda L, Giraldez MD, Takahashi M, et al. A high degree of LINE-1 hypomethylation is a unique feature of early-onset colorectal cancer. PLoS One. 2012;7(9). doi:10.1371/journal.pone.0045357.
- Eisenberg B, Decosse JJ, Harford F, Michalek J. Carcinoma of the colon and rectum: the natural history reviewed in 1704 patients. Cancer. 1982;49(6):1131–4. doi:10.1002/1097-0142(19820315)49:6%3c1131::AID-CNCR2820490611%3e3.0.CO;2-T.
- Talbot IC, Ritchie S, Leighton MH, Hughes AO, Bussey HJ, Morson BC. The clinical significance of invasion of veins by rectal cancer. Br J Surg. 1980;67(6):439–42. doi:10.1002/bjs.1800670619.
- Talbot IC, Ritchie S, Leighton MH, Hughes AO, Bussey HJ, Morson BC. Spread of rectal cancer within veins. Histologic features and clinical significance. Am J Surg. 1981;141(1):15–7. doi:10.1016/0002-9610(81)90004-0.
- Krasna MJ, Flancbaum L, Cody RP, Shneibaum S, Ben Ari G. Vascular and neural invasion in colorectal carcinoma. Incidence and prognostic significance. Cancer. 1988;61(5):1018–23. doi:10.1002/1097-0142(19880301)61:5%3c1018::AID-CNCR2820610527%3e3.0.CO;2-H.
- Rich T, Gunderson LL, Lew R, Galdibini JJ, Cohen AM, Donaldson G. Patterns of recurrence of rectal cancer after potentially curative surgery. Cancer. 1983;52(7):1317–29. doi:10.1002/1097-0142(19831001)52:7%3c1317::AID-CNCR2820520731%3e3.0.CO;2-6.
- Ouchi K, Sugawara T, Ono H, Fujiya T, Kamiyama Y, Kakugawa Y, Mikuni J, Tateno H. Histologic features and clinical significance of venous invasion in colorectal carcinoma with hepatic metastasis. Cancer. 1996;78(11):2313–7. doi:10.1002/(SICI)1097-0142(19961201)78:11%3c2313::AID-CNCR7%3e3.0.CO;2-N.
- Taylor F, Mangat N, Swift IR, Brown G. Proforma-based reporting in rectal cancer. Cancer Imaging. 2010;10(1A):S142–S150. doi:10.1102/1470-7330.2010.9092.
- NICE. Colorectal cancer: diagnosis and management. [CG131]. 2014; Available from: https://www.nice.org.uk/guidance/CG131.
- Smith NJ, Barbachano Y, Norman AR, Swift RI, Abulafi AM, Brown G. Prognostic significance of magnetic resonance imaging-detected extramural vascular invasion in rectal cancer. Br J Surg. 2008;95(2):229–36. doi:10.1002/bjs.5917.
- MERCURY Study Group. Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: prospective observational study. BMJ. 2006;333(7572):779. doi:10.1136/bmj.38937.646400.55.
- Puli SR, Reddy JB, Bechtold ML, Choudhary A, Antillon MR, Brugge WR. Accuracy of endoscopic ultrasound to diagnose nodal invasion by rectal cancers: a meta-analysis and systematic review. Ann Surg Oncol. 2009;16(5):1255–65. doi:10.1245/s10434-009-0337-4.
- Taylor FG, Quirke P, Heald RJ, Moran B, Blomqvist L, Swift I, Sebag-Montefiore DJ, Tekkis P, Brown G, MERCURY study group. Preoperative high-resolution magnetic resonance imaging can identify good prognosis stage I, II, and III rectal cancer best managed by surgery alone: a prospective, multicenter, European study. Ann Surg. 2011;253(4):711–9. doi:10.1097/SLA.0b013e31820b8d52.
- Smith NJ, Shihab O, Arnaout A, Swift RI, Brown G. MRI for detection of extramural vascular invasion in rectal cancer. AJR Am J Roentgenol. 2008;191(5):1517–22. doi:10.2214/AJR.08.1298.
- Brown G, Radcliffe AG, Newcombe RG, Dallimore NS, Bourne MW, Williams GT. Preoperative assessment of prognostic factors in rectal cancer using high-resolution magnetic resonance imaging. Br J Surg. 2003;90(3):355–64. doi:10.1002/bjs.4034.
- Bugg WG, Andreou AK, Biswas D, Toms AP, Williams SM. The prognostic significance of MRI-detected extramural venous invasion in rectal carcinoma. Clin Radiol. 2014;69(6):619–23. doi:10.1016/j.crad.2014.01.010.
- Chand M, Siddiqui MR, Swift I, Brown G. Systematic review of prognostic importance of extramural venous invasion in rectal cancer. World J Gastroenterol. 2016;22(4):1721–6. doi:10.3748/wjg.v22.i4.1721.
- Patel UB, Brown G, Machado I, Santos-Cores J, Pericay C, Ballesteros E, Salud A, Isabel-Gil M, Montagut C, Maurel J, et al. MRI assessment and outcomes in patients receiving neoadjuvant chemotherapy only for Primary Rectal Cancer: longterm results from the GEMCAD 0801 trial. Ann Oncol. 2017;28(2):344–353. doi:10.1093/annonc/mdw616.
- Chand M, Evans J, Swift RI, Tekkis PP, West NP, Stamp G, Heald RJ, Brown G. The prognostic significance of postchemoradiotherapy high-resolution MRI and histopathology detected extramural venous invasion in rectal cancer. Ann Surg. 2015;261(3):473–9. doi:10.1097/SLA.0000000000000848.
- Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM, Bos JL. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319(9):525–32. doi:10.1056/NEJM198809013190901.
- Jass JR. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology. 2007;50(1):113–30. doi:10.1111/j.1365-2559.2006.02549.x.
- Dienstmann R, Vermeulen L, Guinney J, Kopetz S, Tejpar S, Tabernero J. Consensus molecular subtypes and the evolution of precision medicine in colorectal cancer. Nat Rev Cancer. 2017;17(2):79–92. doi:10.1038/nrc.2016.126.
- Gunal A, Hui P, Kilic S, Xu R, Jain D, Mitchell K, Robert M, Kenney B. KRAS mutations are associated with specific morphologic features in colon cancer. J Clin Gastroenterol. 2013;47(6):509–14. doi:10.1097/MCG.0b013e3182703030.
- Nazemalhosseini Mojarad E, Farahani RK, Haghighi MM, Aghdaei HA, Kuppen PJ, Zali MR. Clinical implications of BRAF mutation test in colorectal cancer. Gastroenterol Hepatol Bed Bench. 2013;6(1):6–13.
- Missiaglia E, Jacobs B, D'Ario G, Di Narzo AF, Soneson C, Budinska E, Popovici V, Vecchione L, Gerster S, Yan P, et al. Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. Ann Oncol. 2014;25(10):1995–2001. doi:10.1093/annonc/mdu275.
- Yamauchi M, Morikawa T, Kuchiba A, Imamura Y, Qian ZR, Nishihara R, Liao X, Waldron L, Hoshida Y, Huttenhower C, et al. Assessment of colorectal cancer molecular features along bowel subsites challenges the conception of distinct dichotomy of proximal versus distal colorectum. Gut. 2012;61(6):847–54. doi:10.1136/gutjnl-2011-300865.
- Williamson JS, Harris DA, Beynon J, Jenkins GJ. Review of the development of DNA methylation as a marker of response to neoadjuvant therapy and outcomes in rectal cancer. Clin Epigenetics. 2015;7(1):70. doi:10.1186/s13148-015-0111-3.
- Jo P, Jung K, Grade M, Conradi LC, Wolff HA, Kitz J, Becker H, Rüschoff J, Hartmann A, Beissbarth T, et al. CpG island methylator phenotype infers a poor disease-free survival in locally advanced rectal cancer. Surgery. 2012;151(4):564–70. doi:10.1016/j.surg.2011.08.013.
- Toyota M, Ahuja N, Ohe-Toyota M, Herman JG, Baylin SB, Issa JP. CpG island methylator phenotype in colorectal cancer. Proc Natl Acad Sci U S A. 1999;96(15):8681–6. doi:10.1073/pnas.96.15.8681.
- Weisenberger DJ, Siegmund KD, Campan M, Young J, Long TI, Faasse MA, Kang GH, Widschwendter M, Weener D, Buchanan D, et al. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet. 2006;38(7):787–93. doi:10.1038/ng1834.
- Ogino S, Kawasaki T, Kirkner GJ, Kraft P, Loda M, Fuchs CS. Evaluation of markers for CpG island methylator phenotype (CIMP) in colorectal cancer by a large population-based sample. J Mol Diagn. 2007;9(3):305–14. doi:10.2353/jmoldx.2007.060170.
- Kaneda A, Yagi K. Two groups of DNA methylation markers to classify colorectal cancer into three epigenotypes. Cancer Sci. 2011;102(1):18–24. doi:10.1111/j.1349-7006.2010.01712.x.
- Loughrey MB, Quirke P, Shepherd NA. G049: Standards and datasets for reporting cancers dataset for colorectal cancer histopathology reports, R.C.O. Pathologists, Editor. London; 2014. https://www.rcpath.org/resourceLibrary/dataset-for-colorectalcancer-histopathology-reports--3rd-edition-.html.
- Maughan NJ, Morris E, Forman D, Quirke P. The validity of the Royal College of Pathologists' colorectal cancer minimum dataset within a population. Br J Cancer. 2007;97(10):1393–8. doi:10.1038/sj.bjc.6604036.
- Yagi K, Akagi K, Hayashi H, Nagae G, Tsuji S, Isagawa T, Midorikawa Y, Nishimura Y, Sakamoto H, Seto Y, et al. Three DNA methylation epigenotypes in human colorectal cancer. Clin Cancer Res. 2010;16(1):21–33. doi:10.1158/1078-0432.CCR-09-2006.
- Williamson JS, Jones HG, Williams N, Griffiths AP, Jenkins G, Beynon J, Harris DA. Extramural vascular invasion and response to neoadjuvant chemoradiotherapy in rectal cancer: Influence of the CpG island methylator phenotype. World J Gastrointest Oncol. 2017;9(5):209–17. doi:10.4251/wjgo.v9.i5.209.
- Kim CH, Huh JW, Kim HR, Kim YJ. CpG island methylator phenotype is an independent predictor of survival after curative resection for colorectal cancer: A prospective cohort study. J Gastroenterol Hepatol. 2017;32(8):1469–1474. doi:10.1111/jgh.13734.
- Cha Y, Kim KJ, Han SW, Rhee YY, Bae JM, Wen X, Cho NY, Lee DW, Lee KH, Kim TY, et al. Adverse prognostic impact of the CpG island methylator phenotype in metastatic colorectal cancer. Br J Cancer. 2016;115(2):164–71. doi:10.1038/bjc.2016.176.
- Gallois C, Laurent-Puig P, Taieb J. Methylator phenotype in colorectal cancer: A prognostic factor or not? Crit Rev Oncol Hematol. 2016;99:74–80. doi:10.1016/j.critrevonc.2015.11.001.
- Kahari VM, Saarialho-Kere U. Matrix metalloproteinases and their inhibitors in tumour growth and invasion. Ann Med. 1999;31(1):34–45. doi:10.3109/07853899909019260.
- Araki Y, Mimura T. Matrix Metalloproteinase Gene Activation Resulting from Disordred Epigenetic Mechanisms in Rheumatoid Arthritis. Int J Mol Sci. 2017;18(5):pii: E905. doi:10.3390/ijms18050905.
- Lin HF, Hsi E, Huang LC, Liao YC, Juo SH, Lin RT. Methylation in the matrix metalloproteinase-2 gene is associated with cerebral ischemic stroke. J Investig Med. 2017;65(4):794–9. doi:10.1136/jim-2016-000277.
- Tsang JS, Vencken S, Sharaf O, Leen E, Kay EW, McNamara DA, Deasy J, Mulligan ED. Global DNA methylation is altered by neoadjuvant chemoradiotherapy in rectal cancer and may predict response to treatment – A pilot study. Eur J Surg Oncol. 2014;40(11):1459–66. doi:10.1016/j.ejso.2014.06.008.
- McCarthy K, Pearson K, Fulton R, Hewitt J. Pre-operative chemoradiation for non-metastatic locally advanced rectal cancer. Cochrane Database Syst Rev. 2012;12:Cd008368.
- Reggiani Bonetti L, Lionti S, Domati F, Barresi V. Do pathological variables have prognostic significance in rectal adenocarcinoma treated with neoadjuvant chemoradiotherapy and surgery? World J Gastroenterol. 2017;23(8):1412–23. doi:10.3748/wjg.v23.i8.1412.
- Sinclair RCF, Sumpter K, Griffin SM. Fitness after chemotherapy. Br J Anaesth. 2016;116(1):140. doi:10.1093/bja/aev419.
- Kretschmer C, Sterner-Kock A, Siedentopf F, Schoenegg W, Schlag PM, Kemmner W. Identification of early molecular markers for breast cancer. Mol Cancer. 2011;10(1):15. doi:10.1186/1476-4598-10-15.
- Wang M, Gu H, Wang S, Qian H, Zhu W, Zhang L, Zhao C, Tao Y, Xu W. Circulating miR-17-5p and miR-20a: molecular markers for gastric cancer. Mol Med Rep. 2012;5(6):1514–20.
- Hartmann C, Hentschel B, Tatagiba M, Schramm J, Schnell O, Seidel C, Stein R, Reifenberger G, Pietsch T, von Deimling A, et al. Molecular markers in low-grade gliomas: predictive or prognostic? Clin Cancer Res. 2011;17(13):4588–99. doi:10.1158/1078-0432.CCR-10-3194.
- Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ, Juan T, Sikorski R, Suggs S, Radinsky R, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26(10):1626–34. doi:10.1200/JCO.2007.14.7116.
- Yokoi K, Yamashita K, Ishii S, Tanaka T, Nishizawa N, Tsutsui A, Miura H, Katoh H, Yamanashi T, Naito M, et al. Comprehensive molecular exploration identified promoter DNA methylation of the CRBP1 gene as a determinant of radiation sensitivity in rectal cancer. Br J Cancer. 2017;116(8):1046–1056. doi:10.1038/bjc.2017.65.
- Shiovitz S, Bertagnolli MM, Renfro LA, Nam E, Foster NR, Dzieciatkowski S, Luo Y, Lao VV, Monnat RJ Jr, Emond MJ, et al. CpG island methylator phenotype is associated with response to adjuvant irinotecan-based therapy for stage III colon cancer. Gastroenterology. 2014;147(3):637–45. doi:10.1053/j.gastro.2014.05.009.
- Donada M, Bonin S, Barbazza R, Pettirosso D, Stanta G. Management of stage Ii colon cancer – the use of molecular biomarkers for adjuvant therapy decision. BMC Gastroenterol. 2013:36. doi:10.1186/1471-230X-13-36.
- Jover R, Nguyen TP, Pérez-Carbonell L, Zapater P, Payá A, Alenda C, Rojas E, Cubiella J, Balaguer F, Morillas JD, et al. 5-fluorouracil adjuvant chemotherapy does not increase survival in patients with CpG island methylator phenotype colorectal cancer. Gastroenterology. 2011;140(4):1174–81. doi:10.1053/j.gastro.2010.12.035.
- Harada H, Miyamoto K, Yamashita Y, Taniyama K, Ohdan H, Okada M. Methylated DLX4 Predicts Response to Pathologic Stage I Non-Small Cell Lung Cancer Resection. Ann Thorac Surg. 2015;99(5):1746–54. doi:10.1016/j.athoracsur.2014.12.058.
- Wu F, Lu M, Qu L, Li DQ, Hu CH. DNA methylation of hMLH1 correlates with the clinical response to cisplatin after a surgical resection in Non-small cell lung cancer. Int J Clin Exp Pathol. 2015;8(5):5457–63.
- Montenegro MF, González-Guerrero R, Sánchez-del-Campo L, Piñero-Madrona A, Cabezas-Herrera J, Rodríguez-López JN. Targeting the epigenetics of the DNA damage response in breast cancer. Cell Death Dis. 2016;7:e2180. doi:10.1038/cddis.2016.85.
- Larijani L, Madjd Z, Samadikuchaksaraei A, Younespour S, Zham H, Rakhshan A, Mohammadi F, Rahbari A, Moradi A. Methylation of O6-methyl guanine methyltransferase gene promoter in meningiomas–comparison between tumor grades I, II, and III. Asian Pac J Cancer Prev. 2014;15(1):33–8. doi:10.7314/APJCP.2014.15.1.33.
- van den Bent MJ, Erdem-Eraslan L, Idbaih A, de Rooi J, Eilers PH, Spliet WG, den Dunnen WF, Tijssen C, Wesseling P, Sillevis Smitt PA, et al. MGMT-STP27 methylation status as predictive marker for response to PCV in anaplastic Oligodendrogliomas and Oligoastrocytomas. A report from EORTC study 26951. Clin Cancer Res. 2013;19(19):5513–22. doi:10.1158/1078-0432.CCR-13-1157.
- Andrade AF, Borges KS, Castro-Gamero AM, Silveira VS, Suazo VK, Oliveira JC, Moreno DA, de Paula Queiroz RG, Scrideli CA, Tone LG. Zebularine induces chemosensitization to methotrexate and efficiently decreases AhR gene methylation in childhood acute lymphoblastic leukemia cells. Anticancer Drugs. 2014;25(1):72–81. doi:10.1097/CAD.0000000000000028.
- Tripathi P, Rao SX, Zeng MS. Clinical value of MRI-detected extramural venous invasion in rectal cancer. J Dig Dis. 2017;18(1):2–12. doi: 10.1111/1751-2980.12439.