ABSTRACT
Human epidermal growth factor receptor 2 (HER2) positive is a unique molecular subtype of breast cancer (BC) characterized by high malignancy and poor prognosis. Bilateral primary breast cancer (BPBC) harboring HER2 gene amplification is available to be detected among the BC survivors due to the increasing use of anti-HER2 adjuvant therapy. However, owing to the paucity of cases reported, knowledge of treating HER2-positive BPBC patients including the clinical behavior, histopathologic characteristics, anti-HER2 therapeutic response and disease outcome are not fully understood. Here we report a case of its kind receiving nonstandardized treatment during adjuvant stage. Upon tumor recurrence with liver metastasis, she received trastuzumab combined with chemotherapy and reached a PFS of 14.5 months in first-line treatment. While maintained trastuzumab plus carboplatin as second-line treatment progressed promptly, re-treatment of trastuzumab after lapatinib administration in third line can still benefit the patient. The present case report delineates an anti-HER2 path for a particular characterized patient, and also provides new evidence of trastuzumab re-usage after disease progression of prior anti-HER2 therapy.
Introduction
Breast cancer (BC) is one of the most frequently diagnosed cancers worldwide and the major cause of cancer-related death in women.Citation1,Citation2 According to the estimation of American Cancer Society, the BC incidence and mortality of the Asian/Pacific Islanders (APIs) are 88.3/100,000 and 11.4/100,000, respectively.Citation2 Human epidermal growth factor receptor 2 (HER2) gene amplification positive is a unique molecular subtype occurring in 18% of APIs patients.Citation2 Anti-HER2 therapy significantly prolonged the survival and successfully changed the natural course of BC patients with HER2 positive.Citation3,Citation4 However in China, a certain number of patients with this kind of disease did not receive standard anti-HER2 adjuvant treatment because of economic burden and hindrance in propagation. Hence, these patients might experience an increased risk of developing a contralateral BC as compared to the general female population. Here we report a rare case of HER2-positive bilateral primary breast cancer (BPBC). The patient underwent nonstandardized adjuvant treatment, experienced a contralateral BC, developed liver metastasis in a short time from the secondary primary, and received multi-line anti-HER2 therapies for metastatic disease. The clinicopathological features and prognostic characteristics of the patient were provided, and the efficacy and value of re-treatment of trastuzumab after multi-line targeted therapy resistance were also analyzed.
Case report
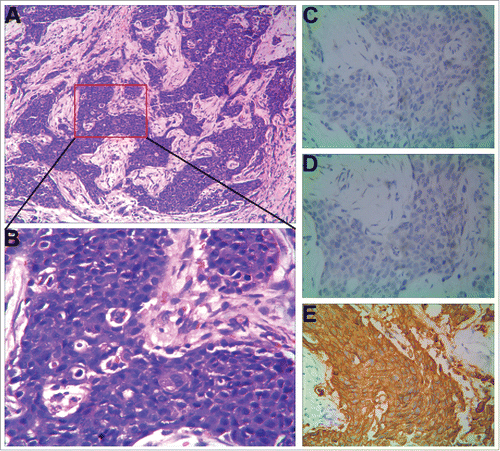
A 51-year-old premenopausal woman was admitted to the local hospital to evaluate for a progressively painful, enlarging left breast mass for approximately 3 months and was found to have BC in March 2014. She received modified radical mastectomy of the left breast with axillary dissection. Histopathological analysis revealed infiltrating ductal carcinoma (IDC), Nottingham grade II out of III ( and ), tumor diameter of 4.5 cm. Axillary dissection revealed 12 lymph nodes, 2 involved with carcinoma. Tumor was found to be estrogen receptor (ER) negative, progesterone receptor (PR1) negative, and HER2 2+∼3+ by immunohistochemistry (). Without fluorescence in situ hybridization (FISH) analysis to confirm the status of HER2 gene, she received adjuvant chemotherapy with epirubicin and docetaxel for 6 cycles (every 3 weeks for 1 cycle), and completed chest wall and regional nodal irradiation in September 2014.
Figure 1. Histopathologic characteristics of the first primary BC of the patient. (A) Hematoxylin and eosin staining (× 100). (B) Hematoxylin and eosin staining (× 400). (C-E) Immunohistochemical staining (× 400). ER (−), PR1 (−), HER2 (2+∼3+).
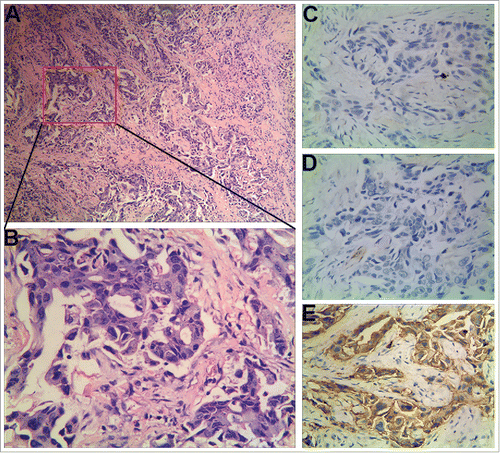
In January 2015, the patient presented to the local hospital for first review and was found to have new BC in external lower quadrant of the right breast. She received modified radical mastectomy of the right breast with pathologic report showing IDC, Nottingham grade II out of III ( and ), tumor diameter of 1.5 cm, and axillary nodes 0/17. Immunohistochemistry showed ER(−), PR1(−), HER2 (2+) for the second BC (). She began chemotherapy consisting of vinorelbine and cisplatin for 4 cycles and completed treatment on April 2015.
Figure 2. Histopathologic characteristics of the second primary BC of the patient. (A) Hematoxylin and eosin staining (× 100). (B) Hematoxylin and eosin staining (× 400). (C-E) Immunohistochemical staining (× 400). ER (−), PR1 (−), HER2 (2+).
The patient was referred to our institution in August 2015 due to “epigastric discomfort for 2 weeks”. Abdominal computed tomography (CT) scan revealed multiple hepatic nodules ( and ). As experiencing the second surgical operation for not a long time, she refused to undergo liver biopsy. The HER2 status in tumor of second BC was proved gene amplification by FISH analysis (). Additional imaging examinations in other regions were negative for signs of metastatic disease. The patient had an Eastern Cooperative Oncology Group (ECOG) score of 0, and showed transaminase elevation including ALT 111 U/L (normal range 9–60) and AST 153 U/L (normal range 15–45) by liver function test. Blood, renal function, urine tests and EKG were normal. Serum CEA was 2.96 ng/ml (normal <5 ng/ml), and CA153 425.20 IU/ml (normal <28 IU/ml).
Figure 3. Imaging and molecular analysis of the patient at the first diagnosis of liver metastasis. (A and B) Abdominal CT scan showed multiple lesions in the liver (indicated by red arrow). (C) FISH assay revealed HER2 gene amplification of the second BC. (D) After treatment with trastuzumab-based triple-drug combination regimen for 3 cycles.
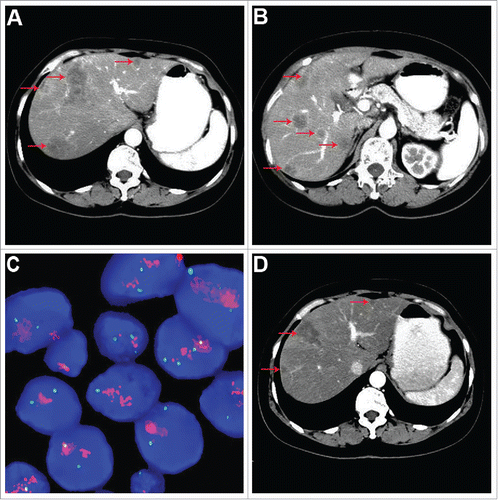
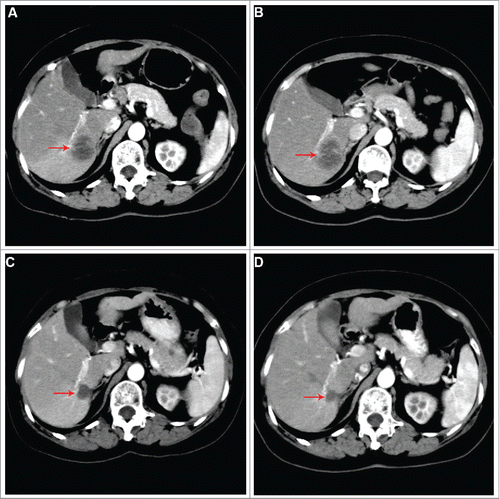
According to the molecular findings, prior adjuvant therapies, the patient's good health condition and strong willingness to treatment, trastuzumab combined with gemcitabine and capecitabine was started as per our protocol. This treatment ameliorated her clinical symptoms significantly and promptly. Transaminase and CA153 levels were returned to normal at 1 month after treatment. Results of scans demonstrated liver target lesions partial response (PR2) after 3 cycles (), and sustained PR2 after 6 cycles. However, she suffered from repeated myelosuppression (CTCAE grade 1–3) and intolerable hand-foot syndrome (CTCAE grade 3) during the treatment of this triple-drug combination regimen. We switched to maintenance treatment with trastuzumab and docetaxel for 9 cycles, at which time the follow-up CT in November 2016 revealed liver tumor progression (), with the progression free survival (PFS) time up to 14.5 months in the first-line treatment. Subsequently, trastuzumab combined with carboplatin was administered as second-line treatment, whereas the liver target lesion progressed rapidly after 2 cycles’ treatment (). For third-line treatment, lapatinib combined with weekly paclitaxel was administrated, leading to liver tumor shrinkage after 2 cycles, and satisfactory tumor response after 5 cycles (). However, intolerable lapatinib-related rash in trunk (CTCAE grade 3) occurred at that time, and the therapy was accordingly ceased. In May 2017, we re-treated trastuzumab plus three-weekly paclitaxel as maintenance strategy for metastatic disease, and PR2 of liver target lesions was maintained (). Currently, she is still on trastuzumab-based treatment with good tolerance.
Figure 4. Abdominal CT scans showing dynamic changes of liver metastatic lesions during multi-line treatments. (A) Progression to regimen of trastuzumab combined with docetaxel as first-line maintenance treatment. (B) Progression to regimen of trastuzumab combined with carboplatin as second-line treatment. (C) After third-line treatment with lapatinib plus paclitaxel regimen. (D) On third-line treatment with trastuzumab plus paclitaxel.
Discussion
In this article, we reported a rare case with metastatic bilateral primary breast cancer (BPBC) whose molecular phenotype is ER/PR1 (−), HER2 (+), and also depicted growing problems in the real world of China in the management of patients with HER2-positive BC: how to deal with the disease of patient who received nonstandardized prior therapy, and also after failure of trastuzumab when taking drug availability into account? Several difficulties and doubtful points in the clinical course of this case remained to be discussed and elucidated.
(1) Metachronous BPBC or metastatic contralateral BC?
BPBC refers to independent primary cancer of each breast developing at the same time or successively, occurring in 4.4% to 8.0% of all the patients with BC.Citation5,Citation6 Generally, bilateral breast cancer (BBC) was categorized as synchronous bilateral breast cancer (sBBC) and metachronous bilateral breast cancer (mBBC). Domestic experts usually distinguish these two kinds of disease with 6 months of interval,Citation7 whereas foreign scholars take 3 months as the boundary.Citation6 According to the guideline of WHO 2012, BBC is defined as sBBC when contralateral BC is diagnosed within 3 months. Currently, the diagnostic criteria for BPBC are primarily based on 5 lines of followed provisions proposed by RobbinsCitation8 in 1964 and supplemented by KanCitation9 in 1993: (a) Location: Primary cancer frequently locates in the parenchyma of the external lateral quadrant of the contralateral breast, while metastasis usually the adipose tissue around the breast or near the lineae mediana; (b) Histological type: Tumor histological types or nuclear differentiation degrees between the first and second primary BC are usually different, however, same phenotype cannot be excluded in certain cases; (c) In situ lesions: The presence of carcinoma in situ or evolution from in situ carcinoma to invasive cancer, which can be found in tissues around the neoplasia, is considered the most reliable evidence of primary cancer; (d) Growth pattern: Primary cancer usually has solitary and invasive growth, while metastatic carcinoma grows with the characteristics of multiple and expansive; (e) Time of the first primary: No evidence of local recurrence or distant metastasis of the first BC over 5 years supports the secondary carcinoma as primary one. For the patient in this case, considering the newly diagnosed cancer located in the parenchyma and external quadrant of the right breast; the same as that of the first cancer in histologic type; in solitary and invasive growth; developed more than 9 months after the first primary, thus although the evidence of in situ lesion cannot be obtained, the multidisciplinary team (MDT) in our institution is more inclined to diagnose of the secondary lesion of the right breast as a primary and metachronous BC.
(2) Does metastatic BC with nonstandardized adjuvant treatment still stand a chance?
The development of trastuzumab is a major breakthrough of anti-HER2 therapy in BC. Several randomized controlled trials have affirmed that trastuzumab-based therapies can significantly prolong survival both in adjuvant treatment in early stage or systemic treatment in advanced stage.Citation3,Citation4 Moreover, HER2 remains an effective therapeutic target even after failure of trastuzumab in HER2-positive metastatic BC, thus NCCN guidelineCitation10 and several researchesCitation11-13 suggest cross-line treatment of anti-HER2 drugs to benefit BC patients.
HER2-positive BCs may result in an unfavorable prognosis without standard anti-HER2 treatment in early stage, and this notion was confirmed by the performance of the patient to develop the secondary cancer less than 1 year from the first primary. In the previous studies, first-line treatment of trastuzumab-based triple-drug combination regimen provided 11–18 months PFS benefit for HER2-overexpressing BC patients,Citation4,Citation14 while trastuzumab plus gemcitabine and capecitabine as alternative first-line option in our case rendered a parallel PFS of 14.5 months. The efficacy is particular satisfactory because the patient once missed the best therapeutic strategy and anti-HER2 treatment timing. Based on this case report, we consider that without standardized treatment in the early stage, anti-HER2 therapy in advanced stage still has the potential to change the disease outcome of BC.
(3) How to deal with multi-line targeted therapy resistance in HER2-positive mBC in developing countries without various anti-HER2 drugs?
In China, lapatinib has been approved by Food and Drug Administration (CFDA) for HER2-positive advanced BC previously treated with anthecycline, taxanes and trastuzumab in 2013.Citation15 Nevertheless, with unavailability of TDM-1 and pertuzumab, effective approaches to treat patients progressing to application of trastuzumab and lapatinib are always hot spots. Lapatinib, by inducing stabilization and accumulation of inactive HER2 receptor at the cell surface, could enhanced immune-mediated trastuzumab-dependent cytotoxicity.Citation16 Gori et al.Citation17 has evaluated the efficacy and safety of re-treatment of trastuzumab after multi-line progression and concluded that the continuation of anti-HER2 treatment is associated with improved clinical outcome in HER2-overexpressing mBC patients progressing to lapatinib-based therapy. Bian et al.Citation18 demonstrated that interval from discontinuation of lapatinib to trastuzumab administration is an independent prognostic factor, which stresses the importance of patients with HER2-positive mBC to receive continuous anti-HER2 targeted therapy. In this case, given the long period of trastuzumab in the first-line treatment, we hypothesized the rapid failure of second-line treatment might be reasonably attributed to the down-regulation or degradation of HER2. While interfered with lapatinib by third-line treatment, the patient responded again to trastuzumab by exhibiting an excellent tumor response and no serious treatment-associated side effects.
Conclusion
BPBC is a rare and special subgroup of BC with low incidence. However, it has a big amount of patients due to the large population in China. To distinguish BPBC and metastatic BC is important while difficult, and further studies and additional case reports are warranted to focus on this field. In China and most developing countries, due to unavailability of a variety of novel target drugs as well as economic reasons, a certain number of patients with HER2-positive BC fail to receive standard anti-HER2 therapy in adjuvant treatment. However, anti-HER2 approach in metastatic stage of BC is potent to produce a satisfactory therapeutic effect and even reverse the disease outcome. More importantly, patients can still benefit from re-treatment of trastuzumab after prior trastuzumab or lapatinib-based therapies.
Abbreviations
| APIs | = | Asian/Pacific Islanders |
| BBC | = | bilateral breast cancer |
| BC | = | breast cancer |
| BPBC | = | bilateral primary breast cancer |
| CFDA | = | China Food and Drug Administration |
| CT | = | computed tomography |
| ER | = | estrogen receptor |
| FISH | = | fluorescence in situ hybridization |
| HER2 | = | human epidermal growth factor receptor 2 |
| IDC | = | infiltrating ductal carcinoma |
| mBBC | = | metachronous bilateral breast cancer |
| mBC | = | metastatic breast cancer |
| MDT | = | multidisciplinary team |
| PFS | = | progression free survival |
| PR1 | = | progesterone receptor |
| PR2 | = | partial response |
| sBBC | = | synchronous bilateral breast cancer. |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Ethical statements
The report of this study was approved by the ethics committee of our institution, and written informed consent was obtained from the patient.
Acknowledgments
The authors sincerely thank the patient for her contribution to the publication of this case report.
Additional information
Funding
References
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics 2012. CA Cancer J Clin. 2015;65:87–108. doi:10.3322/caac.21262.
- Desantis CE, Fedewa SA, Goding SA, Kramer JL, Smith RA, Jemal A. Breast cancer statistics, 2015: Convergence of incidence rates between black and white women. Ca A Cancer Journal for Clinicians. 2016;66:31. doi:10.3322/caac.21320.
- Cameron D, Piccart-Gebhart MJ, Gelber RD, Procter M, Goldhirsch A, de Azambuja E, Castro G Jr, Untch M, Smith I, Gianni L, et al. 11 years' follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet. 2017;389:1195–205. doi:10.1016/S0140-6736(16)32616-2.
- Valero V, Forbes J, Pegram MD, Pienkowski T, Eiermann W, von Minckwitz G, Roche H, Martin M, Crown J, Mackey JR, et al. Multicenter phase III randomized trial comparing docetaxel and trastuzumab with docetaxel, carboplatin, and trastuzumab as first-line chemotherapy for patients with HER2-gene-amplified metastatic breast cancer (BCIRG 007 study): two highly active therapeutic regimens. J Clin Oncol. 2011;29:149–56. doi:10.1200/JCO.2010.28.6450.
- Kheirelseid EA, Jumustafa H, Miller N, Curran C, Sweeney K, Malone C, McLaughlin R, Newell J, Kerin MJ. Bilateral breast cancer: Analysis of incidence, outcome, survival and disease characteristics. Breast Cancer Res Treat. 2011;126:131–40. doi:10.1007/s10549-010-1057-y.
- Jobsen JJ, van der Palen J, Ong F, Riemersma S, Struikmans H. Bilateral breast cancer, synchronous and metachronous; differences and outcome. Breast Cancer Res Treat. 2015;153:277–83. doi:10.1007/s10549-015-3538-5.
- Zhang T, Zhang BN. Bilateral primary breast cancer: a report of 217 cases. Zhonghua Zhong Liu Za Zhi. 2004;26:756–8. doi:10.3760/j.issn:0253-3766.2004.12.015.
- Robbins GF, Berg JW. Bilateral Primary Breast Cancer; a Prospective Clinicopathological Study. Cancer. 1964;17:1501–27. doi:10.1002/1097-0142(196412)17:12%3c1501::AID-CNCR2820171202%3e3.0.CO;2-P.
- Kan X. Clinicopathology of breast cancer [M]. Beijing: Peking Union Medical College Press, 1993:98–102.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Breast Cancer, Version 2. 2017. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
- von Minckwitz G, du Bois A, Schmidt M, Maass N, Cufer T, de Jongh FE, Maartense E, Zielinski C, Kaufmann M, Bauer W, et al. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: A german breast group 26/breast international group 03–05 study. J Clin Oncol. 2009;27:1999–2006. doi:10.1200/JCO.2008.19.6618.
- Blackwell KL, Burstein HJ, Storniolo AM, Rugo H, Sledge G, Koehler M, Ellis C, Casey M, Vukelja S, Bischoff J, et al. Randomized study of Lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol. 2010;28:1124–30. doi:10.1200/JCO.2008.21.4437.
- Extra JM, Antoine EC, Vincent-Salomon A, Delozier T, Kerbrat P, Bethune-Volters A, Guastalla JP, Spielmann M, Mauriac L, Misset JL, et al. Efficacy of trastuzumab in routine clinical practice and after progression for metastatic breast cancer patients: the observational Hermine study. Oncologist. 2010;15:799–809. doi:10.1634/theoncologist.2009-0029.
- Wardley AM, Pivot X, Morales-Vasquez F, Zetina LM, de Fatima Dias Gaui M, Reyes DO, Jassem J, Barton C, Button P, Hersberger V, et al. Randomized phase II trial of first-line trastuzumab plus docetaxel and capecitabine compared with trastuzumab plus docetaxel in HER2-positive metastatic breast cancer. J Clin Oncol. 2010;28:976–83. doi:10.1200/JCO.2008.21.6531.
- Xu BH, Jiang ZF, Chua D, Shao ZM, Luo RC, Wang XJ, Liu DG, Yeo W, Yu SY, Newstat B, et al. Lapatinib plus capecitabine in treating HER2-positive advanced breast cancer: efficacy, safety, and biomarker results from Chinese patients. Chin J Cancer. 2011;30:327–35. doi:10.5732/cjc.010.10507.
- Scaltriti M, Verma C, Guzman M, Jimenez J, Parra JL, Pedersen K, Smith DJ, Landolfi S, Ramon y Cajal S, Arribas J, et al. Lapatinib, a HER2 tyrosine kinase inhibitor, induces stabilization and accumulation of HER2 and potentiates trastuzumab-dependent cell cytotoxicity. Oncogene. 2009;28:803–14. doi:10.1038/onc.2008.432.
- Gori S, Montemurro F, Spazzapan S, Metro G, Foglietta J, Bisagni G, Ferzi A, Silva RR, Gamucci T, Clavarezza M, et al. Retreatment with trastuzumab-based therapy after disease progression following lapatinib in HER2-positive metastatic breast cancer. Ann Oncol. 2012;23:1436–41. doi:10.1093/annonc/mdr474.
- Bian L, Jiang ZF, Wang T, Song JJ, Guo YF, Zhang HQ, Zhang SH, Wu SK, Song ST. Efficacy and prognostic analysis of retreatment with trastuzumab-based therapy after multi-line targeted therapy resistance in HER2-positive metastatic breast cancer. Zhonghua Yi Xue Za Zhi. 2013;93:48–52. doi:10.3760/cma.j.issn.0376-2491.2013.01.015.
