ABSTRACT
A 75-y-old Chinese female patient diagnosed with lung adenocarcinoma with brain metastasis suffered severe nausea and vomiting, and these symptoms were contraindications for CyberKnife treatment. Neither mannitol, nor dexamethasone, relieved the symptoms. However, after the patient received a single dose of bevacizumab (200 mg, 2.9 mg/kg), the patient's symptoms were significantly relieved. The patient subsequently completed a successful CyberKnife treatment. In addition, the patient received an oral treatment of gefitinib. At 15 months post treatment, the patient's brain tumor was controlled. Thus, administration of bevacizumab at a low dose (2.9 mg/kg) may significantly alleviate peri-tumoral brain edema and its symptoms, thereby facilitating radiosurgery treatment.
Introduction
Radiosurgery is widely used in the treatment of brain metastases from non-small cell lung cancer (NSCLC). However, metastatic brain tumors are often surrounded by peri-tumoral brain edema (PTBE), which can lead to neurological symptoms.Citation1 Several medications, including mannitol and steroids, are frequently used to relieve brain edema.Citation2,Citation3 However, the effects of these drugs have been limited in some patients, including patients with diabetes, impaired renal function, or gastrohelcosis.Citation4 It has been hypothesized that vascular endothelial growth factor A (VEGF-A) plays an important role in cerebral edema that is associated with brain tumors. Correspondingly, recent case studies and clinical trialsCitation5-Citation14 have demonstrated that bevacizumab, a monoclonal antibody recognizing VEGF-A, provides an effective treatment for brain edema.
Here, we report the case of a 75-y-old female with a metastatic brain tumor surrounded by peri-tumoral brain edema who experienced severe nausea and vomiting. Following administration of a single dose of bevacizumab (2.9 mg/kg, 200 mg), the neuropathological symptoms experienced by the patient were well controlled. As a result, the patient was able to undergo successful CyberKnife treatment.
Case report
A 75-y-old Chinese female patient underwent resection of the lower lobe of her left lung in October 2014. Two tumors were found with lengths of 2.0 cm and 1.5 cm, respectively. Low grade adenocarcinoma was diagnosed without metastatic lymph nodes (0/21). Immunohistochemistry showed that the tumor cells were positive for TTF-1 and negative for p63; Ki67 was 10%. Sanger's direct sequencing detected mutations in exon 21 of the epidermal growth factor receptor gene (EGFR). Pathologic staging indicated T3N0M0, IIb. The patient subsequently underwent four cycles of adjuvant chemotherapy with pemetrexed and carboplatin.
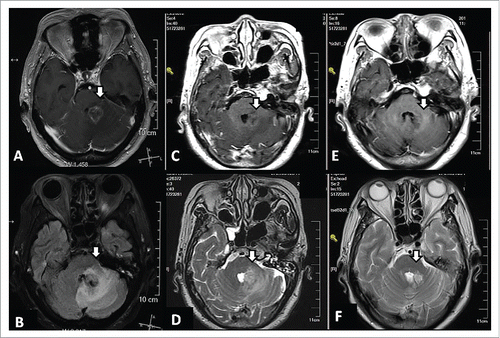
In February 2016, the patient experienced nausea and vomiting, dizziness, fatigue, and headaches. Brain magnetic resonance imaging (MRI) showed a 2.0 cm tumor near the fourth ventricle ( & ). The patient's symptoms were not relieved by mannitol (125 mL, 4 × daily) or dexamethasone (5 mg/d). A lower dose of dexamethasone was administered due to the patient's history of diabetes that extended over the previous 10 y and the potential for high doses of dexamethasone to induce hyperglycemia.
Figure 1. MRI of a 75-y-old female with lung adenocarcinoma and brain metastasis at three time points. At time point 1 (16 February 2016), prior to treatment, a large region of edema was observed in the tissue surrounding the brain metastasis according to gadolinium-enhanced T1-weighted MRI (A) and T2-weighted FLAIR MRI (B). Following administration of a single dose of bevacizumab (2.9 mg/kg) on 19 February 2016, gadolinium-enhanced T1-weighted MRI (C) and T2-weighted MRI (D) were performed at time point 2 (23 February 2016) and the volume of edema was found to be reduced. After the patient received a CyberKnife treatment and gefitinib treatments for an additional 15 months, gadolinium-enhanced T1-weighted MRI (E) and T2-weighted MRI (F) performed at time point 3 (18 July 2017) showed the brain tumor was well controlled.
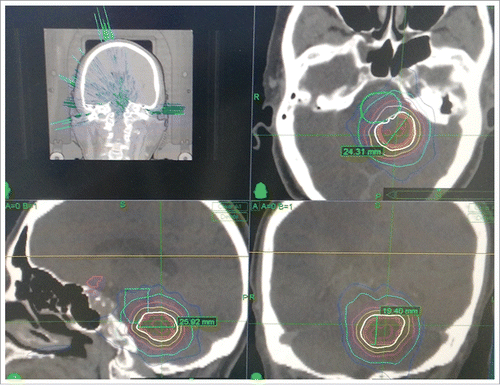
On 19 February 2016, the patient was treated with a single dose of 200 mg bevacizumab (2.9 mg/kg). By the next morning, the nausea and vomiting had stopped. Four d later, MRI showed a significant reduction in edema volume compared with pre-treatment scans ( & ). Six d later, the patient underwent CyberKnife treatment. A brain tumor was found proximal to the brain stem and a relatively low dose of radiation was applied (18 Gy in three fractions, 71% isodose curve), based on our clinical experience (). By 11 March 2016, administration of mannitol and dexamethasone was discontinued and an oral regimen of gefitinib was started. At 15 months follow-up (August 2017), the patient's brain tumor was well controlled ( & ).
Discussion
Peri-tumoral brain edema frequently accompanies metastatic tumors.Citation1 Not only do these symptoms reduce a patient's quality of life, they are also contraindications for a radiotherapy regimen.
Currently, administration of corticoids and mannitol are the main treatments for peritumoral edema. VEGF is a secretory glycoprotein which can specifically act on vascular endothelial cells of tumors to promote angiogenesis, compromise the blood brain barrier, increase the exudation of water and electrolytes, and contribute to edema.Citation15-17 Correspondingly, in previous studies, anti-VEGF drugs were able to mediate significant therapeutic effects on peritumoral edema.Citation18 These effects potentially derive from the ability of anti-VEGF drugs to decrease prostacyclin and nitric oxide production, activate procoagulant pathways, and/or reduce the proliferation and migration of endothelial cells of tumor vessels.Citation15
In a previous study, bevacizumab therapy was administered 3–10 d after completion of CyberKnife radiosurgery for a minimum of two cycles (5 mg/kg at 2-week intervals). This regimen was found to be a promising and safe treatment for brain metastases with extensive cerebral edema. Moreover, radiosurgery was safely combined with bevacizumab in patients with non-squamous NSCLC with brain metastases.Citation19 Despite these encouraging results, however, adverse reactions have been associated with bevacizumab treatments, including severe bleeding and hypertension. The dose of bevacizumab that is prescribed for the treatment of brain necrosis-induced brain edema is generally 5 mg/kg for 1–3 weeks, and this dose is lower than the anti-tumor dose of bevacizumab (5 mg/kg/week) that is approved by the US Food and Drug Administration.Citation20,Citation21 It remains unclear whether lower doses of bevacizumab can alleviate peritumoral brain edema, and this is an important consideration for future studies. However, the present case report demonstrates that a low dose of bevacizumab (2.9 mg/kg) has the potential to relieve vomiting and nausea and facilitate CyberKnife treatment. As a result, excellent short-term and long-term survival benefits could be obtained.
Abbreviations
| EI | = | Edema index |
| IMRT | = | Intensity-modulated radiation therapy |
| MRI | = | Magnetic resonance imaging |
| NSCLC | = | Non-small cell lung cancer |
| PTBE | = | Peritumoral brain edema |
| VEGF-A | = | Vascular endothelial growth factor A |
Conflicts of interest
The authors have no potential conflicts of interest.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- Lemercier P, Paz Maya S, Patrie JT, Flors L, Leiva-Salinas C. Gradient of apparent diffusion coefficient values in peritumoral edema helps in differentiation of glioblastoma from solitary metastatic lesions. AJR Am J Roentgenol. 2014;203:163–169. doi:10.2214/AJR.13.11186. PMID:24951211.
- Palma L, Bruni G, Fiaschi AI, Mariottini A. Passage of mannitol into the brain around gliomas: a potential cause of rebound phenomenon. A study on 21 patients. J Neurosurg Sci. 2006;50:63–66.
- Jeon YS, Koh YC, Song SW, Cho J, Lim SD. Palliative Resection of Metastatic Brain Tumors Previously Treated by Stereotactic Radiosurgery. Brain Tumor Res Treat. 2016;4:116–123. doi:10.14791/btrt.2016.4.2.116. PMID:27867922.
- Ly KI, Wen PY. Clinical Relevance of Steroid Use in Neuro-Oncology. Curr Neurol Neurosci Rep. 2017;17:5. doi:10.1007/s11910-017-0713-6. PMID:28138871.
- Gonzalez J, Kumar AJ, Conrad CA, Levin VA. Effect of bevacizumab on radiation necrosis of the brain. Int J Radiat Oncol Biol Phys. 2007;67:323–326. doi:10.1016/j.ijrobp.2006.10.010. PMID:17236958.
- Pillay Smiley N, Alden T, Hartsell W, Fangusaro J. Severe Radiation Necrosis Successfully Treated With Bevacizumab in an Infant with Low-Grade Glioma and Tumor-Associated Intractable Trigeminal Neuralgia. Pediatr Blood Cancer. 2016;63:1671–1673. doi:10.1002/pbc.26055. PMID:27187113.
- Furuse M, Nonoguchi N, Kawabata S, Miyata T, Toho T, Kuroiwa T, Miyatake S. Intratumoral and peritumoral post-irradiation changes, but not viable tumor tissue, may respond to bevacizumab in previously irradiated meningiomas. Radiat Oncol. 2015;10:156. doi:10.1186/s13014-015-0446-0. PMID:26223253.
- Sadraei NH, Dahiya S, Chao ST, Murphy ES, Osei-Boateng K, Xie H, Suh JH, Peereboom DM, Stevens GH, Ahluwalia MS. Treatment of cerebral radiation necrosis with bevacizumab: the Cleveland clinic experience. Am J Clin Oncol. 2015;38:304–310. doi:10.1097/COC.0b013e31829c3139. PMID:23799286.
- Bitzer M, Opitz H, Popp J, Morgalla M, Gruber A, Heiss E, Voigt K. Angiogenesis and brain oedema in intracranial meningiomas: influence of vascular endothelial growth factor. Acta Neurochir (Wien). 1998;140:333–340. doi:10.1007/s007010050106. PMID:9689324.
- Furuse M, Kawabata S, Kuroiwa T, Miyatake S. Repeated treatments with bevacizumab for recurrent radiation necrosis in patients with malignant brain tumors: a report of 2 cases. J Neurooncol. 2011;102:471–475. doi:10.1007/s11060-010-0333-3. PMID:20694573.
- Benoit A, Ducray F, Cartalat-Carel S, Psimaras D, Ricard D, Honnorat J. Favorable outcome with bevacizumab after poor outcome with steroids in a patient with temporal lobe and brainstem radiation necrosis. J Neurol. 2011;258:328–329. doi:10.1007/s00415-010-5747-5. PMID:20862487.
- Williams BJ, Park DM, Sheehan JP. Bevacizumab used for the treatment of severe, refractory perilesional edema due to an arteriovenous malformation treated with stereotactic radiosurgery. J Neurosurg. 2012;116:972–977. doi:10.3171/2012.1.JNS111627. PMID:22324417.
- Berghoff AS, Sax C, Klein M, Furtner J, Dieckmann K, Gatterbauer B, Widhalm G, Rudas M, Zielinski CC, Bartsch R, et al. Alleviation of brain edema and restoration of functional independence by bevacizumab in brain-metastatic breast cancer: a case report. Breast Care (Basel). 2014;9:134–136. doi:10.1159/000360930. PMID:24944558.
- Wang Y, Wang E, Pan L, Dai J, Zhang N, Wang X, Liu X, Mei G, Sheng X. A new strategy of CyberKnife treatment system based radiosurgery followed by early use of adjuvant bevacizumab treatment for brain metastasis with extensive cerebral edema. J Neurooncol. 2014;119: 369–376. doi:10.1007/s11060-014-1488-0. PMID:24879376.
- Kamba T, McDonald DM. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br J Cancer. 2007;96:1788–1795. doi:10.1038/sj.bjc.6603813. PMID:17519900.
- Longo R, Sarmiento R, Fanelli M, Capaccetti B, Gattuso D, Gasparini G. Anti-angiogenic therapy: rationale, challenges and clinical studies. Angiogenesis. 2002;5: 237–256. doi:10.1023/A:1024532022166. PMID:12906317.
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi:10.1038/nm0603-669. PMID:12778165.
- Fraum TJ, Kreisl TN, Sul J, Fine HA, Iwamoto FM. Ischemic stroke and intracranial hemorrhage in glioma patients on antiangiogenic therapy. J Neurooncol. 2011;105: 281–289. doi:10.1007/s11060-011-0579-4. PMID:21603965.
- Guinde J, Carron R, Tomasini P, Greillier L, Régis J, Barlesi F. Bevacizumab plus radiosurgery for non-squamous non-small cell lung cancer patients with brain metastases: a safe combination? World Neurosurg. 2017;107:1047.e1–1047.e4. doi:10.1016/j.wneu.2017.07.185. PMID:28803179.
- Giantonio BJ, Catalano PJ, Meropol NJ, O'Dwyer PJ, Mitchell EP, Alberts SR, Schwartz MA, Benson AB 3rd, Eastern CooperativeOncology Group Study E3200. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–1544. doi:10.1200/JCO.2006.09.6305. PMID:17442997.
- Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, Mannel RS, Homesley HD, Fowler J, Greer BE. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365:2473–2483. doi:10.1056/NEJMoa1104390. PMID:22204724.