ABSTRACT
Epithelial growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) have markedly improved the response of non-small cell lung cancer (NSCLC) with EGFR-mutant patients. However, these patients inevitably come cross acquired resistance to EGFR-TKIs. The transformation of lung adenocarcinoma to small cell lung cancer (SCLC) following treatment with EGFR-TKIs is rare, which leads to resistance to EGFR-TKIs.
The present case concerns a case of a 38-year-old man presenting with cough and dyspnea. Radical resection was performed and confirmed an EGFR exon 21 L858R lung adenocarcinoma. However, the patient suffered pleural metastasis after successful treatment with surgery and adjuvant treatment. So, erlotinib was administered with 18 months. Because of enlarged pleural nodule, repeat biopsy identified an SCLC and chemotherapy was started. However, despite the brief success of chemotherapy, our patient suffered brain metastasis.
Our case emaphsizes both the profile of transformation from NSCLC to SCLC and the importance of repeat biopsy dealing with drug resistance. We also summarize the clinical characteristics, mechanisms, predictors of SCLC transformation, treatment after transformation and other types of transformation to SCLC.
Introduction
Epithelial growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) have exhibited remarkable responses compared with standard cytotoxic chemotherapy in patients with EGFR-positive non–small cell lung cancer.Citation1,Citation2 Despite the favourable responses, these patients will virtually develop disease progression after a median time of about 12 months.Citation3 Several resistance mechanisms have been identified.Citation4–8 Transformation to small-cell lung cancer (SCLC) has been described as a mechanism of resistance to EGFR-TKI treatment in approximately 5% of patients.Citation9 Hence, we report a case of TKI resistance due to SCLC transformation and review the literature to summarize the clinical features, mechanisms, predictors of SCLC transformation, treatment after transformation and other types of transformation to SCLC.
Case presention
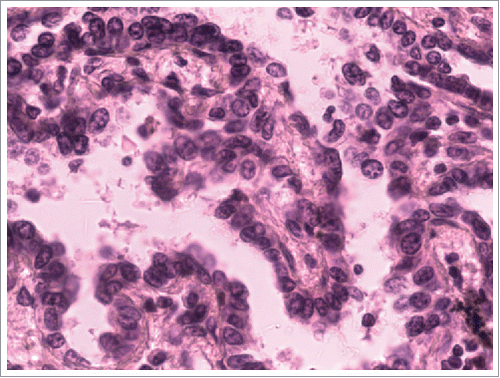
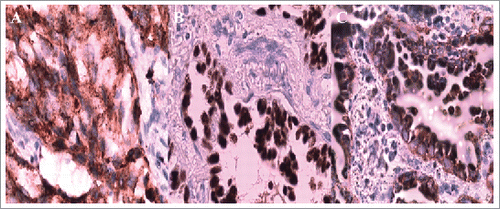
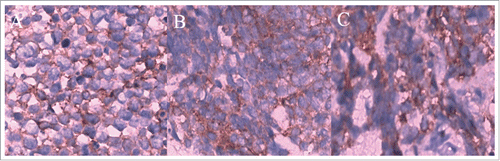
A 38-year-old Chinese man with no smoking history presented with cough and dyspnoea in September 2014. Table summarizes the clinical characteristic of this patient. Computed tomography (CT) scans of the chest with contrast revealed a 4.5 × 4.5 cm left lower lobe mass with left hilar and mediastinal lymphadenopathies. Physical examination suggested no significant abnormalities. Laboratory findings were within the normal range, except for the carcinoembryonic antigen (CEA) level of 3.99 ng/mL (normal range, 0 to 3.4 ng/mL) in the serum. Other exams showed no positive signs. So, complete resection was performed. The pathological diagnosis of the specimens was lung adenocarcinoma, and we confirmed the metastasis of mediastinum lymph nodes (4/12) and the pleura involved was identified (). The results of immunohistochemistry (IHC) staining were positive for Napsin A, TTF-1, and CK7 () and negative for p40,Syn. Epidermal growth factor receptor (EGFR) exon 21 L858R mutation was identified using the amplification refractory mutation system (ARMS) method.
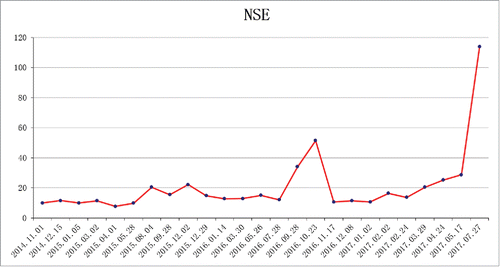
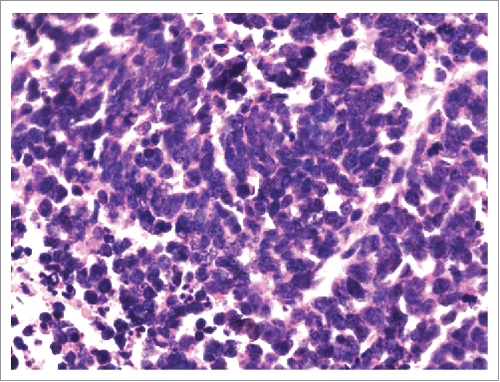
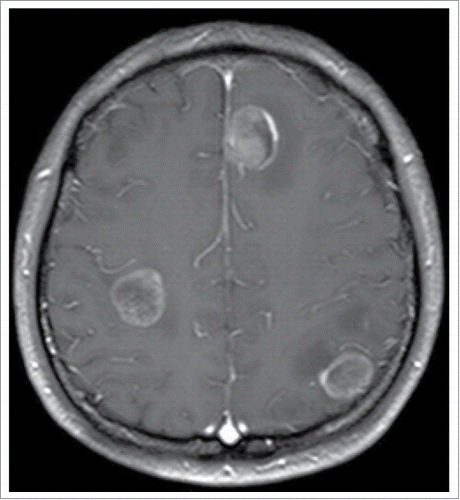
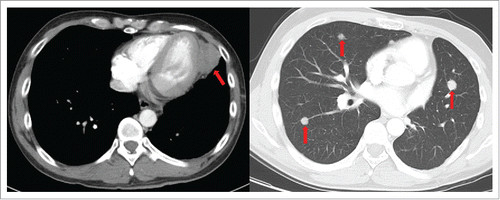
Therefore, the patient received four cycles of adjuvant chemotherapy with pemetrexed and cisplatin. However, adjuvant radiotherapy was rejected by this patient. Five months later after surgery (March 2015), CT scan showed a 1.0 × 1.2 cm left pleural nodule. () So, erlotinib was administered because of progressive disease. Within 18 months of erlotinib treatment, disease decreased and remained persistently stable () according to the Response Evaluation Criteria in Solid Tumors. After reimaging in September 2016, CT revealed an enlarged pleural mass (4.1 × 3.4 cm) and bilateral lung nodules (), and neuron-specific enolase (NSE) increased to 33.96 ng/mL (normal range, 0 to 17 ng/mL) (). A repeat biopsy of pleural nodules was performed, and histologic analysis showed small cell lung cancer (SCLC) (). Immunohistochemical staining was confirmed as positive for CgA, NSE, and Syn () and negative for Napsin A, TTF-1. The patient was treated with etoposide and cisplatin for 6 cycles. At this duration, the nodule decreased. In July 2017, brain magnetic resonance imaging suggested cerebral metastases (). This patient refused chemotherapy and joined a clinical trial.
Figure 3. Five months later after surgery (March 2015), CT scan showed a 1.0 × 1.2cm left pleural nodule.
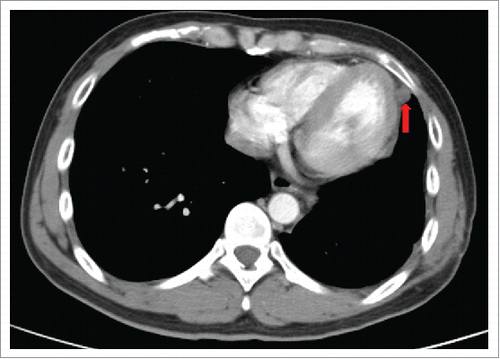
Figure 4. Within 18 months of erlotinib treatment, disease decreased and remained stable persistently.
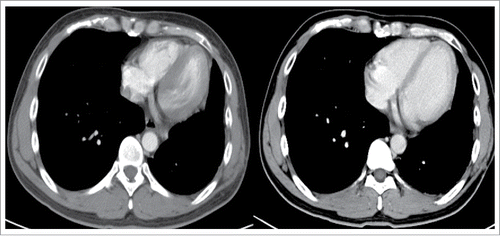
Figure 5. CT revealed the enlarged pleural mass (4.1 × 3.4cm), bilateral lung nodules in September 2016.
The patient consented for the publication of the present case report.
Discussion
Clinical features
SCLC transformation is more likely to develop in lung adenocarcinomas that have EGFR-activating mutations than in EGFR wild-type tumours.Citation9 A recent study revealed that SCLC transformation occurred more frequently in lung adenocarcinomas with a deletion in EGFR exon 19 (50.0%, 9/18) than in those with exon 21 L858R mutation (27.8%, 5/18).Citation10 The majority of the SCLC transformations retained the same gene mutation as the primary lung adenocarcinoma (83.3%, 15/18), with only a small proportion (16.7%, 3/18) losing the original gene mutation or gaining another type of gene mutation.
From a study that included 39 patients from published case studies, the results showed that the median time from initial diagnosis of lung adenocarcinoma to the SCLC transformation was 19 months (range 1–61 months).Citation11 The median survival after SCLC diagnosis was 6 months. Female gender was significantly associated with longer SCLC transformation at the multivariable analysis.
In addition, the time from the first diagnosis of lung adenocarcinoma to the onset of SCLC phenotype was long (19 months, range 1–61 months) and seems to be longer than the progression-free survival reported in patients with TKI treatment in prospective clinical trials (8–11 months).Citation11 In our case, the interval between TKI treatment and SCLC transformation is 18 months. These findings suggest that long-term exposure to TKI may be needed for SCLC transformation, and patients obtaining a long-term benefit from these drugs are at a higher risk of trans-differentiation to the SCLC phenotype at progression.
Mechanisms
The potential mechanisms underlying SCLC phenotype conversion after TKI therapy remain unclear.
Historically, SCLC was believed to develop from neuroendocrine cells of central airways, whereas adenocarcinoma derives from the alveolar type II cells located in the alveolar surface area. Several studies have shown that alveolar type II cells may be common precursors of both lung adenocarcinoma and SCLC.Citation9,Citation12 Therefore, lung adenocarcinoma arising from these alveolar type II cells and harbouring EGFR mutations might trans-differentiate to SCLC under the selective pressure of TKI therapy. So, EGFR-mutant lung cancers that stemmed from alveolar type II cells may have the potential to transform into SCLC during disease progression.
The alternate hypothesis is that initial tumours consisted of the combined histology of NSCLC and SCLC. As the number of NSCLC cells decreased due to treatment, the SCLC component of the initial tumour became dominant.Citation9
Therefore, it can be concluded that SCLC cells may trans-differentiate from primary adenocarcinoma cells, originating from the minor pre-existent SCLC under the selection pressure of EGFR-TKIs, or arise from multi-potent cancer stem cells. This phenomenon emphasizes the importance of repeat biopsies in the clinical design of a treatment regimen.
In addition, a prior study reported that retinoblastoma protein (Rb) expression was lost in cases where the histological phenotype had changed from NSCLC to SCLC after EGFR-TKI therapy, whereas Rb was rarely lost in those that retained the NSCLC phenotype. Rb expression loss is considered to be one of the molecular mechanisms of SCLC transformation.Citation13 Moreover, research showed that TP53 status in EGFR TKI-treated EGFR mutant lung adenocarcinoma is also informative in predicting small-cell transformation.Citation14
Predictors of SCLC transformation
Therapeutic strategies for SCLC and NSCLC differ substantially. Therefore, identification of a non-invasive method before re-biopsy to find potential disease transformation is important.
A rapid increase in the serum NSE and a poor response to EGFR-TKIs is usually an indication of transformation from adenocarcinoma to SCLC.Citation15 In this case, the tumour progressed rapidly in spite of TKI treatment, along with a marked increase in NSE level. In the present case, the remarkably increased serum levels of NSE highlighted the necessity of repeat biopsies. The present case indicates that patients could benefit from routine testing of the serum NSE levels to monitor SCLC transformation.
NSE is an important marker for SCLC and may play potential roles in the diagnosis of SCLC transformation from EGFR-positive lung carcinoma patients receiving EGFR-TKI treatment. However, little attention has been paid to the NSE levels in serum, as NSE is not a clinical routine test for NSCLC patients.
Furthermore, the pro-gastrin releasing-peptide (pro-GRP) has also been recommended as a tumour marker for the early prediction of disease transformation from adenocarcinoma to SCLC.Citation16 When and how this process started warrants further investigation, as does the predictive value of some specific tumour markers.
Therefore, routine and dynamic tests of NSE and pro-GRP levels in the serum may be helpful to screen patients with SCLC transformation before invasive biopsies. The rapidly increased levels and tumour growth may point to SCLC transformation and support further biopsies, although whether they can accurately predict SCLC transformation remains to be determined.
In addition, an investigation revealed that SCLC tended to occur later in the earlier stage of adenocarcinoma (I, II, IIIA) than in advanced ones (IIIB, IV).Citation10 An average interval is 59 months (range, 32–120 months) in the former and 20 months (range, 14–31 months) in the latter, which suggests that disease stage could be a risk factor for SCLC transformation.
Treatment after transformation
Most cases of SCLC transformation have exhibited neuroendocrine differentiation with increased chemosensitivity and respond well to initial etoposide and cisplatin (EP) chemotherapy. Based on the available evidence, patients with SCLC transformed from adenocarcinoma should be given standard therapies.Citation17
Repeat biopsy may be needed to confirm the dominant histology that was not identified at the initial diagnosis, when tumours do not respond as initially expected or when the responses among two or more different lesions are discordant in advanced lung adenocarcinoma. In clinical practice, identification of small cell components is crucial since the treatment option can be switched to the EP regime against SCLC.
Other types of transformation to SCLC
EGFR–wild-type adenocarcinomas also occur in small cell lung cancer transformation, which suggests that transformation to SCLC is not unique to EGFR-mutated tumours, nor does it exclusively result from TKI treatment.Citation18 Transformation to SCLC is also reported as a mechanism of acquired resistance to crizotinib in ALK rearranged lung cancer.Citation19 In addition, a rare case described SCLC transformation from NSCLC without EGFR mutation during immunotherapy with nivolumab.Citation20 Therefore, more attention should be paid to strategies of SCLC transformation to NSCLC.
Conclusion
In conclusion, we report a case of the EFGR-mutant NSCLC that transformed to SCLC during treatment with EGFR-TKI. The possibility of transformation to SCLC should be considered when treating EGFR-mutant adenocarcinoma with EGFR-TKI, especially when serum NSE increases. Re-biopsy should be considered when managing drug resistance, and frequent surveillance may be needed.
Table 1. Treatment detail of SCLC transformation patient.
References
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Eng J Med. 2009;361(10):947–57. doi:10.1056/NEJMoa0810699
- Rossi A, Di Maio M. LUX-Lung: determining the best EGFR inhibitor in NSCLC? Lancet Oncol. 2015;16(2):118–9. doi:10.1016/S1470-2045(14)71196-9. PMID:25589190.
- Engelman JA, Janne PA. Mechanisms of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer. Clin Cancer Res. 2008;14(10):2895–9. doi:10.1158/1078-0432.CCR-07-2248. PMID:18483355.
- Kobayashi S, Boggon TJ, Dayaram T, Jänne PA, Kocher O, Meyerson M, Johnson BE, Eck MJ, Tenen DG, Halmos B. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352(8):786–92. doi:10.1056/NEJMoa044238. PMID:15728811
- Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, Lindeman N, Gale CM, Zhao X, Christensen J, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316(5827):1039–43. doi:10.1126/science.1141478. PMID:17463250.
- Yano S, Wang W, Li Q, Matsumoto K, Sakurama H, Nakamura T, Ogino H, Kakiuchi S, Hanibuchi M, Nishioka Y, et al. Hepatocyte growth factor induces gefitinib resistance of lung adenocarcinoma with epidermal growth factor receptor-activating mutations. Cancer Res. 2008;68(22):9479–87. doi:10.1158/0008-5472.CAN-08-1643. PMID:19010923.
- Yamamoto C, Basaki Y, Kawahara A, Nakashima K, Kage M, Izumi H, Kohno K, Uramoto H, Yasumoto K, Kuwano M, et al. Loss of PTEN expression by blocking nuclear translocation of EGR1 in gefitinib-resistant lung cancer cells harboring epidermal growth factor receptor-activating mutations. Cancer Res. 2010;70(21):8715–25. doi:10.1158/0008-5472.CAN-10-0043. PMID:20959484.
- Suda K, Tomizawa K, Fujii M, Murakami H, Osada H, Maehara Y, Yatabe Y, Sekido Y, Mitsudomi T. Epithelial to mesenchymal transition in an epidermal growth factor receptor-mutant lung cancer cell line with acquired resistance to erlotinib. J Thorac Oncol. 2011;6(7):1152–61. doi:10.1097/JTO.0b013e318216ee52. PMID:21597390.
- Oser MG, Niederst MJ, Sequist LV, Engelman JA. Transformation from non-small-cell lung cancer to small-cell lung cancer: molecular drivers and cells of origin. Lancet Oncol. 2015;16(4):e165–172. doi:10.1016/S1470-2045(14)71180-5. PMID:25846096.
- Jiang SY, Zhao J, Wang MZ, Huo Z, Zhang J, Zhong W, Xu Y. Small-cell lung cancer transformation in patients with pulmonary adenocarcinoma: A case report and review of literature. Medicine. 2016;95(6):e2752. doi:10.1097/MD.0000000000002752. PMID:26871823.
- Roca E, Gurizzan C, Amoroso V, Vermi W, Ferrari V, Berruti A. Outcome of patients with lung adenocarcinoma with transformation to small-cell lung cancer following tyrosine kinase inhibitors treatment: A systematic review and pooled analysis. Cancer Treat Rev. 2017;59:117–22. doi:10.1016/j.ctrv.2017.07.007. PMID:28806542.
- Shi X, Duan H, Liu X, Zhou L, Liang Z. Genetic alterations and protein expression in combined small cell lung cancers and small cell lung cancers arising from lung adenocarcinomas after therapy with tyrosine kinase inhibitors. Oncotarget. 2016;7(23):34240–9. doi:10.18632/oncotarget.9083. PMID:27145273.
- Niederst MJ, Sequist LV, Poirier JT, Mermel CH, Lockerman EL, Garcia AR, Katayama R, Costa C, Ross KN, Moran T, et al. RB loss in resistant EGFR mutant lung adenocarcinomas that transform to small-cell lung cancer. Nat commun. 2015;6:6377. doi:10.1038/ncomms7377. PMID:25758528.
- Lee JK, Lee J, Kim S, Kim S, Youk J, Park S, An Y, Keam B, Kim DW, Heo DS, et al. Clonal history and genetic predictors of transformation into small-cell carcinomas from lung adenocarcinomas. J clin oncol. 2017;35(26):3065–74. doi:10.1200/JCO.2016.71.9096. PMID:28498782.
- Zhang Y, Li XY, Tang Y, Xu Y, Guo WH, Li YC, Liu XK, Huang CY, Wang YS, Wei YQ. Rapid increase of serum neuron specific enolase level and tachyphylaxis of EGFR-tyrosine kinase inhibitor indicate small cell lung cancer transformation from EGFR positive lung adenocarcinoma? Lung Cancer. 2013;81(2):302–5. doi:10.1016/j.lungcan.2013.04.005. PMID:23683536.
- Norkowski E, Ghigna MR, Lacroix L, Le Chevalier T, Fadel É, Dartevelle P, Dorfmuller P, Thomas de Montpréville V. Small-cell carcinoma in the setting of pulmonary adenocarcinoma: new insights in the era of molecular pathology. J Thor Oncol. 2013;8(10):1265–71. doi:10.1097/JTO.0b013e3182a407fa
- van Meerbeeck JP, Fennell DA, De Ruysscher DK. Small-cell lung cancer. Lancet. 2011;378(9804):1741–55. doi:10.1016/S0140-6736(11)60165-7. PMID:21565397.
- Ahn S, Hwang SH, Han J, Choi YL, Lee SH, Ahn JS, Park K, Ahn MJ, Park WY. Transformation to small cell lung cancer of pulmonary adenocarcinoma: Clinicopathologic analysis of six cases. J Pathol Transl Med. 2016;50(4):258–63. doi:10.4132/jptm.2016.04.19. PMID:27160687.
- Miyamoto S, Ikushima S, Ono R, Awano N, Kondo K, Furuhata Y, Fukumoto K, Kumasaka T. Transformation to small-cell lung cancer as a mechanism of acquired resistance to crizotinib and alectinib. Jpn J Clin Oncol. 2016;46(2):170–3. PMID:26613679.
- Imakita T, Fujita K, Kanai O, Terashima T, Mio T. Small cell lung cancer transformation during immunotherapy with nivolumab: A case report. Respir Med Case Rep. 2017;21:52–5. PMID:28393006.