ABSTRACT
Gastric carcinoma (GC) is a common gastrointestinal malignancy with high incidence and mortality worldwide, and most patients are diagnosed in the late stages of disease. Palliative chemotherapy provides a survival benefit for patients with inoperable advanced GC. However, elderly patients who are unable to tolerate chemotherapy had worse prognosis due to lack of effective treatment. Herein we reported a Chinese elderly GC patient using next generation sequencing (NGS)-based tumor DNA analysis. Valuable gene variants of vascular endothelial growth factor (VEGF) A gene amplification were detected. Additionally, a novel NOTCH1-BPHL fusion has been identified. He received antiangiogenic drug apatinib and showed both good clinical and radiographic response, but eventually died of non-cancer related cause, with progression free survival time (PFS) and overall survival time (OS) up to 9.53 months. This was the first GC case with apatinib usage as first-line treatment under the guidance of NGS gene profiling.
Introduction
Gastric carcinoma (GC) is one of the most commonly diagnosed malignancies throughout the world. China has high-incidence of GC, and both the incidence and mortality rates of GC ranked second of all malignant tumors in China in 2015.Citation1 For patients with advanced GC, a comprehensive treatment strategy based on chemotherapy is adopted in clinical practice. The chemotherapeutic regimen generally involves a platinum-based double or triple-drug combination regimens,Citation2-3 however, the overall efficacy was not satisfactory. The most favourable results had been seen in the randomized controlled, phase 3 ToGA trial showing a median survival of 13.8 months in Her 2-overexpression or amplification advanced GC patients treated with chemotherapy plus trastuzumab.Citation4 Drugs targeting angiogenesis are promising for the treatment of various types of cancer including GC. Among them, apatinib mesylate, which is an orally administered small-molecule vascular endothelial growth factor receptor-2 (VEGFR-2) tyrosine kinase inhibitor (TKI), has been approved by China Food and Drug Administration (CFDA) for the treatment of patients with advanced gastric or gastroesophageal junction (GOJ) adenocarcinoma who failed second-line chemotherapy. Elderly GC patients are a special subgroup whose organ functions are usually poor, and some might present with a couple of underlying diseases. Hence, these patients should not or cannot undergo high-intensity chemotherapy. Therefore, this presented a lack of effective treatment for the elderly patients. In order to fulfill this gap, we herein reported a case of an elderly GC patient who was treated with apatinib as a first-line treatment driven by next generation sequencing (NGS)-based comprehensive molecular characterization.
Case report
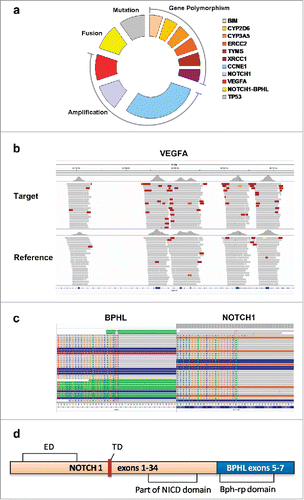
A 76-year-old Chinese male presented to our institution in September 2016 due to “epigastric discomfort for more than a year, and a left neck mass for half a year”. He was a farmer and had a history of smoking and drinking, as well as history of chronic bronchitis and carried hepatitis B virus. The patient had poor appetite and weight loss of 10 kg since the onset of disease. On physical examination, he showed anemic appearance, multiple, nontender, and immobile mass on the left neck (2 × 4 cm), epigastric tenderness (+), and no positive signs were found for other systems. The patient refused to undergo gastroscopy, but received Positron emission tomography/computed tomography (PET-CT) examination. Imaging results showed that the fundus and cardia of stomach were obviously thickened with irregular soft tissue masses, with fluorodeoxyglucose (18F-FDG) uptake up to SUVmax 16.7. Other findings include multiple metastatic nodules in the left neck, mediastinum, bilateral pulmonary hilum, peripancreatic space and sides of bronchi, lesser curvature and ventral aorta (SUVmax = 11.6) ( and ). Biopsy of the left neck mass revealed poorly differentiated adenocarcinoma with massive necrosis ( and ). The patient had an Eastern Cooperative Oncology Group (ECOG) score of 2, and showed ischemic ECG changes. Blood test showed severe anemia (hemoglobin 60.0 g/L). Liver and renal function tests, and urine test were normal. Serum CEA was 260.7 ng/ml (normal <5 ng/ml). Hepatitis B test showed HBsAg (+), HBeAb (+), HBcAb (+), and HBV DNA<500.0 copies. He was clinically diagnosed with metastatic gastric adenocarcinoma, anemia (severe), and chronic hepatitis B (inactive phase). He was given the best supportive treatment of blood transfusion and nutrition supplement. The patient was unable to tolerate chemotherapy due to his advanced age and poor health condition. After fully communicating with the patient and his family members, NGS-based tumor DNA profiling was performed to look for potentially targeted therapeutic opportunities. Additionally, plasma for circulating tumor DNA (ctDNA) analysis was collected for comparison. Targeted NGS (Cancer driver genes panel, Geneseeq Biotechnology Inc., Nanjing, China) of the tumor () revealed no aberrations of HER-2, EGFR, MET, RAS, BRAF, PIK3CA or CDH1 genes; microsatellite instability (MSI) (−); but VEGFA gene amplification (2.1 times, ), NOTCH1 gene amplification (2.6 times), CCNE1 gene amplification (8.7 times); NOTCH1-BPHL fusion at 2% mutant allele frequency (MAF) (), and C135F mutation of TP53 gene. However, NGS of the ctDNA detected only TP53 gene with C135F mutation. When screening the patient's paraffin-embedded biopsy specimens to validate the NGS results, we found no enough sample for further fluorescent in situ hybridization (FISH) analysis for VEGFA gene.
Figure 1. Imaging and histopathologic characteristics of the patient at the first diagnosis. (a and b) Positron emission tomography/computed tomography (PET-CT) scans showing heterogeneous fluorodeoxyglucose (FDG) uptake in multiple lymphadenopathies and gastric lesions. (c and d) Hematoxylin and eosin staining of biopsy tissues of the left neck lymph nodes confirmed metastatic adenocarcinoma (400 × magnification and 100 × magnification, respectively).
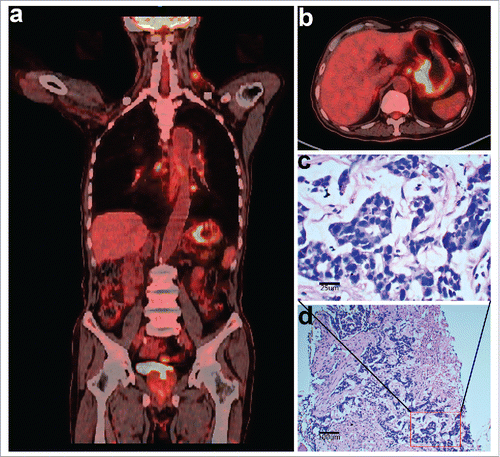
Figure 2. Molecular gene variations of the patient using targeted NGS-based tumor DNA profiling. Sequencing library was prepared by Illumina TruSeq DNA PCR-Free Sample Preparation Kit and target capture was sequenced on Illumina MiSeq NGS platform according to its instruction. Copy number variations (CNVs) were identified using tested sample and normal human hapmap DNA NA18535 average read depths at each captured region (exonic region). (a) Different forms of variation for the indicated reporters were shown. (b) VEGFA gene amplification as demonstrated by CNVs detection. (c) Paired-end sequencing data indicated somatic interchromosomal NOTCH1-BPHL fusion as demonstrated by Integrative Genomics Viewer program. (d) Schematic diagram of predicted domains of NOTCH1-BPHL fusion protein. ED: Extracellular domain; NICD: NOTCH1 intracellular domain; TD: Transmembrane domain.
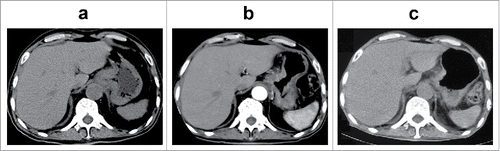
Based on the molecular findings, drugs available in China, patient's condition and treatment willingness, apatinib (500 mg daily) for antiangiogenic treatment was recommended. This treatment ameliorated his clinical symptoms significantly and promptly. One week after treatment, mass on the left neck was decreased to 1 × 3 cm. The mass almost disappeared and CEA was returned to normal at 1 month after treatment. Results of scans demonstrated gastric tumor shrinkage after 2 months (), and satisfactory response after 7 months (). Tumor was excellently controlled until the patient died due to accidental fall injury on 13th July, 2017, with a progression free survival (PFS) and overall survival (OS) time of 9.53 months. The treatment was well tolerated, and no dose adjustments were performed.
Discussion
For the past 10 years, first-line/second-line chemotherapy ± targeted therapy has been the standard treatment for metastatic GC.Citation4,Citation5 However, patients enrolled in the clinical studies are usually younger and generally in good condition, and hence it is not clear as to whether the elderly patients who are generally in poor health condition or with complications can benefit from such therapy. Apatinib mesylate, an orally bioavailable small-molecule VEGFR-2 TKI, demonstrated a survival benefit for patients with metastatic GC who received two or more lines of prior chemotherapy.Citation6 The most common adverse events associated with apatinib treatment include hand-foot syndrome, proteinuria, and hypertension, which were similar to the other antiangiogenic agents. Due to its simplicity, compliance, and less associated side effects, apatinib has been used in more and more clinical practices for advanced metastatic GC treatment and also other solid tumors.Citation7
The patient in this case was at advanced age and in poor health condition, so it is hard for him to tolerate chemotherapy. Given no standard treatment in this setting, we recommended targeted NGS of both tumor and ctDNA to uncover the potential therapeutic targets. Molecular results between these two samples demonstrated histologic examination is more sensitivity in revealing tumor gene variants. We speculated that this might be related to more tumor enrichment in tissues and relatively low sensitivity for plasma NGS testing. Therefore, tumor tissues are still considered as the most ideal specimens for NGS genetic analysis. NGS of the tumor revealed various genetic variations, and among these, CCNE1 gene amplification caused chromosomal instability and led to the occurrence of tumor, as demonstrated in a variety of tumors including GC.Citation8 C135F mutation of TP53 gene that is located in the DNA-binding domain caused the inactivation of TP539 and is also considered as a common somatic mutation in several solid tumors. NOTCH pathway activation, which might be caused by NOTCH1 gene amplification, can promote cell proliferation, inhibit apoptosis and participate in tumor development.Citation10 Meantime, we detected that exon 34 of NOTCH1 and intron 4 of BPHL were fractured and fused, and the fusion product might be a novel recombinant protein by encoded amino acids of exon 1–34 of NOTCH1 and exon 5–7 of BPHL (). Compared with normal NOTCH1 protein, the NOTCH1 in the fusion protein missed 2075–2555 amino acids. Since the NOTCH1-BPHL fusion has not been reported in tumors, the significance of this novel rearrangement is unclear, and further studies are warranted.
Another important molecular event identified is VEGFA gene amplification. As a member of VEGF family, VEGFA is a key regulator of angiogenesis and associated with increased tumor aggressiveness and reduced survival in patients with GC.Citation11 Studies have shown discrete results regarding VEGFA as a predictive biomarker for antiangiogenic agent, bevacizumab in lung cancer.Citation12 In metastatic breast cancer patients, retrospective studies have showed that VEGFA has the potential to predict clinical benefit of bevacizumab,Citation13 but MERiDiAN prospective study with baseline plasma VEGFA as stratification factor ended in failure.Citation14 However, in the studies of GC, AVAGAST clinical trial showed that plasma VEGFA was a strong candidate biomarker in the identification of patients benefitting most from bevacizumab.Citation15 The correlation between VEGFA and apatinib efficacy remains unclear. However, activation of VEGF pathway and the research experience of bevacizumab demonstrated that VEGFA gene amplification in tumor samples provided some evidence for apatinib treatment in this case. Treatment outcome of the drug produced an excellent tumor response, which finally confirmed our assumption. In the previous studies, first-line chemotherapy provided 5–7 months PFS benefit for patients with advanced GC,Citation2-4 while apatinib treatment in this case provided a better PFS of 9.53 months. More importantly, the patient avoided painful chemotherapy, required no hospitalization, maintained the quality of life without virus activation and other serious treatment-associated side effects. The patient would live longer if no accident occurred. Thus, VEGFA has the potential to predict the efficacy of apatinib, but the value of CCNE1 or NOTCH1 gene amplification, NOTCH1-BPHL fusion or even apatinib's off-target effects (eg. targeting PDGFR, c-Kit and c-Src) in this case cannot be excluded.
To sum up, this case reported an advanced GC in elderly male patient treated with apatinib as first-line therapy. This attributed to valuable molecular events as discovered by NGS-based tumor DNA profiling, and substantial benefits were ultimately evidenced from patient's response. This proof-of-concept study is worthy to be promoted in clinical practice. The efficacy and safety of apatinib as first-line therapy, especially in elderly patients with advanced GC, should be confirmed in a larger group. Hence, we have launched a phase II clinical trial (NCT03219593), with the aim to provide a better evaluation and explore potential biomarkers.
Ethical statements
The report of this study was approved by the ethics committee of our institution, and written informed consent was obtained from the family member of the patient.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thank Fu-feng Wang of Geneseeq Technology Inc. for technical assistance.
Additional information
Funding
References
- Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–32. doi.org/10.3322/caac.21338. PMID:26808342.
- Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, Rodrigues A, Fodor M, Chao Y, Voznyi E, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24(31):4991–7. doi.org/10.1200/JCO.2006.06.8429. PMID:17075117.
- Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, Middleton G, Daniel F, Oates J, Norman AR. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358(1):36–46. doi.org/10.1056/NEJMoa073149. PMID:18172173.
- Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–97. doi.org/10.1016/S0140-6736(10)61121-X. PMID:20728210.
- Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15(11):1224–35. doi.org/10.1016/S1470-2045(14)70420-6. PMID:25240821.
- Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y, Liu W, Tong J, Liu Y, Xu R, et al. Randomized, Double-Blind, Placebo-Controlled Phase III trial of Apatinib in patients with Chemotherapy-Refractory advanced or metastatic Adenocarcinoma of the stomach or Gastroesophageal junction. J Clin Oncol. 2016;34(13):1448–54. doi.org/10.1200/JCO.2015.63.5995. PMID:26884585.
- Scott AJ, Messersmith WA, Jimeno A. Apatinib: a promising oral antiangiogenic agent in the treatment of multiple solid tumors. Drugs Today (Barc). 2015;51(4):223–9. doi.org/10.1358/dot.2015.51.4.2320599. PMID:26020064.
- Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513(7517):202–9. doi.org/10.1038/nature13480. PMID:25079317.
- Gonin-Laurent N, Gibaud A, Huygue M, Lefevre SH, Le Bras M, Chauveinc L, Sastre-Garau X, Doz F, Lumbroso L, Chevillard S, et al. Specific TP53 mutation pattern in radiation-induced sarcomas. Carcinogenesis. 2006;27(6):1266–72. doi.org/10.1093/carcin/bgi356. PMID:16492679.
- Kim SJ, Lee HW, Baek JH, Cho YH, Kang HG, Jeong JS, Song J, Park HS, Chun KH. Activation of nuclear PTEN by inhibition of Notch signaling induces G2/M cell cycle arrest in gastric cancer. Oncogene. 2016;35(2):251–60. doi.org/10.1038/onc.2015.80. PMID:25823029.
- Suzuki S, Dobashi Y, Hatakeyama Y, Tajiri R, Fujimura T, Heldin CH, Ooi A. Clinicopathological significance of platelet-derived growth factor (PDGF)-B and vascular endothelial growth factor-A expression, PDGF receptor-beta phosphorylation, and microvessel density in gastric cancer. BMC Cancer. 2010;10:659. doi.org/10.1186/1471-2407-10-659. PMID:21118571.
- Lambrechts D, Lenz HJ, de Haas S, Carmeliet P, Scherer SJ. Markers of response for the antiangiogenic agent bevacizumab. J Clin Oncol. 2013;31(9):1219–30. doi.org/10.1200/JCO.2012.46.2762. PMID:23401453.
- Miles DW, de Haas SL, Dirix LY, Romieu G, Chan A, Pivot X, Tomczak P, Provencher L, Cortés J, Delmar PR, et al. Biomarker results from the AVADO phase 3 trial of first-line bevacizumab plus docetaxel for HER2-negative metastatic breast cancer. Br J Cancer. 2013;108(5):1052–60. doi.org/10.1038/bjc.2013.69. PMID:23422754.
- Miles D, Cameron D, Bondarenko I, Manzyuk L, Alcedo JC, Lopez RI, Im SA, Canon JL, Shparyk Y, Yardley DA, et al. Bevacizumab plus paclitaxel versus placebo plus paclitaxel as first-line therapy for HER2-negative metastatic breast cancer (MERiDiAN): A double-blind placebo-controlled randomised phase III trial with prospective biomarker evaluation. Eur J Cancer. 2017;70:146–55. doi.org/10.1016/j.ejca.2016.09.024. PMID:27817944.
- Van Cutsem E, de Haas S, Kang YK, Ohtsu A, Tebbutt NC, Ming Xu J, Peng Yong W, Langer B, Delmar P, Scherer SJ, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a biomarker evaluation from the AVAGAST randomized phase III trial. J Clin Oncol. 2012;30(17):2119–27. doi.org/10.1200/JCO.2011.39.9824. PMID:22565005.