ABSTRACT
CD5-positive (CD5+) diffuse large B-cell lymphoma (DLBCL) is associated with poor survival compared with CD5-negative DLBCL. The clinical characteristics of CD5+ DLBCL are different from both CD5-negative DLBCL and other CD5+ B cell lymphomas. There is currently no promising chemotherapy for CD5+ DLBCL. Herein, we report a 49-year-old Asian male with refractory CD5+ DLBCL. He complained of aggravated abdominal pain and weight loss. Computed tomography scan revealed abdominal masses, widespread lymphadenopathy, splenomegaly, and intussusception of the ileocecal junction with bowel wall thickening. Core needle aspiration biopsy of an abdominal mass was performed and immunohistochemistry revealed DLBCL of nongerminal center type. In this report, the dose-intensified R-Hyper CVAD (A) regimen as salvage therapy was introduced but failed to result in substantial improvement over the initially standard R-CHOP regimen. Next, the R-GDP regimen was administered as second-line treatment, but only resulted in a partial response. However, the addition of lenalidomide to R-GDP (R2-GDP) resulted in complete remission. The clinical features, pathogenesis, and possible mechanism of action of lenalidomide in CD5+ DLBCL have been described in the literature. The results of the present case report and literature searches indicate that CD5+ DLBCL may share a common pathway with activated B-cell like (ABC) DLBCL as determined by gene expression profiling. Lenalidomide is expected to induce favorable responses in patients with CD5+ DLBCL.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common and heterogeneous B-cell neoplasm. The expression of CD5 by neoplastic cells in DLBCL is rare and predicts poor prognosis. Survival is worse in CD5-positive (CD5+) DLBCL patients than in those with CD5-negative (CD5−) DLBCL. Moreover, the clinical characteristics of CD5+ DLBCL are different from both CD5− DLBCL and other CD5+ B cell lymphomas such as chronic lymphocytic leukemia (CLL), Richter's transformation of CLL, and mantle cell lymphoma (MCL). DLBCL patients with CD5 expression are predominantly older with a female preponderance. Furthermore, these patients typically present with poor performance status, elevated serum lactate dehydrogenase (LDH), advanced disease stage with increased extranodal involvements, high international prognostic index (IPI) score, and frequent central nervous system (CNS) involvement.Citation1
Previous studies have reported that CD5+ DLBCL is associated with poorer outcome than CD5− DLBCL with conventional CHOP chemotherapy.Citation1 Even following the introduction of rituximab, the poor prognosis of DLBCL patients with CD5 expression has not been improved.Citation2-4 The effect of rituximab is only evident in CD5− DLBCL. Despite undergoing transplantations (autologous or allogeneic stem cell transplantations), the overall survival and response rate of patients with CD5+ DLBCL cannot be improved.Citation5-7 To our knowledge, there is currently no promising chemotherapy for the treatment of CD5+ DLBCL. Herein, we report a patient with CD5+ DLBCL who was resistant to R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone) as first-line chemotherapy and the intensive R-Hyper CVAD (A) regimen (rituximab plus cyclophosphamide, vincristine, doxorubicin, and dexamethasone), and who quickly achieved a complete response after the administration of additional lenalidomide to R-GDP (rituximab plus gemcitabine, dexamethasone, cisplatin).
Case report
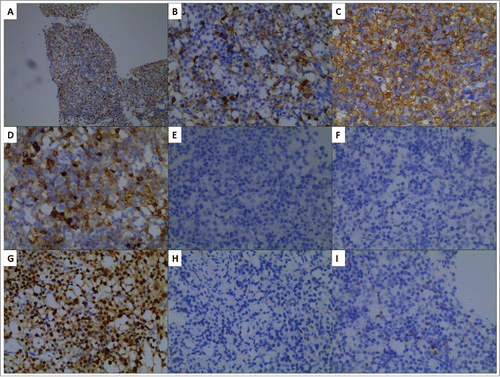
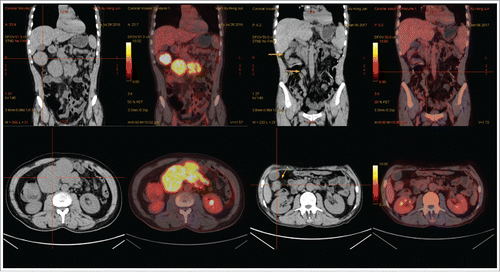
A 49-year-old male presented to the general surgery department of the Affiliated Hospital of Nantong University in July 2016 following 1 month of umbilical pain. He complained of aggravated abdominal pain and weight loss. The patient did not experience night sweats or fever during this period of time. His past medical history was normal. He was not taking any medications and did not drink or smoke. Upon physical examination, the main sign was tenderness in the middle abdomen. Computed tomography (CT) scan of the abdomen revealed abdominal masses, widespread lymphadenopathy, splenomegaly, and intussusception of the ileocecal junction with bowel wall thickening. Laboratory results showed normal ranges for complete blood cell counts, liver function tests, serum LDH, renal function tests, and coagulation tests. The test for hepatitis B virus (HBV) revealed that HBsAg and HBeAg were negative, while HBsAb, HBeAb, and HBcAb were positive. The HBV-DNA test by polymerase chain reaction was negative. Core needle aspiration biopsy of an abdominal mass was performed, and histologic analysis revealed the tissue samples were LCA+, CD20+, CD79a+, CD5+, CD2−, CD3−, CD10−, BCL6−, MUM1+, CD30−, CKpan−, CK18−, FoxP1−, CD38−, CD138−, Cyclin D1− and 40% positive for Ki-67 (). Immunohistochemistry demonstrated the presence of DLBCL of nongerminal center type (non-GCB). Bone marrow smear and biopsy were both normal. Regarding staging, upon positron emission tomography (PET)/CT scan, the patient was shown to have multiple enlarged retroperitoneal lymph nodes, and the largest abdominal mass of 80 × 57 mm with high standard uptake value of 13.0 (). The patient was then diagnosed with DLBCL (stage II, bulky) of non-GCB type, age-adjusted IPI score 1, low-intermediate risk. The traditional R-CHOP regimen was chosen as initial treatment for the patient (CD5+ DLBCL), but there was no response. In August 2016, he developed intestinal obstruction induced by the tumor, which prompted application of the replacement regimen, R-Hyper CVAD (A) chemotherapy. Unexpectedly, the largest abdominal mass still was 77 × 50 mm, core needle biopsy of which was performed again. According to immunohistochemistry, the immunophenotype of the neoplastic cells was identical to those analyzed previously, and MYC expression was negative. We next treated the patient with standard-dose R-GDP, and found that the large abdominal mass reduced in size to 59 × 32 mm after one cycle of R-GDP. However, the abdominal mass was stable in size to 60 × 43 mm after the second cycle of R-GDP. Then, oral lenalidomide 25mg per day on days 1 through 10 in combination with R-GDP (R2-GDP) every 21 days was administered. The patient responded very well to R2-GDP with obvious reduction of the bulky mass. After three cycles of R2-GDP, PET/CT scan was performed and confirmed that complete remission was achieved (). The patient then received the fourth cycle of R2-GDP tailored with lenalidomide (10 mg/day by oral administration on days 1 through 10, every 28 days) as maintenance therapy. Four doses of intrathecal methotrexate, cytarabine, and dexamethasone were administered as CNS prophylaxis. The patient was followed-up every 3 months, and remission was sustained.
Discussion
CD5 is a cell surface glycoprotein, and is mainly expressed by T cells and a small subset of normal B cells. The roles of CD5 in B cells have been investigated. CD5 has an immunoreceptor tyrosine-based inhibitory motif. It inhibits the T cell response and protects B cells from B cell receptor (BCR) signaling-mediated apoptosis. In general, CD5 is observed in the lymphoma cells of patients with CLL and MCL. In addition, CD5 has been shown to be expressed in DLBCL, accounting for 5–10% of all cases of DLBCL.Citation1 Patients with CD5+ DLBCL have aggressive clinical features, poor prognosis, and short survival time compared with patients lacking CD5 expression, indicating that CD5 may regulate specific signaling pathways involved in tumor progression. B cells include B1 cells and B2 cell subsets. B1 cells with constitutive CD5 antigen expression are distinct from B2 cells in terms of gene usage, function, phenotype, and anatomical localization. Multiple studies demonstrated that malignant B cells in CLL belong to the group of B1 cells.Citation8,Citation9 However, newly diagnosed CD5+ DLBCL patients have different clinical characteristics from those of CLL patients. Additionally, CD5 is not only a marker of B1 cells, but is observed in B2 cells after BCR activation. The cellular origin of CD5+ DLBCL remains unknown.Citation10,Citation11
Biological studies can further our understanding of the pathogenesis of CD5+ DLBCL. Immunohistochemical analyses and tests for immunoglobulin mutations in CD5+ DLBCL suggest that it originates from the non-GCB stage of somatically mutated B cells. It was reported that the signaling pathways mediated by CD5 can protect B cells from BCR-mediated apoptosis by producing autocrine interleukin-10, thereby providing a survival factor for B cells.Citation11 This observation was thought to explain how CD5 induced by BCR stimulation can prolong the survival of malignant B cells, thereby playing an important role in B cell lymphoma. Similarly, gene expression profiling (GEP) has shown that CD5+ DLBCL is different from CD5− DLBCL, and is closely correlated with activated B-cell like (ABC) DLBCL.Citation12 GEP was performed for 90 DLBCL patients, including 33 CD5+ and 57 CD5− patients. GEP showed that the top five upregulated genes in CD5+ DLBCL were different from those in CD5− DLBCL (SH3BP5, SLC6A4, USH1C, SFTA2, and ART3 in CD5+ DLBCL and CYP4Z1, CCDC67, MDM2, C2orf69, and ASCC3 in CD5− DLBCL). SH3BP5, the most relevant gene associated with CD5+ DLBCL, was shown to be a signature gene for ABC DLBCL. GEP of CD5+ DLBCL included many genes related to neurological function. Furthermore, the results may provide rational insights into the cause of the high rates of CNS relapse in CD5+ DLBCL.Citation13
Patients with CD5+ DLBCL have poor outcomes, even when treated with R-CHOP or R-CHOP-like chemotherapy (). Currently, no known therapeutic strategies can improve the outcome in these patients. Alinari et al. reported on 102 patients with de novo CD5+ DLBCL who received R-CHOP or R-CHOP-like chemotherapy in a large multicenter study. Twenty-eight patients underwent autologous or allogeneic transplantation. Unfortunately, 71% (20/28) of the patients relapsed post-transplantation.Citation6 The development of a more effective induction strategy is necessary for improving the outcomes of patients with CD5+ DLBCL. In the present report, a dose-intensified cytotoxic regimen was introduced. However, R-Hyper-CVAD (A) therapy failed to provide substantial improvement over the standard R-CHOP regimen. Next, the R-GDP regimen was administered as second-line treatment, and induced only a partial response. Given that most cases of CD5+ DLBCL are ABC DLBCL, we believe that therapeutic strategies for ABC DLBCL may also be effective for the treatment of CD5+ DLBCL.
Table 1. Outcomes of CD5+ DLBCL treated with rituximab containing chemotherapy.
Lenalidomide, a potent immunomodulatory agent, has been shown to be effective for treating several cases of ABC DLBCL by increasing natural killer (NK) cell number, and by enhancing NK cell-mediated cytotoxicity and antibody-dependent cellular cytotoxicity (ADCC). It also plays an important role in the tumor microenvironment by upregulating the expression of interferon-γ, tumor necrosis factor-α, and perforin.Citation14,Citation15 Based on experimental studies, lenalidomide was first administrated as a single-agent, and demonstrated potent activity in patients with relapsed/refractory indolent or aggressive non-Hodgkin's lymphoma, including CLL, MCL, and DLBCL.Citation16 Compared with patients with GCB DLBCL, lenalidomide resulted in improved responses in those with ABC DLBCL. The antineoplastic mechanism of lenalidomide in the ABC subtype was shown to be related to BCR-dependent NF-κB activity by downregulating interferon regulatory factor 4.Citation17 Combinations of lenalidomide with other treatments have been investigated. It was reported that lenalidomide enhanced rituximab-induced apoptosis by enhancing Fc-receptor signaling, and contributed to ADCC by enhancing Fas ligand and granzyme B expression after rituximab binding to the Fc-receptor.Citation18 Moreover, the preclinical mechanistic rationales of combination treatments include elevated NK cell number and activity, activated caspase-3 and caspase-9, arrest of malignant B cells in the G0/G1 phase, and antiangiogenic activity.Citation19 Given its distinct mechanisms, lenalidomide is expected to provide complementary effects with rituximab treatment, and overcome resistance to rituximab.Citation16 According to a recent study, lenalidomide combined with R-CHOP (R2-CHOP) showed promising effects in the treatment of DLBCL, and the addition of lenalidomide appears to improve the poor outcome of ABC patients,Citation15 suggesting lenalidomide may have similar activity in CD5+ DLBCL patients. In this report, the traditional R-CHOP regimen was selected as initial treatment for our CD5+ DLBCL patient, but induced no response. The patient then received the intensive high-dose R-Hyper-CVAD (A) regimen as salvage therapy, although he failed to respond to this treatment as well. However, the patient demonstrated remarkable tumor regression and successfully achieved complete remission in response to the addition of lenalidomide to R-GDP. Regarding the unchangeable adverse outcomes with stem cell transplantation and attractive efficacy of lenalidomide, low-dose lenalidomide was chosen as maintenance therapy for this patient with CD5+ DLBCL.
Conclusion
CD5+ DLBCL has an aggressive clinical course and poor outcome. The case report indicates that the clinical benefit of CD5+ DLBCL may depend on selection of a combined regiment including agents with a novel mechanism of action rather than dose-intensified chemotherapy. In this report, the clinical results highlight the potential activity of lenalidomide for this refractory CD5+ DLBCL patient. However, further studies are required to confirm if lenalidomide and its use in combination is effective for the treatment of CD5+ DLBCL, including longer follow-up time and increased number of observations for this patient.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- Jain P, Fayad LE, Rosenwald A, Young KH, O'Brien S. Recent advances in de novo CD5+ diffuse large B cell lymphoma. Am J Hematol. 2013;88:798–802. doi:10.1002/ajh.23467. PMID:23695956.
- Ennishi D, Takeuchi K, Yokoyama M, Asai H, Mishima Y, Terui Y, Takahashi S, Komatsu H, Ikeda K, Yamaguchi M, et al. CD5 expression is potentially predictive of poor outcome among biomarkers in patients with diffuse large B-cell lymphoma receiving rituximab plus CHOP therapy. Ann Oncol. 2008;19:1921–6. doi:10.1093/annonc/mdn392. PMID:18573805.
- Hyo R, Tomita N, Takeuchi K, Aoshima T, Fujita A, Kuwabara H, Hashimoto C, Takemura S, Taguchi J, Sakai R, et al. The therapeutic effect of rituximab on CD5-positive and CD5-negative diffuse large B-cell lymphoma. Hematol Oncol. 2010;28:27–32. PMID:19358143.
- Niitsu N, Okamoto M, Tamaru JI, Yoshino T, Nakamura N, Nakamura S, Ohshima K, Nakamine H, Hirano M. Clinicopathologic characteristics and treatment outcome of the addition of rituximab to chemotherapy for CD5-positive in comparison with CD5-negative diffuse large B-cell lymphoma. Ann Oncol. 2010;21:2069–74. doi:10.1093/annonc/mdq057. PMID:20231297.
- Miyazaki K, Yamaguchi M, Suzuki R, Kobayashi Y, Maeshima AM, Niitsu N, Ennishi D, Tamaru JI, Ishizawa K, Kashimura M, et al. CD5-positive diffuse large B-cell lymphoma: a retrospective study in 337 patients treated by chemotherapy with or without rituximab. Ann Oncol. 2011;22:1601–7. doi:10.1093/annonc/mdq627. PMID:21199885.
- Alinari L, Gru A, Quinion C, Huang Y, Lozanski A, Lozanski G, Poston J, Venkataraman G, Oak E, Kreisel F, et al. De novo CD5+ diffuse large B-cell lymphoma: Adverse outcomes with and without stem cell transplantation in a large, multicenter, rituximab treated cohort. Am J Hematol. 2016;91:395–9. doi:10.1002/ajh.24299. PMID:26800311.
- Thakral B, Medeiros LJ, Desai P, Lin P, Yin CC, Tang G, Khoury JD, Hu S, Xu J, Loghavi S, et al. Prognostic impact of CD5 expression in diffuse large B-cell lymphoma in patients treated with rituximab-EPOCH. Eur J Haematol. 2017;98:415–21. doi:10.1111/ejh.12847. PMID:28039906.
- Griffin DO, Holodick NE, Rothstein TL. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+ CD27+ CD43+ CD70. J Exp Med. 2011;208:67–80. doi:10.1084/jem.20101499. PMID:21220451.
- Rosen A, Bergh AC, Gogok P, Evaldsson C, Myhrinder AL, Hellqvist E, Rasul A, Björkholm M, Jansson M, Mansouri L, et al. Lymphoblastoid cell line with B1 cell characteristics established from a chronic lymphocytic leukemia clone by in vitro EBV infection. Oncoimmunology. 2012;1:18–27. doi:10.4161/onci.1.1.18400. PMID:22720208.
- Taniguchi M, Oka K, Hiasa A, Yamaguchi M, Ohno T, Kita K, Shiku H. De novo CD5+ diffuse large B-cell lymphomas express VH genes with somatic mutation. Blood. 1998;91:1145–51. PMID:9454743.
- Gary-Gouy H, Harriague J, Bismuth G, Platzer C, Schmitt C, Dalloul AH. Human CD5 promotes B-cell survival through stimulation of autocrine IL-10 production. Blood. 2002;100:4537–43. doi:10.1182/blood-2002-05-1525. PMID:12393419.
- Xu-Monette ZY, Tu M, Jabbar KJ, Cao X, Tzankov A, Visco C, Nagarajan L, Cai Q, Montes-Moreno S, An Y, et al. Clinical and biological significance of de novo CD5+ diffuse large B-cell lymphoma in Western countries. Oncotarget. 2015;6:5615–33. doi:10.18632/oncotarget.3479. PMID:25760242.
- Miyazaki K, Yamaguchi M, Imai H, Kobayashi K, Tamaru S, Kobayashi T, Shiku H, Katayama N. Gene expression profiling of diffuse large B-Cell lymphomas supervised by CD5 expression. Int J Hematol. 2015;102:188–94. doi:10.1007/s12185-015-1812-2. PMID:26009281.
- Semeraro M, Vacchelli E, Eggermont A, Galon J, Zitvogel L, Kroemer G, Galluzzi L. Trial Watch: Lenalidomide-based immunochemotherapy. Oncoimmunology. 2013;2:e26494. doi:10.4161/onci.26494. PMID:24482747.
- Nowakowski GS, LaPlant B, Macon WR, Reeder CB, Foran JM, Nelson GD, Thompson CA, Rivera CE, Inwards DJ, Micallef IN, et al. Lenalidomide combined with R-CHOP overcomes negative prognostic impact of non-germinal center B-cell phenotype in newly diagnosed diffuse large B-Cell lymphoma: a phase II study. J Clin Oncol. 2015;33:251–7. doi:10.1200/JCO.2014.55.5714. PMID:25135992.
- Garciaz S, Coso D, Schiano de Colella JM, Bouabdallah R. Lenalidomide for the treatment of B-cell lymphoma. Expert Opin Investig Drugs. 2016;25:1103–16. doi:10.1080/13543784.2016.1208170. PMID:27414850.
- Zhang LH, Kosek J, Wang M, Heise C, Schafer PH, Chopra R. Lenalidomide efficacy in activated B-cell-like subtype diffuse large B-cell lymphoma is dependent upon IRF4 and cereblon expression. Br J Haematol. 2013;160:487–502. doi:10.1111/bjh.12172. PMID:23252516.
- Wu L, Adams M, Carter T, Chen R, Muller G, Stirling D, Schafer P, Bartlett JB. lenalidomide enhances natural killer cell and monocyte-mediated antibody-dependent cellular cytotoxicity of rituximab-treated CD20+ tumor cells. Clin Cancer Res. 2008;14:4650–7. doi:10.1158/1078-0432.CCR-07-4405. PMID:18628480.
- Gribben JG, Fowler N, Morschhauser F. Mechanisms of Action of Lenalidomide in B-Cell Non-Hodgkin Lymphoma. J Clin Oncol. 2015;33:2803–11. doi:10.1200/JCO.2014.59.5363. PMID:26195701.