ABSTRACT
Objective: This study was conducted to investigate the effects of ADP dependent glucokinase antisense RNA 1 (ADPGK-AS1)/ miR-205-5p/ zinc finger E-box binding homeobox 1 (ZEB1) on PC cells.
Methods: Differentially expressed lncRNAs and miRNAs in pancreatic cancer (PC) were identified by microarray analysis. In silico ceRNA analysis was conducted to find out the interactions among lncRNAs, miRNAs and mRNAs. Quantitative real-time PCR (qRT-PCR) was utilized to examine the expression of miR-205-5p and lncRNA ADPGK-AS1 in PC and non-cancerous cells. The association between miR-205-5p and ADPGK-AS1 as well as miR-205-5p and ZEB1 was determined by dual-luciferase reporter gene assay. After manipulating the expression of ADPGK-AS1, mir-205-5p and ZEB1 in PANC-1 and SW-1990 cells, cell proliferation, migration, invasion and apoptosis were respectively confirmed by cell counting kit-8 (CCK-8) assay, transwell assay and TUNEL. Western blot was applied to examine the expression of Epithelial-mesenchymal Transition-related proteins. In vivo experiment was conducted to further determine the effect of miR-205-5p/ZEB1 on tumorigenic ability of PC cells.
Results: MiR-205-5p was low-expressed while ZEB1 and ADPGK-AS1 were high-expressed in PC tissues and cells compared with the normal. Dual-luciferase reporter gene assay proved that ADPGK-AS1 could directly target miR-205-5p and miR-205-5p could directly target ZEB1 3′UTR. The expression of MiR-205-5p was negatively correlated with proliferation, migration and invasion, and positively correlated with apoptosis rate of PC cells, while ZEB1 and ADPGK-AS1 had an inversed effect. Further in vitro and in vivo investigation indicated that epithelial-mesenchymal transition (EMT) could be restrained by miR-205-5p through targeting ZEB1. ADPGK-AS1 strongly promoted the tumorigenesis via downregulating miR-205-5p expression and induced the EMT process in vivo.
Conclusion: ADPGK-AS1 inhibited miR-205-5p and therefore promoted PC progression through activating ZEB1-induced EMT.
Introduction
Pancreatic cancer (PC) is currently the fourth leading cause of cancer-related deaths in western countries and at the same time the sixth in China.Citation1 Although a lot of progresses have reached to better understand the mechanism of PC during the past decade, the five-year overall survival rate of intermediate and advanced PC in both genders still remains 7%.Citation2 The lack of early symptoms, diagnostic and prognostic markers is the main reason for the high mortality rate of PC.Citation3 Conventional surgical resection and chemotherapy remained the major option for most PC patients, in spite of limited overall survival benefit for its powerlessness to prevent a recurrence.Citation4 It is generally thought that multimodal therapy, including effective adjuvant (or neoadjuvant) therapy for pancreas cancer offers the best hope for meaningful long-term survival.Citation5 Therefore, novel therapeutic treatment of PC needs to be developed.
As a hot area of tumor biology research, endogenous small non-coding RNAs, miRNAs, can result in degradation or inhibition of the target mRNAs.Citation6 Aberrant expression of miRNAs has effects on carcinogenesis by enhancing the proto-oncogenes or inhibiting the tumor suppressor genes.Citation7 Several studies showed that miR-205, which was first studied by Gregory et al.,Citation8 has been known to play vital roles in oncogenesis and tumor metastasis in many kinds of carcinomas,Citation9-12 thus could become a promising strategy for PC treatment.Citation13
Long non-coding RNAs (lncRNAs), defined as non-coding RNA more than 200 nt with limited or no protein coding ability, are gaining prominence. The function of lncRNAs has been characterized in many kinds of carcinomas.Citation14-16 LncRNAs exert their function mainly at three levels: transcription, post-transcription and epigenetic modification.Citation17 Previous studies have reported that lncRNAs could function as competing endogenous RNA (ceRNA) and interact with encoding genes or other classes of no-coding RNAs, including miRNAs, and regulate their expressions.Citation18-21 Based on the result of bioinformatic analysis, we aimed at the lncRNA ADPGK-AS1, which was high-expressed in PC and had a predicted relationship with miR-205.
Reported as a crucial differentiation and morphogenetic process, epithelial-mesenchymal transition (EMT) can facilitate cell movements and generation of new tissue types during embryogenesis.Citation22 The expression of genes that promote cell-cell contact, such as E-cadherin and the miR-200 family were lost during the acquisition of EMT characteristics, while migration and invasion of various cancer cells, such as PC cells could be enhanced by the expression of mesenchymal markers, including fibronectin and N-cadherin.Citation23 EMT was discovered to be an essential phenotypic conversion during the development of embryonic, the remodeling of tissues, the healing of wound, and cancer metastasis.Citation23-25 The EMT inducer ZEB1 is a transcriptional repressor of epithelial gene such as E-cadherin.Citation26 Depletion of ZEB1 attenuates stemness, colonization capacity and also phenotypic/metabolic plasticity of PC cells.Citation27 Moreover, it has been confirmed that EMT could be suppressed by miR-205-5p targeting ZEB1 and SIP1 (ZEB2) [Philip et al., 2008]. Thus, we put our hypothesis that ADPGK-AS1 might function through miR-205-5p and regulating ZEB1-induced EMT process.
This study investigated the role of miR-205-5p, ADPGK-AS1 and ZEB1 in pancreatic carcinogenesis. We found that downregulation of ADPGK-AS1 facilitated the miR-205-5p, which could suppress PC cell invasion, migration and proliferation by inhibiting ZEB1-induced EMT. These findings revealed that inhibiting ADPGK-AS1 might be a potential treatment for PC.
Materials and methods
Cell culture
Normal pancreatic ductal epithelial cell line HPDEC, human embryo kidney cell line HEK-293T, human pancreatic cancer cell lines PANC-1 and SW-1990 were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). Dulbecco's Modified Eagle Medium (DMEM) (Gibco, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS) (Gibco) was used to culture the cells at 37°C in a humidified chamber containing 5% CO2.
Transfection
Transient transfection for in vitro experiments: miR-205-5p mimics, miR-205-5p inhibitor, the plasmids containing ADPGK-AS1, ZEB1 cDNA or ZEB1 shRNA were purchased from RiboBio (Guangzhou, China). Cells were cultured with DMEM containing 10% FBS until the convergence rate reached 80–90%. All the compounds were transfected into the cells using Lipofectamine 2000 reagent and Opti-MEM serum-free medium (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol. Cells were cultured for another 48 h after transfection.
Stable transfection for in vivo experiments: Lentivirus vectors containing ADPGK-AS1 or pre-miR-205 were purchased from RiboBio. Lentivirus vectors, pMDLg/pRRE, pRSV-Rev and pMD2.G plasmids were co-transfected into HEK-293T cells to obtain lentivirus particles. PANC-1 cells were seeded onto 24-well plates and cultured for 24 h, then lentivirus suspension was added (MOI = 20) with polybrene (5 μg/mL) present. Cells were incubated for another 24 h, and then medium containing lentivirus particles was removed and replaced as fresh medium. Transfected cells were observed under the fluorescence microscope (Thermo Fisher Scientific, Waltham, MA, USA) to estimate the transfection efficiency.
Microarray analysis
The expression profiles of lncRNAs and miRNAs were obtained from The Cancer Genome Atlas (TCGA) dataset. Bioconductor R (https://www.r-project.org/) was used to analyze the expression profiles and filter differential-expressed lncRNAs and miRNAs. Restricted conditions were foldchange > 2 and adjusted P value < 0.05. The results were presented in the form of volcano plot and heatmap.
CeRNA analysis and target prediction
We constructed a lncRNA-miRNA-mRNA network using Cytoscape (http://www.cytoscape.org/) to visualize their interactions based on our lncRNA microarray data and miRNA microarray data. In the network, we predicted the lncRNA/miRNA interaction with miRNA target prediction software (Arraystar's home-made) established from TargetScan (http://www.targetscan.org/) and miRanda (http://www.microrna.org/). The network was based on the theory of ceRNA that the lncRNA shared the same miRNA with mRNA or other non-coding RNAs in one triplet.
qRT-PCR
Total RNA was extracted with Trizol reagent (Beyotime, Shanghai, China). The reverse transcription of lncRNA, mRNA and miRNA were carried out in accordance with the protocol of Prime Script RT Master Mix (TaKaRa) and amiRcute miRNA cDNA kit (Tiangen, Beijing, China) respectively. QRT-PCR was performed by SYBR kit (Takara, Tokyo, Japan) and Mastercycler nexus X2 PCR (Eppendorf, Hamburg, Germany). U6 and GAPDH were used as the endogenous reference for RNA expression. The relative quantities of RNAs were calculated using 2−ΔΔCt method. Primer sequences were shown in .
Table 1. Primers for qRT-PCR.
Western blot
Cell proteins were extracted with RIPA lysis buffer (Beyotime). BCA protein assay kit (Beyotime) was used to measure the protein concentration. Afterwards, the proteins were dissociated using SDS-PAGE and then placed onto PVDF membranes (Invitrogen). The membranes were subsequently sealed with 5% skim milk for 1 h, and then incubated with primary antibodies rabbit anti-ZEB1 (1:1000, Abcam, Cambridge, MA, USA), anti-E-Cadherin (1:1000, Abcam), anti-N-Cadherin (1:1000, Abcam) and anti-β-actin (1:500, Abcam) for 24 h at 4°C. Secondary antibody HRP-conjugated goat anti-rabbit IgG (1:2000, Abcam) was added to incubate the membranes at 37°C for 1 h after washing the membrane with tris buffer saline-Tween 20 (TBST) for 10 min. Then the membranes were washed with TBST three times. The enhanced chemiluminescence (ECL) detection reagent (Beyotime) was utilized to visualize the proteins, and the immunoblot strips were analyzed using Image J 1.48u.
Cell counting kit-8 (CCK-8) assay
Cells were seeded in a 96-well plate (2 × 103 cells/well) and cultured for 24/48/72/96 h, then 10 μL CCK-8 solution (Dojindo, Kumamoto, Japan) was added to each well and the cells were incubated at 37°C for 1 h. A microplate reader (Biotek, Winooski, Vermont, USA) was used to quantify the absorbance of each well at the wavelength of 450 nm. All reactions were repeated in triplicate.
TUNEL
TUNEL was performed using In Situ Cell Death Detection Kit (Roche, Basel, Switzerland). Cell suspension was smeared on the coverslip, then 4% paraformaldehyde was dropwise added to fix the cells for 1 h. Fixed cells were then treated by 0.1% Triton X-100 (Beyotime) at 4°C for 3 min, and 50 μL TUNEL solution was added. After 1 h incubation at 37°C in the dark room, the fluorescence intensity was observed under the fluorescence microscope.
Dual-luciferase reporter gene assay
The wild type (wt) or mutant type (mut) 3' UTR of ZEB1 as well as ADPGK-AS1 were amplified and subcloned into pmiR-REPORT luciferase vector (RiboBio, Guangzhou, China). MiR-205-5p mimics or control mimics and wt or mut 3′UTR of ZEB1 or ADPGK-AS1 were simultaneously transfected into HEK-293T cells with Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA). 24 h later, the luciferase activity was observed by dual-luciferase reporter assay system (Promega, Madison, WI, USA) in compliance with the producer's protocol.
Transwell assay
The cells were placed on 12-well plates. After diluted using 100 μL serum-free medium, Matrigel (BD Biosciences, San Jose, CA, USA) was added to the upper chamber of transwell. 2 × 105 cells were seeded into the upper chambers with serum-free medium. The lower chambers were added with DMEM containing 10% FBS. When examining cell migration, Matrigel was not used. After incubation in a 5% CO2 humidified atmosphere at 37°C for 1 h, we fixed the migrating or invading cells with 4% paraformaldehyde for 10 min and then stained these cells with 1% crystal violet. The invasion cells were counted under the optical microscope (Nikon, Tokyo, Japan).
Tumor xenograft
The Institutional Animal Care and Treatment Committee of the Qilu Second Hospital of Shandong University (Shandong, China) authorized all animal protocols. The mice (4-week-old BALB/c mice, 5 per group) were subcutaneously injected with PANC-1 cells (1 × 107) stably transfected with miR-205-5p mimics or ADPGK-AS1, and the tumor volume was recorded every 5 days. All the mice were sacrificed at 30th day and the tumor weight were measured. Western blot was conducted to analyze the expression of ZEB1, N-cadherin and E-cadherin in mice tumor tissues.
Immunohistochemistry
The fresh tumor tissues were extracted after sacrificing the mice at 30th day, then fixed in 4% paraformaldehyde and embedded in paraffin. The 5-µm-thick sections were dewaxed twice in xylene for 10 min and rehydrated in ethanol. After washed with PBS 3 times, the slides were boiled in 10 mM/L citrate buffer for antigen retrieval and blocked by immersion in 3% methanolic peroxide for 15 min. Afterwards, sections were stained with primary antibody Ki-67 (1:500-1:1000, BOSTER) at 4°C overnight and washed by PBS for 3 times. Incubation with HRP-labeled secondary antibody anti-APS IgG (BOSTER) was performed at room temperature for 30 min. After washed with water, diaminobenzidine (DAB, Sigma-Aldrich) was utilized for 5 min then hematoxylin (Richard Allan Scientific, Kalamazoo, MI, USA) was added for another 2 min.
Statistical analysis
SPSS version 21.0 (SPSS Inc, Chicago, IL, USA) was applied to statistical analyses and all results were calculated as mean ± standard deviation (SD). Student's t-test was utilized to analyze the differences between groups while one-way ANOVA was utilized for multiple groups. P < 0.05 represented statistically significance.
Results
LncRNA and miRNA expression profiles in pancreatic cancer
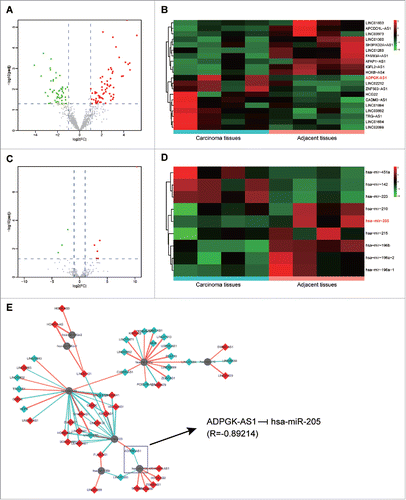
The expression profiles of lncRNAs and miRNAs in PC carcinomas and adjacent tissues were obtained from TCGA. Foldchanges (> 2) and adjusted P values (< 0.05) were observed to assess the differential expression of genes. The volcano plots showed the variation of lncRNA and miRNA expression (, ). 20 most marked differentially expressed lncRNAs and all the differentially expressed miRNAs identified by microarray analysis were presented in the heatmap (, ). It was shown that lncRNA ADPGK-AS1 was up-regulated in PC carcinoma tissues compared with the adjacent tissues while miR-205 was down-regulated. In the network created by Cytoscape (), lncRNA/miRNA interaction was predicted using Arraystar's home-made miRNA target prediction software. All these analysis results revealed that lncRNA ADPGK-AS1 potentially targeted miR-205.
Figure 1. LncRNA and miRNA expression profiles in pancreatic cancer (A) Volcano plot of LncRNA expression profile in PC; (B) Heatmap of LncRNA expression profile in PC; (C) Volcano plot of miRNA expression profile in PC; (D) Heatmap of lncRNA expression profile in PC; (E) The lncRNA/miRNA interaction, predicted an direct association between miR-205-5p and ADPGK-AS1.
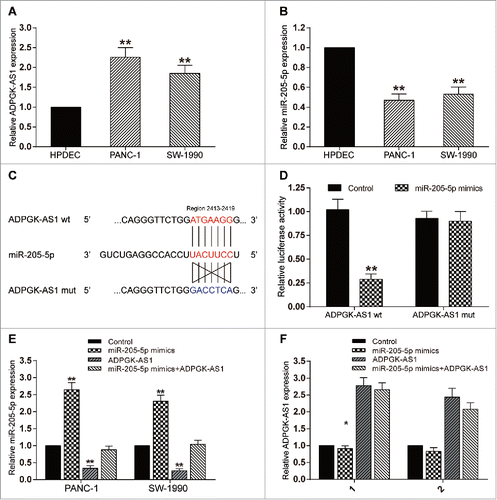
LncRNA ADPGK-AS1 directly targeted miR-205-5p
Compared with HPDEC cell line (negative control), the expression of lncRNA ADPGK-AS1 in PANC-1 and SW-1990 cell lines was significantly up-regulated (P < 0.01, ) while the expression of miR-205-5p was down-regulated (P < 0.01, ). The result of dual-luciferase reporter assay showed that co-transfection of ADPGK-AS1 (wt) and miR-205-5p mimics could significantly reduce luciferase activity with respect to other groups (P < 0.01, ). Further experiments indicated that overexpression of ADPGK-AS1 significantly suppressed miR-205-5p expression, while miR-205-5p had little effect on the expression of ADPGK-AS1(P < 0.01, ), confirming that ADPGK-AS1 could efficiently sponge miR-205-5p and suppress its expression.
Figure 2. LncRNA ADPGK-AS1 directly targeted miR-205-5p (A) Relative expression level of ADPGK-AS1 in PANC-1 and SW-1990 cells; (B) Relative expression level of miR-205-5p in PANC-1 and SW-1990 cells; (C) The predicted binding site between miR-205-5p and ADPGK-AS1; (D) The relative luciferase activity of ADPGK-AS1+miR-205-5p was significantly lower; (E) miR-205-5p expression could be promoted by miR-205-5p mimics transfection while suppressed by ADPGK-AS1 transfection; (F) ADPGK-AS1 expression could be promoted by ADPGK-AS1 transfection while miR-205-5p mimics transfection had little effect on ADPGK-AS1 expression. (*P < 0.05, **P < 0.01, compared with control group).
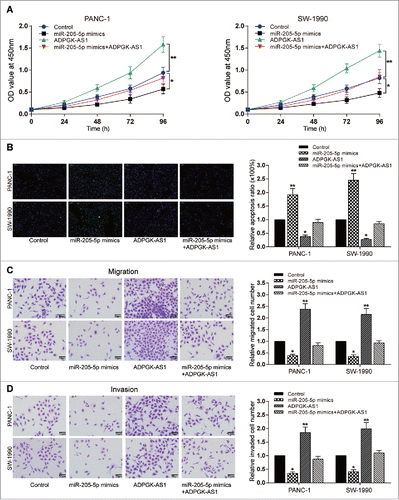
ADPGK-AS1 promoted proliferation, migration and invasion, and suppress apoptosis of PC cells through inhibiting miR-205-5p
Cellular experiments were conducted to explore the effects of ADPGK-AS1 and miR-205-5p on PC cell viability. Higher proliferation ratio and lower cell apoptosis rate were detected in ADPGK-AS1 overexpression group, while upregulation of miR-205-5p (P < 0.05, ). Transwell assay results showed that ADPGK-AS1 overexpression could promote the migration and invasion capacity of PC cells, whereas miR-205-5p overexpression suppressed the migration and invasion of PC cells (P < 0.01, ). Moreover, in the group which ADPGK-AS1 and miR-205-5p were simultaneously overexpressed, no significant difference was observed in all results compared to the control group ().
Figure 3. LncRNA ADPGK-AS1 induced proliferation, decrease of apoptosis rate, migration, and invasion of PC cells (A) CCK-8: PANC-1/SW-1990 cell viability could be promoted by ADPGK-AS1 while suppressed by miR-205-5p mimics; (B) TUNEL: PANC-1/SW-1990 cell apoptosis could be reduced by ADPGK-AS1 while enhanced by miR-205-5p mimics; (C) Transwell: PANC-1/SW-1990 cell migration could be promoted by ADPGK-AS1 while suppressed by miR-205-5p mimics; (D) Transwell: PANC-1/SW-1990 cell invasion could be promoted by ADPGK-AS1 while suppressed by miR-205-5p mimics. (*P < 0.05, **P < 0.01, compared with control group).
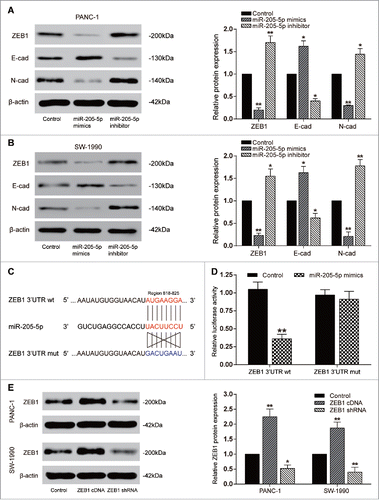
MiR-205-5p directly targeted ZEB1
At 48 hours post transfection, cells were harvested and total RNA or proteins were extracted and analyzed. Compared with the control group, up-regulation of miR-205-5p in PANC-1 and SW-1990 cell lines could significantly up-regulate E-cadherin expression but down-regulate ZEB1 and N-cadherin expression (P < 0.01, ). Besides, as dual-luciferase reporter gene assay revealed, co-transfection of ZEB1 (wt) and miR-205-5p showed a remarkably decreased luciferase activity compared with other groups, which verified the predicted binding site between miR-205-5p and ZEB1 (P < 0.01, ). To investigate the effect of ZEB1 in PC cells, we used ZEB1 cDNA or shRNA to regulate its expression, and qRT-PCR results verified their effect that ZEB1 cDNA or shRNA could respectively promote or inhibit the expression of ZEB1 (P < 0.01, ).
Figure 4. MiR-205-5p directly targeted ZEB1 (A-B) Western blot: in PANC-1 and SW-1990 cells, E-cadherin expression was promoted by miR-205-5p mimics while suppressed by miR-205-5p inhibitor; ZEB1 and N-cadherin expression was suppressed by miR-205-5p mimics while promoted by miR-205-5p inhibitor; (C) The predicted binding site between miR-205-5p and ZEB1;(D) The relative luciferase activity of ZEB1 3′UTR wt+miR-205-5p mimics was remarkably lower; (E) Western blot: in PANC-1 and SW-1990 cells, ZEB1 expression was promoted by ZEB1 cDNA while suppressed by ZEB1 shRNA. (*P < 0.05, **P < 0.01, compared with control group).
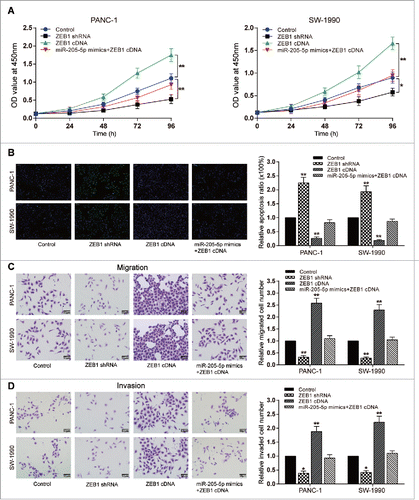
MiR-205-5p reversed ZEB1-induced PC cell proliferation, migration, invasion and decrease of apoptosis
Higher proliferation ratio and lower cell apoptosis rate were detected in ZEB1 cDNA group (P < 0.05, ), while in ZEB1 shRNA group, lower proliferation ratio and higher cell apoptosis rate were detected (P < 0.05, ). Transwell assay results showed that ZEB1 cDNA could induce the migration and invasion capacity of PC cells, whereas ZEB1 shRNA inhibited the migration and invasion of both two PC cell lines (P < 0.01, ). Simultaneous overexpression of ZEB1 and miR-205-5p showed no significant difference in all results compared with the control group ().
Figure 5. MiR-205-5p reversed ZEB1-induced proliferation, decrease of apoptosis rate, migration, and invasion of PC cells (A) CCK-8: PANC-1/SW-1990 cell viability could be promoted by ZEB1 cDNA while suppressed by ZEB1 shRNA; (B) TUNEL: PANC-1/SW-1990 cell apoptosis could be reduced by ZEB1 cDNA while enhanced by ZEB1 shRNA; (C) Transwell: PANC-1/SW-1990 cell migration could be promoted by ZEB1 cDNA while suppressed by ZEB1 shRNA; (D) Transwell: PANC-1/SW-1990 cell invasion could be promoted by ZEB1 cDNA while suppressed by ZEB1 shRNA. (*P < 0.05, **P < 0.01, compared with control group).
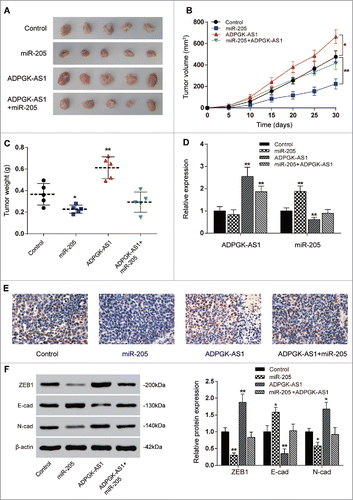
ADPGK-AS1/miR-205-5p regulated PC tumor growth through EMT in vivo
Tumor xenograft in nude mice was conducted to explore the effect of ADPGK-AS1/miR-205-5p in vivo. Stably transfected PANC-1 cells were subcutaneously injected and the mice were sacrificed at thirty days post-injection. In ADPGK-AS1 group, tumor volume and weight were apparently higher in comparison with that in control group, while all the results were inversed in miR-205 group (P < 0.01, ). Total RNA was isolated from tumor tissues and qRT-PCR was utilized to measure the expression of ADPGK-AS1 and miR-205-5p. Expectedly, the ADPGK-AS1 expression was up-regulated in ADPGK-AS1 group and ADPGK-AS1+miR-205 group compared with the control group which injected with untreated PANC-1 cells (P < 0.01, ). Ki-67 staining confirmed the stronger tumorigenesis in ADPGK-AS1 group and less cell viability in miR-205 group compared to the control (P < 0.01, ). However, there was no differences in Ki-67 positive score between control and ADPGK-AS1+miR-205 group, suggesting the regulatory relationship between ADPGK-AS1 and miR-205-5p. In miR-205 group, ADPGK-AS1 expression showed no significant difference compared with control group while miR-205-5p expression increased. On the other hand, stably transfected ADPGK-AS1 downregulated the miR-205-5p expression (P < 0.01), while the downregulation was rescued by co-transfection of pre-miR-205. Total proteins were also extracted for western blot. The results indicated that E-cadherin expression was down-regulated in ADPGK-AS1 group while which was up-regulated in miR-205 group; expression of N-cadherin and ZEB1 was up-regulated in ADPGK-AS1 group while which was down-regulated in miR-205-5p group, indicating that ADPGK-AS1 or miR-205-5p could respectively promote or inhibit tumor formation through regulating EMT connected proteins (P < 0.01, ).
Figure 6. ADPGK-AS1/miR-205-5P regulated PC tumor growth through EMT in vivo (A) Images of the excised tumors at 30th day were presented; (B) Tumor volume of miR-205-5p group was significantly lower while which of ADPGK-AS1 group was significantly higher; (C) Tumor weight of miR-205-5p group was significantly lower while which of ADPGK-AS1 group was significantly higher; (D) Total RNA in tumor tissues was extracted and qRT-PCR was utilized to measure the expression of ADPGK-AS1 and miR-205-5p. (E) Immunohistochemistry staining of Ki-67 was performed to value the cell viability during tumorigenesis. (F) Western blot: In tumor tissues, E-cadherin expression was promoted by miR-205-5p while suppressed by ADPGK-AS1; ZEB1 and N-cadherin expressions were suppressed by miR-205-5p while promoted by ADPGK-AS1. (*P < 0.05, **P < 0.01, compared with control group).
Discussion
The present study showed that the overexpression of lncRNA ADPGK-AS1 could induce the proliferation and metastasis of PANC-1 and SW-1990 cell lines through inhibiting miR-205-5p and subsequently facilitating the expression of ZEB1. ZEB1 overexpression could promote PC cell migration, invasion and proliferation but suppress apoptosis, which could be rescued by enforced expression of miR-205-5p. In vivo experiments also validated the in vitro experiments, showing that ADPGK-AS1 down-regulation, miR-205-5p up-regulation or ZEB1 inhibition efficiently suppressed PC tumor growth via restraining EMT.
This is the first paper to evaluate the expression level of ADPGK-AS1 in PC cell lines as we know. In the present study, we found high-expressed ADPGK-AS1 and its potential relationship with low-expressed miR-205-5p through bioinformatic analysis. To verify the analysis result, we performed transfection and manipulated ADPGK-AS1 and miR-205-5p expression in two PC cell lines. The expression of miR-205-5p could be downregulated by ADPGK-AS1 and further dual luciferase reporting assay confirmed that miR-205-5p was directly sponged by ADPGK-AS1. Previous studies have showed that miR-205 expression was down-regulated in PC cells and its overexpression could suppress the metastasis of PC.Citation28,Citation29 Several lncRNAs have been reported to act as a ceRNA against miR-205-5p and affect the tumorigenesis, including MALAT1, GAS5 and SNHG5.Citation30-32 Our study showed the parallel results that the overexpression of ADPGK-AS1 was found to sponge miR-205-5p and therefore induce the migration and proliferation of PC cells.
Our study also explored the effect of ADPGK-AS1/miR-205-5p on regulating ZEB1, which could induce EMT process, enhance the metastasis and propagation and at the same time inhibit the apoptosis of PC cells. As widely reported, cell migration and invasiveness could be promoted by EMT, which has important effects in development of cancer cell metastasis and drug resistance, including in pancreatic cancer.Citation33 MiR-205-5p and ZEB1 have been known to be closely associated with EMT pathway.Citation8 This axis has been confirmed to participate in PC development and progression in the study.Citation11,Citation34,Citation35 However, this miRNA-mRNA relationship needs further studies, such as the mechanism of miRNA regulation in PC progression, which might be affected by lncRNA. Actually, researches about lncRNA-miRNA-mRNA relationship have been widely performed. For instance, a former study have proved that there exists a pathway of lncRNA-TUG1/miR-382/EZH2 in PC cells, which could also take part in proliferation and migration through EMT.Citation36 Herein, we found a high-expressed lncRNA ADPGK-AS1, explored its targeting relationship with miR-205-5p and unraveled the mechanism that underlines how ADPGK-AS1/miR-205-5p/ZEB1 axis regulates pancreatic carcinogenesis. However, our study only provided a lncRNA-miRNA-mRNA pathway. Actually, ZEB1 could be inhibited by many miRNAs, including miR-200 family,Citation8,Citation37 and those miRNAs could also interact with other non-coding RNAs, which ought to be further explored.
In summary, we have proved that ADPGK-AS1 was up-regulated in PC cells and the upregulation of ADPGK-AS1 could promote the migration and invasion as well as suppress the apoptosis of PC cells. Moreover, ADPGK-AS1 was found directly targeting on miR-205-5p, acting as a ceRNA to promote the expression of ZEB1, which could induced the EMT process, promote the proliferation, migration and invasion of PC cells. We performed an in vivo experiment to further prove the effect of ADPGK-AS1/miR-205-5p/ZEB1 on the inhibition of PC tumorigenesis. Our findings helped to better understand the function of ADPGK-AS1/miR-205-5p/ZEB1 in PC progression, which may provide new insights into in-time diagnosis and therapeutic strategies.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed by the authors.
Author contributions
Research conception and design: Suzhen Song and Weihua Yu. Data analysis and interpretation: Sen Lin and Mingbao Zhang. Statistical analysis: Teng Wang and Shuang Guo. Drafting of the manuscript: Suzhen Song. Critical revision of the manuscript: Suzhen Song and Weihua Yu. Receiving grant: Hongbo Wang. Approval of final manuscript: all authors.
Additional information
Funding
References
- Li Y, Vandenboom TG, 2nd, Wang Z, Kong D, Ali S, Philip PA, Sarkar FH. miR-146a suppresses invasion of pancreatic cancer cells. Cancer Res. 2010;70(4):1486–95. doi:10.1158/0008-5472.CAN-09-2792. PMID:20124483.
- Ali S, Dubaybo H, Brand RE, Sarkar FH. Differential expression of MicroRNAs in tissues and plasma Co-exists as a biomarker for pancreatic cancer. J Cancer Sci Ther. 2015;7(11):336–46. doi:10.4172/1948-5956.1000372. PMID:26819679.
- Chakraborty S, Baine MJ, Sasson AR, Batra SK. Current status of molecular markers for early detection of sporadic pancreatic cancer. Biochim Biophys Acta. 2011;1815(1):44–64. doi:10.1016/j.bbcan.2010.09.002. PMID:20888394.
- Peng T, Zhou W, Guo F, Wu HS, Wang CY, Wang L, Yang ZY. Centrosomal protein 55 activates NF-kappaB signalling and promotes pancreatic cancer cells aggressiveness. Sci Rep. 2017;7(1):5925. doi:10.1038/s41598-017-06132-z. PMID:28724890.
- Paulson AS, Tran Cao HS, Tempero MA, Lowy AM. Therapeutic advances in pancreatic cancer. Gastroenterology. 2013;144(6):1316–26. doi:10.1053/j.gastro.2013.01.078. PMID:23622141.
- Harazono Y, Muramatsu T, Endo H, Uzawa N, Kawano T, Harada K, Inazawa J, Kozaki K. miR-655 Is an EMT-suppressive microRNA targeting ZEB1 and TGFBR2. PLoS One. 2013;8(5):e62757. doi:10.1371/journal.pone.0062757. PMID:23690952.
- Szafranska AE, Davison TS, John J, Cannon T, Sipos B, Maghnouj A, Labourier E, Hahn SA. MicroRNA expression alterations are linked to tumorigenesis and non-neoplastic processes in pancreatic ductal adenocarcinoma. Oncogene. 2007;26(30):4442–52. doi:10.1038/sj.onc.1210228. PMID:17237814.
- Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10(5):593–601. doi:10.1038/ncb1722. PMID:18376396.
- Torres A, Kozak J, Korolczuk A, Rycak D, Wdowiak P, Maciejewski R, Torres K. Locked nucleic acid-inhibitor of miR-205 decreases endometrial cancer cells proliferation in vitro and in vivo. Oncotarget. 2016;7(45):73651–63. doi:10.18632/oncotarget.12043. PMID:27655663.
- Zhong G, Xiong X. miR-205 promotes proliferation and invasion of laryngeal squamous cell carcinoma by suppressing CDK2AP1 expression. Biol Res. 2015;48:60. doi:10.1186/s40659-015-0052-5. PMID:26515287.
- Niu K, Shen W, Zhang Y, Zhao Y, Lu Y. MiR-205 promotes motility of ovarian cancer cells via targeting ZEB1. Gene. 2015;574(2):330–6. doi:10.1016/j.gene.2015.08.017. PMID:26275944.
- Bai J, Zhu X, Ma J, Wang W. miR-205 regulates A549 cells proliferation by targeting PTEN. Int J Clin Exp Pathol. 2015;8(2):1175–83. PMID:25973003.
- Mittal A, Chitkara D, Behrman SW, Mahato RI. Efficacy of gemcitabine conjugated and miRNA-205 complexed micelles for treatment of advanced pancreatic cancer. Biomaterials. 2014;35(25):7077–87. doi:10.1016/j.biomaterials.2014.04.053. PMID:24836307.
- Pan Y, Li C, Chen J, Zhang K, Chu X, Wang R, Chen L. The emerging roles of long noncoding RNA ROR (lincRNA-ROR) and its possible mechanisms in human cancers. Cell Physiol Biochem. 2016;40(1–2):219–29. doi:10.1159/000452539. PMID:27855392.
- Zhang A, Xu M, Mo YY. Role of the lncRNA-p53 regulatory network in cancer. J Mol Cell Biol. 2014;6(3):181–91. doi:10.1093/jmcb/mju013. PMID:24721780.
- Martens-Uzunova ES, Bottcher R, Croce CM, Jenster G, Visakorpi T, Calin GA. Long noncoding RNA in prostate, bladder, and kidney cancer. Eur Urol. 2014;65(6):1140–51. doi:10.1016/j.eururo.2013.12.003. PMID:24373479.
- Li X, Wu Z, Fu X, Han W. lncRNAs: insights into their function and mechanics in underlying disorders. Mutat Res Rev Mutat Res. 2014;762:1–21. doi:10.1016/j.mrrev.2014.04.002. PMID:25485593.
- Zhang Y, Xu Y, Feng L, Li F, Sun Z, Wu T, Shi X, Li J, Li X. Comprehensive characterization of lncRNA-mRNA related ceRNA network across 12 major cancers. Oncotarget. 2016;7(39):64148–67. doi:10.18632/oncotarget.11637. PMID:27580177.
- Liang WC, Fu WM, Wong CW, Wang Y, Wang WM, Hu GX, Zhang L, Xiao LJ, Wan DC, Zhang JF, et al. The lncRNA H19 promotes epithelial to mesenchymal transition by functioning as miRNA sponges in colorectal cancer. Oncotarget. 2015;6(26):22513–25. doi:10.18632/oncotarget.4154. PMID:26068968.
- Song X, Cao G, Jing L, Lin S, Wang X, Zhang J, Wang M, Liu W, Lv C. Analysing the relationship between lncRNA and protein-coding gene and the role of lncRNA as ceRNA in pulmonary fibrosis. J Cell Mol Med. 2014;18(6):991–1003. doi:10.1111/jcmm.12243. PMID:24702795.
- Jalali S, Bhartiya D, Lalwani MK, Sivasubbu S, Scaria V. Systematic transcriptome wide analysis of lncRNA-miRNA interactions. PLoS One. 2013;8(2):e53823. doi:10.1371/journal.pone.0053823. PMID:23405074.
- Moustakas A, Heldin CH. Signaling networks guiding epithelial-mesenchymal transitions during embryogenesis and cancer progression. Cancer Sci. 2007;98(10):1512–20. doi:10.1111/j.1349-7006.2007.00550.x. PMID:17645776.
- Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420–8. doi:10.1172/JCI39104. PMID:19487818.
- Wu Y, Zhou BP. Snail: More than EMT. Cell Adh Migr. 2010;4(2):199–203. PMID:20168078.
- Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–90. doi:10.1016/j.cell.2009.11.007. PMID:19945376.
- Meidhof S, Brabletz S, Lehmann W, Preca BT, Mock K, Ruh M, Schuler J, Berthold M, Weber A, Burk U, et al. ZEB1-associated drug resistance in cancer cells is reversed by the class I HDAC inhibitor mocetinostat. EMBO Mol Med. 2015;7(6):831–47. doi:10.15252/emmm.201404396. PMID:25872941.
- Krebs AM, Mitschke J, Lasierra Losada M, Schmalhofer O, Boerries M, Busch H, Boettcher M, Mougiakakos D, Reichardt W, Bronsert P, et al. The EMT-activator Zeb1 is a key factor for cell plasticity and promotes metastasis in pancreatic cancer. Nat Cell Biol. 2017;19(5):518–29. doi:10.1038/ncb3513. PMID:28414315.
- Mondal G, Almawash S, Chaudhary AK, Mahato RI. EGFR-targeted cationic polymeric mixed micelles for codelivery of Gemcitabine and miR-205 for treating advanced pancreatic cancer. Mol Pharm. 2017;14(9):3121–33. doi:10.1021/acs.molpharmaceut.7b00355. PMID:28719220.
- Paik WH, Song BJ, Kim HW, Kim HR, Hwang JH. MicroRNA-200c as a prognostic biomarker for pancreatic cancer. Korean J Gastroenterol. 2015;66(4):215–20. doi:10.4166/kjg.2015.66.4.215. PMID:26493507.
- He B, Bai Y, Kang W, Zhang X, Jiang X. LncRNA SNHG5 regulates imatinib resistance in chronic myeloid leukemia via acting as a CeRNA against MiR-205-5p. Am J Cancer Res. 2017;7(8):1704–13. PMID:28861326.
- Yang W, Hong L, Xu X, Wang Q, Huang J, Jiang L. LncRNA GAS5 suppresses the tumorigenesis of cervical cancer by downregulating miR-196a and miR-205. Tumour Biol. 2017;39(7):1010428317711315. doi:10.1177/1010428317711315. PMID:28671039.
- Hirata H, Hinoda Y, Shahryari V, Deng G, Nakajima K, Tabatabai ZL, Ishii N, Dahiya R. Long noncoding RNA MALAT1 promotes aggressive renal cell carcinoma through Ezh2 and interacts with miR-205. Cancer Res. 2015;75(7):1322–31. doi:10.1158/0008-5472.CAN-14-2931. PMID:25600645.
- Guo Q, Qin W. DKK3 blocked translocation of beta-catenin/EMT induced by hypoxia and improved gemcitabine therapeutic effect in pancreatic cancer Bxpc-3 cell. J Cell Mol Med. 2015;19(12):2832–41. doi:10.1111/jcmm.12675. PMID:26395974.
- Cao LY, Yang J, Fu XG, Lin YH, Lin F, Huang BY. [The MicroRNA miR-205 inhibits epithelial-messenchymal transition in HK-2 cells by down-regulating ZEB1 and ZEB2 expressions]. Nan Fang Yi Ke Da Xue Xue Bao. 2016;36(12):1700–05. PMID:27998868.
- Lee JY, Park MK, Park JH, Lee HJ, Shin DH, Kang Y, Lee CH, Kong G. Loss of the polycomb protein Mel-18 enhances the epithelial-mesenchymal transition by ZEB1 and ZEB2 expression through the downregulation of miR-205 in breast cancer. Oncogene. 2014;33(10):1325–35. doi:10.1038/onc.2013.53. PMID:23474752.
- Zhao L, Sun H, Kong H, Chen Z, Chen B, Zhou M. The Lncrna-TUG1/EZH2 axis promotes pancreatic cancer cell proliferation, migration and EMT phenotype formation through sponging Mir-382. Cell Physiol Biochem. 2017;42(6):2145–58. doi:10.1159/000479990. PMID:28813705.
- Benlhabib H, Guo W, Pierce BM, Mendelson CR. The miR-200 family and its targets regulate type II cell differentiation in human fetal lung. J Biol Chem. 2015;290(37):22409–22. doi:10.1074/jbc.M114.636068. PMID:26203191.
