ABSTRACT
Tumor growth and metastasis are closely related to angiogenesis. Basic fibroblast growth factor(bFGF) is an angiogenic factor, and up-regulated expression of bFGF plays a crucial role in the development and metastasis of melanoma. Therefore, in this study, we sought to achieve antitumor activity by immunity targeting bFGF which would inhibit tumor angiogenesis and simultaneously induce bFGF specific cytotoxic T lymphocytes to kill melanoma cells. A human bFGF protein was used as exogenous antigen, coupled with a saponin-liposome adjuvant formulation to enhance CTL response. The results showed that the immunity induced strong immune response and produced prominent anti-cancer activities. CD31 immunohistochemistry and alginate-encapsulated tumor cell assay displayed that tumor angiogenesis was effectively inhibited. Further, the higher production of IFN-γ and cytotoxic T lymphocyte killing assay suggested that the anti-cancer activities may mainly depend on cellular immune response, which could cause the inhibition of tumor angiogenesis and specific killing of tumor cells by bFGF-specific cytotoxic T lymphocytes. We concluded that immunotherapy targeting bFGF may be a prominent strategy for melanoma, and that the adjuvant formulation of saponin-liposome is very desirable in enhancing cytotoxic T lymphocytes response.
Introduction
Basic fibroblast growth factor(bFGF), also called FGF-2, is one of the 21-member of fibroblast growth factors family known to modulate the growth, differentiation, migration, and survival of a variety of cells.Citation1-4 Besides its mitogenic properties, bFGF is involved in angiogenesis of several types of tumors such as melanoma, glioblastoma, kaposi sarcoma, renal, breast and lung tumors as a potent angiogenic factor. Citation5 In melanoma, basic fibroblast growth factor is the most important autocrine growth factor.Citation6,Citation7 By its mitogenic action on endothelial cells and fibroblasts, the basic fibroblast growth factor produced by melanoma promotes angiogenesis and fibrous stroma formation through paracrine pathways.Citation8 Although there is no expression of bFGF in normal melanocytes, it is always present in melanoma and dysplastic nevi.Citation9-12 Most melanomas show high expression of bFGF and FGFRs, which are critical for the survival and growth of melanoma cells.Citation13,Citation14 Therefore, immunity targeting bFGF may be a promising strategy for the suppression of melanoma. Autologous bFGF may be identified as a self-molecule and could trigger no immune response. So here we employed a human recombinant bFGF protein as an exogenous antigen to break self-tolerance.
As an adjuvant, liposome is a satisfactory antigen delivery system, but the disadvantages of liposome is the lack of immunogenicity and inherent physical instability.Citation15 Saponins, natural glycoside of steroid or triterpene, whose lipophilic acyl side chains have unique abilities to stimulate antigen-specific cytotoxic T lymphocytes production,Citation16 which makes them an ideal adjuvant for cancer vaccines.Citation17,Citation18 Demana P H found that the proper formulation of liposome and saponins can incorporate antigen into a particulate delivery system that can increase the proliferation and activity of T cells,Citation15 since an antigen in particulate form is more immunogenic than a soluble antigen.Citation19,Citation20 The main purpose of most cancer vaccines is to induce specific CTL response,Citation21 so we applied a similar liposome-saponins adjuvant formulation to enhance cytotoxic T lymphocytes response in this work, while Freund's adjuvant was applied as a positive control(because Freund's adjuvant can effectively stimulate cell-mediated immunity and lead to the enhancement of T-helper cells, resulting in some immunoglobulin and effector T cells).Citation22 Our results demonstrate that the immunity targeting bFGF effectively stimulates the bFGF specific immune response and produces potent anti-cancer activities.
Materials and methods
Animals and cell lines. Six-week-old female C57BL/6 mice were purchased from Slack experimental animal Co., LTD(Shanghai, China). Murine melanoma cell B16-F10 from American Type Culture Collection was cultured in DMEM supplemented with 15% fetal bovine serum.
Preparation of lipo-saponins/bFGF vaccine
The vaccine was prepared as described.Citation23,Citation24 In brief, liposomes(liposome2000, Invitrogen, Carlsbad, CA, USA) were added to chloroform dissolved in cholesterol and evaporated to dryness at 37°C for 1 h, then human-derived recombinant bFGF antigen protein (Nanhai longtime Pharmaceutical Co. Ltd., Guangdong, China) was incorporated at 4°C, and finally mixed with saponin(dissolved in dimethyl sulfoxide) by an injector (The saponin, cholesterol and liposome was mixed at the ratio of 1:2:20 in weight).
Immunization
C57BL/6 mice were subcutaneously injected near both armpits once a week for 5 consecutive weeks per mouse with: (a) normal saline(N.S group); (b) bFGF: 10 μg bFGF protein; (c) saponins/bFGF: 10 μg bFGF protein, 5 μg saponins(Yuanye biotechnology Co., Ltd, Shanghai, China) and 10 μg cholesterol; (d) lipo/bFGF: 10 μg bFGF protein, 10 μg cholesterol and 100 μg liposome(liposome2000, Invitrogen, Carlsbad, CA, USA); (e) lipo-saponins/bFGF: 10 μg bFGF protein, 5 μg saponins, 10 μg cholesterol and 100 μg liposome(The saponin, cholesterol and liposome was mixed at the ratio of 1:2:20 in weight); (f) Freund's/bFGF: 10 μg bFGF and Freund's adjuvant(V/V = 1:1). Blood was collected from the immunized mice on day 7 after the last immunization and sera were separated at 1500 rpm centrifugation.
Tumor models
After 5 immunizations, the mice were subcutaneously inoculated with 1 × 106 tumor cells(in 200 μl DMEM medium with no serum) in the right anterior axillary. Tumor dimensions were measured every 3 d with calipers and tumor volumes were calculated by the formula: widthCitation2 × length × 0.52.
In addition, to investigate the effects of the immunity on cancer metastasis, a lung metastasis model of melanoma was established. Briefly, the mice were injected intravenously with 1 × 104 B16-F10/100 μl cells on day 7 after 5 immunizations. Two weeks later, mice were sacrificed and lung surface nodules(the black spots) were counted.
ELISA determination of anti-bFGF antibody
Serum bFGF specific antibodies were detected by ELISA. Ninety-six-well micro titer plate(Falcon) coated with 10 μg /ml bFGF in 50 mM carbonate buffer was kept at 4°C overnight. The plate was washed three times with 0.1% Tween's PBS and sealed with 5% skimmed milk for 1h at 37°C, and then washed again. A serial dilution of mice sera were added to the microtiter wells and incubated for 2h at 37°C. After washes, the wells were incubated with goat anti-mouse IgG antibodies conjugated with horseradish peroxidase(HRP)(Sigma) for 1h at 37°C, DAB substrate solution was added after a new wash. 20 min later, the reactions were stopped by 0.5 M sulphuric acid and the absorbances were read at 405 nm with a BioRad microtiter plate reader.
Cytokine assay
The immunized mice were sacrificed and lymphocytes were aseptically separated using lymphocyte separation medium. After two washes, the lymphocytes(4 × 106 cells in 200 µl, n = 5) were resuspended in the cell culture medium and stimulated with 10 µg/mL of bFGF antigen protein at 37°C for 48h. The levels of IFN-γ and IL-4 in the supernatants were measured using ELISA kits(Rockford, IL) according to the manufacturers’ instructions.
CD31 immunohistochemistry
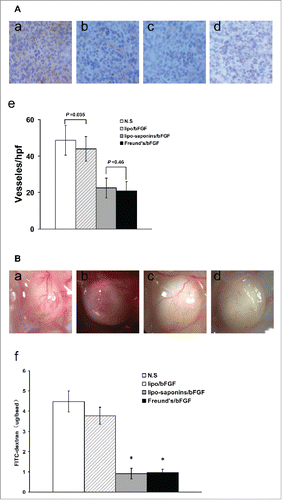
CD31 immunohistochemistry was conducted to determine the angiogenesis in the tumors, using a monoclonal rabbit anti-mouse immunoglobulin. Frozen sections were bathed in cold acetone for 20 min. After two PBS washes, the slices were immersed in 3% hydrogen peroxide to inactivate the endogenous peroxidase. Followed by a new wash, they were blocked with rabbit sera at 37°C for 15 min, then incubated orderly with CD31 specific antibodies(1:500) at 37°C for 2 h, secondary antibody for 40 min, and tertiary antibodies(Horseradish peroxidase-labeled streptavidin) for 40 min. Diaminobenzidine(DAB) was dropped as a chromogen. Tissues were stained and rinsed in water, then counterstained with hematoxylin. Microvessels in each microscopic field were counted at a magnification of × 200.
Alginate-encapsulated tumor cell assay
To further investigate the Inhibition of tumor angiogenesis by the immunity, an alginate-encapsulate tumor cell assay was done. Alginate beads containing 6 × 104 tumor cells per bead were implanted subcutaneously into both dorsal sides of the immunized mice. After two weeks, the mice were injected i.v. with 100ul of 2% fluorescein isothiocyanate(FITC)–dextran(Sigma) solution(100 mg/kg). 20 minutes after the FITC-dextran injection, the alginate beads were surgically removed and photographed. After dissection, alginate beads were transferred to tubes containing 2 ml of saline. The tubes were mixed by a vortex for 20 s and centrifuged (1500 rpm, 5 min). The fluorescence of the supernatant was measured.
CTL-mediated cytotoxicity in vitro
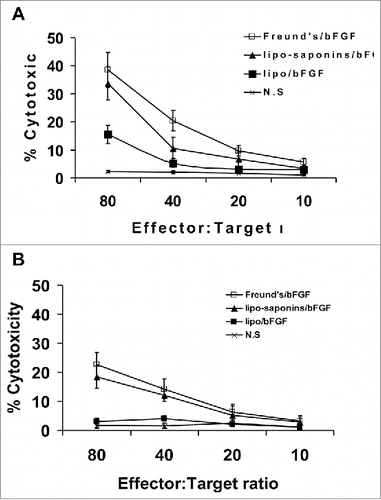
CytoTox 96 NonRa-dioactive Cytotoxicity Assay Kit(Promega, Madison, WI) was used to measure CTL. Respectively, mouse microvascular endothelial cells MS1(ATCC, USA) and B16-F10 cancer cells as target cells were co-cultured with T lymphocytes (Effector cells) from the immunized mice to perform the cell lysis reaction at 37°C. After 8 h, lactate dehydrogenase activity in the supernatant(100 μl) was checked at 490 nm. Cell lysis rate was calculated as suggested by the manufacturer.
Statistical analysis
All data were analyzed using SPSS 22.0. The statistical significance of data was determined by Student's t-test and ANOVA. Differences were considered significant when P < 0.05.
Results
Antibody expression
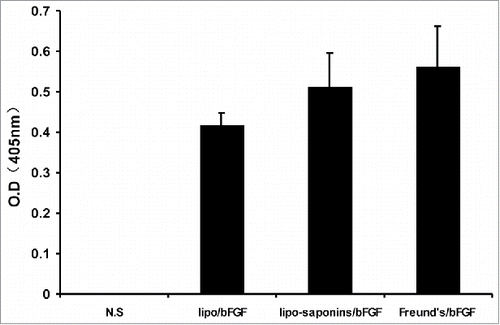
After 5 immunizations, the levels of antibodies in sera were detected by ELISA, using OD values to quantify antibodies levels. There was almost no immune response produced by sapoinins/bFGF and bFGF protein alone(data not shown), Both Freund's/bFGF and lipo-saponins/bFGF elicited very potent humoral immune responses with OD value of 0.562 and 0.513, and the OD value was 0.416 in lipo/bFGF mice ().
Cytokines release
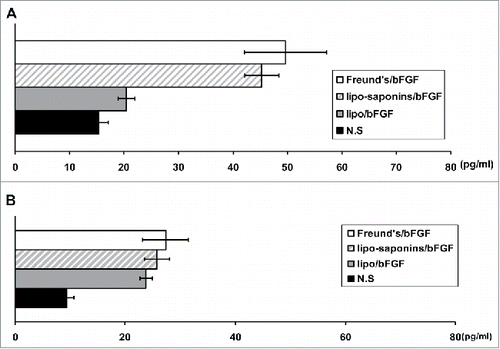
To evaluate the effects of vaccines on Th1 and Th2 type immunity, after the splenocytes of immunized mice were stimulated with 10 µg/ml of bFGF protein in vitro for 48 h, cytokine contents (IL-4 and IFN-γ) in cell-culture supernatants were measured. As shown in , lipo-saponins/bFGF and Freund's/bFGF showed advantages in inducing IFN-γ production than the others (P < 0.01) (). As for the secretion of IL-4, there was no significant difference between lipo-saponins/bFGF and lipo/bFGF group (P = 0.088) ().
Induction of antitumor activity
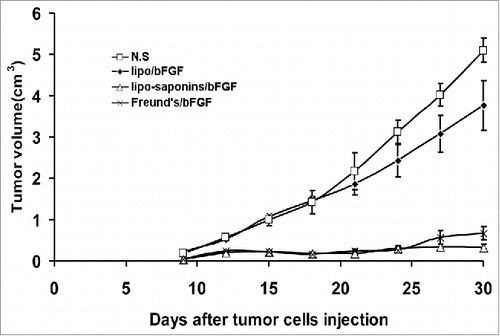
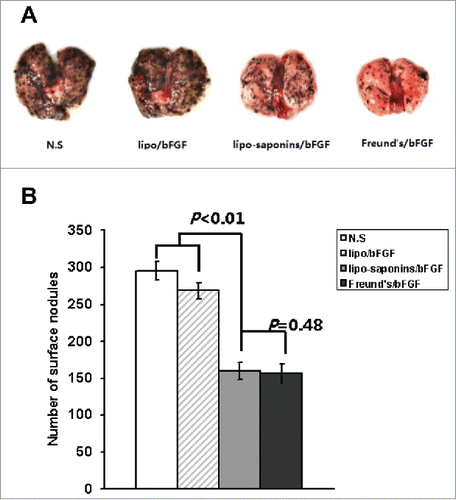
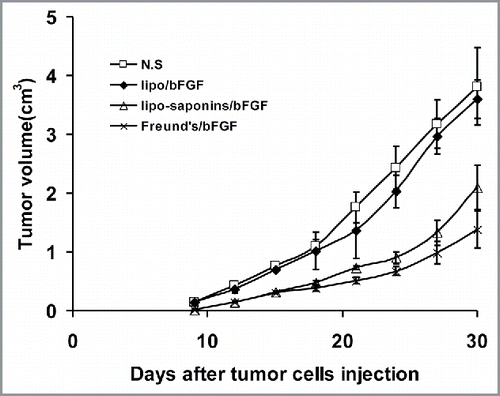
After all the immunizations were completed, mice were inoculated subcutaneously with cancer cells. Within a month of observation, as shown in , the immunities by lipo-saponins/bFGF and Freund's/bFGF showed the strongest inhibitory effect on tumor growth compared with N.S and lipo/bFGF group (P < 0.01), and no very significant difference between lipo-saponins/bFGF and Freund's/bFGF mice (P = 0.052). Lipo/bFGF induced only a slight inhibition(P = 0.013, compared with N.S group), and no antitumor activity was seen in saponins/bFGF and bFGF mice(data not shown). In the lung metastasis model, the fewer lung surface nodules(the black spots) consistently demonstrated a good blockade by lipo-saponins/bFGF and Freund's/bFGF respectively on cancer metastasis, and lipo/bFGF hardly restrained the lung metastasis of cancer cells ().
Effect of immunity on angiogenesis
The effect of immunity on angiogenesis was assessed by CD31 immunohistochemistry of frozen sections. Compared with N.S group, the number of microvessels in lipo/bFGF group reduced a little (P = 0.035), and lipo-saponins/bFGF and Freund's/bFGF mice showed a marked decrease of microvessels(P < 0.01) (). In addition, alginate-encapsulated tumor cell assay further verified the inhibitory effects of different immunizations on angiogenesis. Macroscopic observation showed that the vessels of lipo-saponins/bFGF and Freund's/bFGF mice were obviously sparse, and their FITC–dextran uptakes were significantly decreased, compared with N.S and lipo/bFGF mice ().
Induction of adoptive immunotherapy
To further confirm the anticancer activity, activated T lymphocytes(2 × 106) were adoptively and intravenously transferred to recipient mice. The Next day, the recipient mice were subcutaneously challenged with B16 cancer cells(1 × 106). The results displayed that the immunosuppressive therapy with the T cells of lipo-saponins/bFGF and Freund's/bFGF mice produced visible inhibiting actions on tumor growth ().
Figure 6. Induction of adoptive immunotherapy. Activated T lymphocytes(2 × 106 cells) were transferred from the immunized mice to recipient mice by intravenous injection. After 24h, the recipient mice were subcutaneously inoculated with B16 cells(1 × 106). Visible inhibiting actions on tumor growth were exhibited in lipo-saponins/bFGF and Freund's/bFGF mice(compared with N.S and lipo/bFGF mice, P<0.01).(n = 5).
T cell-mediated cytotoxicity in vitro
In the T lymphocyte toxicity test, we engaged MS1 and B16-F10 cells as target cells respectively. T cells from the mice immunized with lipo-saponins/bFGF and Freund's/bFGF caused the lysis of MS1 () and B16-F10 cells (), the lysis rate of MS1 cells was higher than that of B16-F10 cells().
Side effect of the vaccines
Because human bFGF is highly homologous to mice bFGF(approximately 95% homology), possible side effects of the vaccines such as wound healing, pathological changes in the internal organs, feeding, ruffling of fur, and mortality have been investigated, but no obvious side effects occurred. Meanwhile, no significant hemolytic activity (possibly caused by saponins) was detected in red blood cell count and blood smear test.
Discussion
Protein antigens are usually degraded by lysosome/endosome pathways and presented to MHC II molecules, thus do not induce CTL response. Carrying antigen protein in particulate form can enter the cytoplasm directly, the antigens are processed by proteasome and presented to MHC I molecules, and then induce CTL production.Citation25,Citation26 In our work, although all three of lipo/bFGF, lipo-saponins/bFGF and Freund's/ bFGF elicited potent antibody response (), the contents of IFN-γ in lipo/bFGF mice were significantly lower than those in lipo-saponins/bFGF and Freund's/bFGF mice (). This indicated that the lipo-saponins adjuvant formulation showed its unique advantages in enhancing cellular immunity. This might be explained by Demana P H's findings, Demana P H found that incorporating saponins into liposomes can disrupt the enclosed bilayer of liposomes to form spherical cage-like or ring-like regular particles, and that such particles can enhance the activation of T cells.Citation15
Tumors acquire metabolic needs and oxygen through capillaries, angiogenesis is critical for tumor growth, development and metastasis.Citation27 It has been proved that antiangiogenesis is a very effective strategy for tumor immunotherapy.Citation28,Citation29 In earlier studies, immunotherapy targeting the tumor angiogenic factor bFGF seems to produce potent anti-cancer effects. With bFGF neutralizing antibodies, respectively, Hori A inhibited tumor growthCitation30 and Murai N induced apoptosis of glioma cells.Citation31 Li X succeeded to achieve anti-tumor effects using bFGF-activated fibroblasts as vaccines.Citation32 In our study, CD31 immunohistochemistry and alginate inclusion test showed that microvessel densities in lipo-saponins/bFGF and Freund's/bFGF mice decreased significantly (), and antitumor activities were also very apparent in the two groups. Cytotoxicity experiments showed that T lymphocytes from lipo-saponins/bFGF and Freund's/bFGF mice resulted in the lysis of mice vascular endothelial cells MS1 (), indicating that the immunity inhibited effectively the formation of tumor microvessels and further restricted the growth and metastasis of tumor. Moreover, the T cells from lipo-saponins/bFGF and Freund's/bFGF mice also caused the lysis of B16 cells (), together with the significant increases in IFN-γ productions, suggesting that the immunity not only inhibited angiogenesis, but also brought specific cytotoxicity to cancer cells, which seems to have a dual-targeted function.
Angiogenesis is important for the development and metastasis of melanoma,Citation33 and bFGF is closely related to the angiogenesis and spontaneous metastasis of melanoma.Citation34 Previous studies have also confirmed that bFGF is an estimable target for melanoma therapy. Valesky M blocked the expression of bFGF by antisense technology, resulting in apoptosis of melanoma cell and tumor growth inhibition. Citation14 Vacca A demonstrated that monoclonal antibody of bFGF had remarkable antiangiogenic and anticancer activities in mouse B16 melanoma model.Citation35 Plum SM vaccinated mice with heparin binding domain peptide or receptor binding domain peptide of bFGF, leading to inhibition of tumor angiogenesis and growth.Citation36 Our results also demonstrated that targeting bFGF for melanoma is a correct strategy. In our study, the tumor growth inhibition was induced by the immunity targeting bFGF, and the lung metastasis of cancer cells was also significantly affected. Robust bFGF specific antibody responses were observed in lipo/bFGF mice, lipo-saponins/bFGF and Freund's/ bFGF mice, but obvious anticancer activities appeared only in lipo-saponins/bFGF and Freund's/ bFGF mice, as well as higher IFN-γ productions and effective cytotoxic T cell killing in the two groups, these data suggested that the anti-cancer activities may depend mainly on cellular immunity and humoral immunity may play an auxiliary role.
In terms of side effects of the saponins-based vaccines, Our experience was that the use of large doses of saponins(more than 15µg) could lead to mild hemolysis(contrastive experiments showed that C57BL/6 Mice were more prone to hemolysis than BALB/c mice), but the dose of 5ug we used did not cause hemolysis. Therefore, due to the advantage of saponins in enhancing T lymphocyte toxicity, the adjuvant formulation with a reasonable dose of saponins was a desirable strategy.
In conclusion, our work has shown that the immunity targeting bFGF is very effective in inhibiting melanoma, whether it is solid tumors or lung metastasis. The dual-targeted approach by specific killing of vascular endothelial cells and cancer cells may be a recommendable strategy for tumor immunotherapy, and the adjuvant formulation of saponin-liposome is very desirable in enhancing cytotoxic T lymphocytes response.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- Bikfalvi A, Klein S, Pintucci G, Rifkin DB. Biological roles of fibroblast growth factor-2. Endocrine Reviews. 1997;18(1):26–45. PMID:9034785.
- Dow J K, deVere White R W. Fibroblast growth factor 2: its structure and property, paracrine function, tumor angiogenesis, and prostate-related mitogenic and oncogenic functions. Urology. 2000;55(6):800–806. doi:10.1016/S0090-4295(00)00457-X. PMID:10840080.
- Nugent M A, Iozzo R V. Fibroblast growth factor-2. International Journal of Biochemistry & Cell Biology. 2000;32(3):263. doi:10.1016/S1357-2725(99)00133-8.
- Ornitz D. FGFs, heparan sulfate and FGFRs: complex interactions essential for development. Bioessays. 2000;22(2):108–112.
- Basilico C, Moscatelli D. The FGF family of growth factors and oncogenes. Advances in Cancer Research. 1992;59:115–125. doi:10.1016/S0065-230X(08)60305-X.
- Becker D, Meier C B, Herlyn M. Proliferation of human malignant melanomas is inhibited by antisense oligodeoxynucleotides targeted against basic fibroblast growth factor. Embo J. 1989;8(12):3685–3691. PMID:2684645.
- Wang Y, Becker D. Antisense targeting of basic fibroblast growth factor and dibroblast growth factor receptor-1 in human melanomas blocks intratumoral angiogenesis and tumor growth. Nat Med. 1997;3(8):887–893.
- Shih I M, Herlyn M. Autocrine and paracrine roles for growth factors in melanoma. Vivo. 1994;8(1):113–123.
- Scott G, Stoler M, Sarkar S, Halaban R. Localization of basic fibroblast growth factor mRNA in melanocytic lesions by in situ hybridization. Journal of Investigative Dermatology, 1991;96(3):318–322. doi:10.1111/1523-1747.ep12465203. PMID:2002252.
- Al-Alousi S, Barnhill R, Blessing K, Barksdale S. The prognostic significance of basic fibroblast growth factor in cutaneous malignant melanoma. J Cutan Pathol. 1996;23(6):506–510. doi:10.1111/j.1600-0560.1996.tb01442.x. PMID:9001980.
- Al-Alousi S, Carlson J A, Blessing K, Cook M, Karaoli T, Barnhill RL. Expression of basic fibroblast growth factor in desmoplastic melanoma. J Cutan Pathol. 1996;23(2):118–125. doi:10.1111/j.1600-0560.1996.tb01284.x. PMID:8721445.
- Albino A P, Davis BM, Nanus DM. Induction of Growth Factor RNA Expression in Human Malignant Melanoma: Markers of Transformation. Cancer Res. 1991;51(18):4815–4820. PMID:1716514.
- Halaban R, Kwon B S, Ghosh S, Delli Bovi P, Baird A. bFGF as an autocrine growth factor for human melanomas. Oncogene Res. 1988;3(2):177–186. PMID:3226725.
- Valesky M, Spang AJ, Fisher GW, Farkas DL, Becker D. Noninvasive dynamic fluorescence imaging of human melanomas reveals that targeted inhibition of bFGF or FGFR-1 in melanoma cells blocks tumor growth by apoptosis. Molecular Medicine. 2002;8(2):103–112. PMID:12080186.
- Demana P H, Fehske C, White K, Rades T, Hook S. Effect of incorporation of the adjuvant Quil A on structure and immune stimulatory capacity of liposomes. Immunol Cell Biol. 2004;82(5):547–554. doi:10.1111/j.0818-9641.2004.01276.x.
- Marciani D J, Press J B, Reynolds R C, Pathak AK, Pathak V, Gundy LE, Farmer JT, Koratich MS, May RD. Development of semisynthetic triterpenoid saponin derivatives with immune stimulating activity. Vaccine. 2000;18(27):3141–3151. doi:10.1016/S0264-410X(00)00118-3. PMID:10856794.
- Takahashi H, Takeshfta T, Morein B, Putney S, Germain RN, Berzofsky JA. Induction of CD8+ cytotoxic T cells by immunization with purified HIV-1 envelope protein in ISCOMs. Nature. 1990;344(6269):873–875. doi:10.1038/344873a0. PMID:2184369.
- Soltysik S, Wu J Y, Recchia J, Wheeler DA, Newman MJ, Coughlin RT, Kensil CR. Structure/function studies of QS-21 adjuvant: assessment of triterpene aldehyde and glucuronic acid roles in adjuvant function. Vaccine. 1995, 13(15):1403–1410. doi:10.1016/0264-410X(95)00077-E. PMID:8578817.
- Hong S, Ackerman AL, Virginia C, et al. Enhanced and prolonged cross-presentation following endosomal escape of exogenous antigens encapsulated in biodegradable nanoparticles. Immunology. 2006;117(1):78–88. doi:10.1111/j.1365-2567.2005.02268.x. PMID:16423043.
- Morein F, Bengtsson K L. Functional aspects of iscoms. Immunol Cell Biol. 1998;76(4):295–299. doi:10.1046/j.1440-1711.1998.00756.x.
- Andersen B M, Ohlfest J R. Increasing the efficacy of tumor cell vaccines by enhancing cross priming. Cancer Lett. 2012;325(2):155–164. doi:10.1016/j.canlet.2012.07.012. PMID:22809568.
- Qin HY, Sadelain MW, Hitchon C, Lauzon J, Singh B. Complete Freund's adjuvant-induced T cells prevent the development and adoptive transfer of diabetes in nonobese diabetic mice. J Immunol. 1993;150(5):2072–2080.
- Copland MJ, Rades T, Davies NM. Hydration of lipid films with an aqueous solution of Quil A: a simple method for the preparation of immune-stimulating complexes. Int J Pharm. 2000;196(2):135–139. doi:10.1016/S0378-5173(99)00407-X. PMID:10699704.
- Demana PH, Davies NM, Vosgerau U, Rades T. Pseudo-ternary phase diagrams of aqueous mixtures of Quil A, cholesterol and phospholipid prepared by the lipid-film hydration method. Int J Pharm. 2004, 270(1-2):229–239. doi:10.1016/j.ijpharm.2003.10.020. PMID:14726138.
- Falo LD Jr, Kovacsovicsbankowski M, Thompson K, Rock KL. Targeting antigen into the phagocytic pathway in vivo induces protective tumour immunity. Nat Med. 1995;1(7):649–653. doi:10.1038/nm0795-649. PMID:7585145.
- York IA, Rock K L. Antigen processing and presentation by the class I major histocompatibility complex. Annu Rev Immunol. 1996;14(14):369–396. doi:10.1146/annurev.immunol.14.1.369. PMID:8717519.
- Jain RK, Carmeliet P. SnapShot: Tumor Angiogenesis. Cell. 2012;149(6):1408. doi:10.1016/j.cell.2012.05.025. PMID:22682256.
- Wei YQ, Wang QR, Zhao X, Yang L, Tian L, Lu Y, Kang B, Lu CJ, Huang MJ, Lou YY, et al. Immunotherapy of tumors with xenogeneic endothelial cells as a vaccine. Nat Med. 2000;6(10):1160–1166. doi:10.1038/80506. PMID:11017149.
- Wei Y Q, Huang M J, Yang L, Zhao X, Tian L, Lu Y, Shu JM, Lu CJ, Niu T, Kang B, et al. Immunogene Therapy of Tumors with Vaccine Based on Xenopus Homologous Vascular Endothelial Growth Factor as a Model Antigen. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(20):11545–11550. doi:10.1073/pnas.191112198. PMID:11553767.
- Hori A, Sasada R, Matsutani E, Naito K, Sakura Y, Fujita T, Kozai Y. Suppression of solid tumor growth by immunoneutralizing monoclonal antibody against human basic fibroblast growth factor. Cancer Res. 1991;51(22):6180–184. PMID:1718597.
- Murai N, Ueba T, Takahashi J A, Yang HQ, Kikuchi H, Hiai H, Hatanaka M, Fukumoto M. Apoptosis of human glioma cells in vitro and in vivo induced by a neutralizing antibody against human basic fibroblast growth factor. J Neurosurg. 1996;85(6):1072–1077. doi:10.3171/jns.1996.85.6.1072. PMID:8929497.
- Li X, Wang Y, Zhao Y, Yang H, Tong A, Zhao C, Shi H, Li Y, Wang Z, Wei Y. Immunotherapy of tumor with vaccine based on basic fibroblast growth factor-activated fibroblasts. J Cancer Res Clini Oncol. 2014;140(2):271–280. doi:10.1007/s00432-013-1547-5.
- Mahabeleshwar GH, Byzova TV. Angiogenesis in melanoma. Semin Oncol. 2007;34(6):555–565. doi:10.1053/j.seminoncol.2007.09.009. PMID:18083379.
- Graeven U, Rodeck U, Karpinski S, Jost M, Philippou S, Schmiegel W. Modulation of angiogenesis and tumorigenicity of human melanocytic cells by vascular endothelial growth factor and basic fibroblast growth factor. Cancer Res. 2001;61(19):7282–7290. PMID:11585767.
- Vacca A, Ria R, Ribatti D, Bruno M, Dammacco F. Angiogenesis and tumor progression in melanoma. Recenti Prog Med. 2000;91(11):581–587. PMID:11125952.
- Plum S M, Holaday J W, Ruiz A, Madsen JW, Fogler WE, Fortier AH. Administration of a liposomal FGF-2 peptide vaccine leads to abrogation of FGF-2-mediated angiogenesis and tumor development. Vaccine. 2000;19(9-10):1294–1303. doi:10.1016/S0264-410X(00)00210-3. PMID:11137269.