ABSTRACT
Purpose: To investigate the influence of mutation abundance and sites of epidermal growth factor receptor (EGFR) on therapeutic efficacies of EGFR-tyrosine kinase inhibitor (EGFR-TKIs) treatments of patients with advanced non-small cell lung carcinoma (NSCLC).
Methods: EGFR mutational sites and mutation abundance were analyzed by amplification refractory mutation system (ARMS) in paraffin-embedded tissue sections taken from primary or metastatic tumors of 194 NSCLC patients.
Results: The median progression-free survival (PFS) time of the enrolled patients was 9.3 months (95% CI, 8.2–10.8 months). The PFS was significantly different with EGFR gene mutation abundance after EGFR-TKI therapy (P = 0.014). The median PFS was significantly longer when the cut-off value of EGFR mutation abundance of exon 19 or exon 21, and solely exon 19 was > 26.7% and 61.8%, respectively. For patients who received EGFR-TKI as first-line treatment, the median PFS was significantly longer in the high mutation abundance group than in the low mutation abundance group (12.7 vs 8.7 months, P = 0.002).
Conclusion: The PFS benefits were greater in patients with a higher abundance of exon 19 deletion mutations in the EGFR gene after EGFR-TKI treatment and first line EGFR-TKI treatment led to improved PFS in high mutation abundance patients.
Introduction
About 80% of lung cancer is the non-small cell lung carcinoma (NSCLC) type with adenocarcinoma occuring in more than 50% of NSCLC patients.Citation4,Citation14 Epidermal growth factor receptor (EGFR) mutations are predominantly found in adenocarcinomas and mutations in the tyrosine kinase coding region of EGFR were found to be significantly associated with the therapeutic effects of EGFR-TKI.Citation20,Citation25,Citation8,Citation10,Citation34
However, the exon 19 deletion and the L858R mutation in exon 21, the two common sites of EGFR mutations, showed a great difference in the response to EGFR-TKI. The therapeutic efficacy of EGFR-TKI in patients with exon 19 mutations was superior to that in patients with exon 21 mutations.Citation13,Citation17,Citation26 Clinical trial analysis revealed that after oral EGFR-TKI therapy, patients with an exon 19 deletion showed significantly longer PFS than with conventional chemotherapy, and the overall survival (OS) of patients with exon 19 deletions was also significantly longer compared with chemotherapy (31.7 months vs 20.7 months), while the OS of patients with exon 21 mutations (L858R) was comparable to the chemotherapy (22.1 months vs 26.9 months) group.Citation31
Mutations in exon 19 and exon 21 were the “quality” changes in the EGFR gene. With more studies on EGFR mutation and wide clinical application of EGFR-TKI, “quantity” changes of the EGFR gene mutation on the therapeutic efficacy of EGFR-TKI came to the attention of researchers. Here, the “quantity” changes indicated the abundance of mutations. “Mutation abundance” was first proposed by comparing the EGFR gene mutation status in the same specimen via sequencing and using an amplification refractory mutation system (ARMS). A previous study showed, that after EGFR-TKI therapy, the PFS of patients with high mutation abundance was significantly higher than for patients with low mutation abundance (11.3 months vs 6.9 months, P = 0.014),Citation35 a result which further confirmed that mutation abundance can be considered to be a reliable predictor of PFS during EGFR-TKI therapy.Citation2,Citation33
To further explore the exact relationship between EGFR mutation abundance and the therapeutic efficacy of EGFR-TKI, we retrospectively reviewed data from patients with advanced lung adenocarcinoma, who underwent EGFR mutation abundance testing and we analyzed the influence of mutation abundance and mutational sites of EGFR on patients' PFS.
Patients and methods
The ethical committee of the Henan Cancer Hospital approved this study and written informed consent was obtained from all patients.
Patient eligibility
We retrospectively investigated the medical records of lung adenocarcinoma patients who underwent EGFR mutation and mutation abundance examinations in tumor tissues by ARMS from January 2013 to December 2014 in the Henan Cancer Hospital, a tertiary academic establishment. Additional inclusion criteria were: patients with locally advanced or stage IIIB/IV NSCLC; histopathological examination showing lung adenocarcinoma; ARMS analysis showing sensitive site mutations on the 19 or 21 exons of the EGFR gene, with conclusive mutation abundance data; oral EGFR-TKI therapy was used at any time throughout the course of the disease consisting of the 3 first generation EGFR-TKI drugs Gefitinb (250 mg QD), Erlotinib (150 mg QD) and Icotinib 125 mg TID; regular doctor reviews during EGFR-TKI administration. The meaning of a regular doctor review is that patients accepted chest and abdomen CT, brain MRI and other imaging examinations every 2–3 months to evaluate the potential therapeutic effects on target or non-target lesions. Patients were excluded with mutations on both exon 19 and exon 21 of the EGFR gene or the wild type EGFR gene. Information on gender, age, smoking history, EGFR mutational sites and mutation abundance, and treatment with EGFR-TKI were collected for patients who met the above criteria.
Procedures
EGFR mutational sites and mutation abundance were analyzed by ARMS in paraffin-embedded tissue sections taken from primary or metastatic tumors of eligible patients. For mutation abundance detection, lung tumor tissue in each slice required >50%, and metastatic lymph nodes or other metastases lesions in each slice required >20% changes. Genomic DNA was extracted with a Qiagen 56404 QIAamp DNA FFPE tissue kit (Qiagen Bioscience Co. Ltd., Hilden, Germany). The DNA concentration was measured by a broad-spectrum ultraviolet spectrometer and adjusted to 20–50 ng/mL. DNA samples were stored at −20°C. EGFR mutations were detected using a Human EGFR gene mutation quantitative detection kit for 45 hot genes (Beijing ACCB Biotech Ltd, China) by fluorescence PCR. PCR was performed using a Stratagene Mx3000P real-time PCR system (Agilent Technologies, Santa Clara, USA).
The abundance of the wild type and mutant EGFR genes in the specimens was calculated according to standard curves after the EGFR wild type and mutant alleles were individually amplified. The relative mutation rate was calculated according to the following equation: relative mutation abundance = mutant gene concentration / (mutant gene concentration + wild type gene) concentration) × 100%.Citation33 The primary endpoint of this study was to determine the association between PFS and mutation abundance or mutation sites of eligible patients after EGFR-TKI therapy. PFS was the time from starting administration of oral EGFR-TKI to disease progression. The secondary endpoints included the objective response rate, the association between PFS and age, sex, and whether EGFR-TKI was administered as the first-line treatment. The therapeutic effect of EGFR-TKI was evaluated according to the Response Evaluation Criteria In Solid TumorsCitation6 with Complete Response (CR), disappearance of all target lesions; Partial Response (PR): with at least a 30% decrease in the sum of the longest diameter of target lesions with the baseline sum of the longest diameter as the reference; Progressive Disease (PD) as the ratio between the longest diameter sum of the target lesion and the longest diameter of the smallest target lesion was recorded after starting administration of oral EGFR-TKI, and increased by at least 20% or the presence of one or more new lesions. Objective response rate (ORR) was defined as achieving CR or PR.
Statistical analysis
All statistical analyzes were performed using SPSS for Windows (Version 17.0, SPSS Inc., Chicago, IL, USA). Categorical variables are reported as numbers and percentages, and continuous variables are reported as the mean ± standard deviation. Categorical variables were compared using a chi-square test. Normally distributed continuous variables were subjected to an analysis of variance, and those lacking a normal distribution were compared using the Wilcoxon rank sum test.
Kaplan-Meier and Cox survival regression models were used to analyze the influence of age, gender and other general factors as well as EGFR mutation abundance on survival and prognosis.
ROC curve analysis was used to determine the optimal critical value of mutation abundance diagnosis and the area under the curve (AUC) was calculated. The level of statistical significance was set at P < 0.05.
Results
Characteristics of the enrolled patients
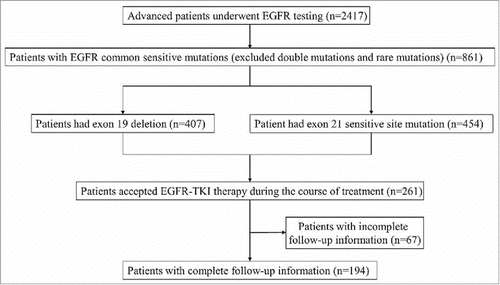
A total of 2,417 patients diagnosed with NSCLC and with an EGFR gene mutation were analyzed with ARMS and collated. Among these patients, 861 had exon 19 deletions (407) or exon 21-sensitive site mutations (454). Of these, 261 patients with stage IIIB/IV lung adenocarcinomas received first generation EGFR-TKI treatment. A total of 194 patients, with regular doctor reviews during EGFR-TKI administration and complete follow-up data, were included in the final analysis ().
The median age was 57 years (range, 26–84 years); of these, 59.8% (116/194) were women, 26.8% had a smoking history, and 58.8% were over 60 years old. There was no significant difference between the EGFR exon 19 deletion patients' distribution ratio (50.5%) and the EGFR exon 21 mutation patients' distribution ratio (49.5%) in 194 patients ().
Table 1. Baseline demographic and clinical characteristics.
A total of 102 patients received the first generation EGFR-TKI drug orally as first-line treatment, and the remaining 92 patients were treated with the same drug as the second line or other treatments. The majority of the patients were pure adenocarcinoma pathological types (190, 97.9%) and in stage IV of TNM (157, 80.9%), as well as with 0–2 ECOG scores (173, 89.2%) during the course of the EGFR-TKI therapy ().
PFS correlated with EGFR mutation abundance or mutation sites after EGFR-TKI therapy
Up to the third of December 2015 after follow-up, 145 of 194 patients treated with the first generation of oral EGFR-TKI drug exhibited disease progression. The median follow-up time was 18.1 months (range, 7.1–33.0 months), with an overall disease control rate of 89.2%. The median PFS was 9.3 months, and the data completed rate was 74.5% (). With further Kaplan-Meier analysis, we found that the PFS duration was related to EGFR mutation abundance, the longest PFS times, 14.8 months, being when the EGFR mutation abundance ≥30% and < 40% (6.70–22.67), with the shortest PFS time being (when the EGFR mutation abundance was <10%) 5.53 months (1.68–9.39). There was a significant difference between the PFS duration in the different EGFR mutation abundance groups (P = 0.014) (). Although the median PFS was increased initially with an increase in the mutation abundance, the median PFS did not continue to increase when the mutation abundance increased more than 40%, and even showed a downward trend (Supplementary Table 1). We respectively tried to use 10%, 20%, 30%, 40% or 50% as the cutoff values to analyze the medium PFS of the high and low abundance groups. The results also revealed that when 10%, 20% or 30% were used as the cutoff values, the medium PFS achieved a statistically significant therapeutic effect in >10%, 20%, 30% compared to <10%, 20% and 30% mutation abundances, respectively. The medium PFS was most significant (10.8 months vs 8.2 months) when a mutation abundance value of 30% was used as the cutoff value (Supplementary Table 1).
Table 2. The association between PFS and mutation abundance after EGFR-TKI therapy.
Risk factor analysis of PFS after EGFR-TKI therapy
Next, we conducted univariate analysis for the general characteristics of age, gender, smoking history, EGFR mutation sites, EGFR mutation abundance and EGFR-TKI treatment as first line or second line by Kaplan-Meier survival analyses. The results showed that these factors were not associated with the median PFS after EGFR-TKI therapy (). In contrast we found that EGFR mutation abundance was associated with the PFS, when EGFR mutation abundance was ≥30%, which was a risk factor for PFS (HR: 1.64, 95% CI: 1.17– 2.31).
Table 3. Risk factors to PFS by univariate analysis.
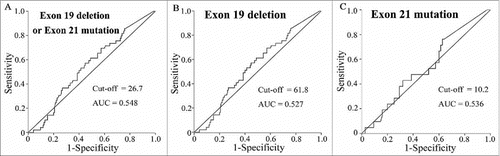
Further, we estimated the cut-off values of EGFR exon 19 deletion or exon 21 mutation abundance groups, as well as only EGFR exon 19 deletion mutation and only EGFR exon 21 mutation abundance by ROC analysis in 194 patients. We found that the cut-off values for them were 26.7, 61.8% and 10.2% and the areas under the curve (AUC) were 0.548 (0.459–0.637) for EGFR exon 19 deletion or exon 21 mutation, 0.527 (0.407–0.646) for solely EGFR exon 19 deletion and 0.536 (0.402–0.670) for solely exon 21 mutations, respectively (). When the cut-off value of EGFR exon 19 deletion or exon 21 mutation abundance group was >26.7%, or EGFR exon 19 deletion mutation abundance was >61.8%, the median of PFS was significantly longer, while exon 21 mutation abundance had no significant effect on PFS differences whether the cut-off value was >10.2% or <10.2% (, ). In addition, we found that the ORRs were higher in high abundance (>26.7%), than in (≤26.7%) low abundance EGFR mutation patients (79.6% vs 45.5%, P < 0.01) and patients with exon 19 deletions had higher ORRs than patients with exon 21 mutations (76.5% vs 46.9%, P < 0.01) (). Also after adjusting for age, gender and other factors in a COX regression survival analysis, EGFR mutation abundance >26.7% of exon 19 deletions and exon 21 mutations kept a significant cut-off value (P = 0.0016) ().
Table 4. The cut-off value of EGFR mutation abundance to the PFS by ROC analysis.
Table 5. Cox regression survival analysis of correlations between EGFR mutation abundance cut-off values and PFS by after adjusting the influence of age, gender and other general factors.
PFS of patients with different treatment lines of EGFR-TKI correlated with EGFR mutation abundance
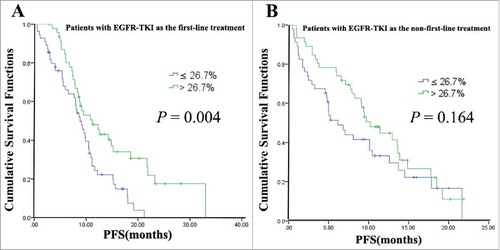
To explore further whether EGFR-TKI applied as first-line and non-first-line treatment would affect survival, first-line and non-first-line treatment of EGFR-TKI were used as stratification factors to analyze the effect of mutation abundance on patient survival. Our data showed that for patients given EGFR-TKI as their first-line treatment, the median PFS was significantly longer in the high mutation abundance group than in the low mutation abundance group (12.7 vs 8.7 months), and the difference was statistically significant (P = 0.002). However, for patients with EGFR-TKI as the non-first-line treatment, there was no difference in the median PFS between the high and low mutation abundance groups (9.7 vs 6.6 months, P = 0.430) ().
The distribution of EGFR mutation sites was related with mutation abundance in all 861 patients
Among the 194 patients included in the final analysis, 98 patients had exon 19 mutations and 96 patients had 21 exon mutations, which was similar to the distribution ratio of the EGFR mutation sites in the initial 861 patients (exon 19 deletion vs exon 21 mutation; 407 vs 454). The proportion of patients with mutations in exon 21 in low abundance EGFR mutations patients was higher than that in patients with EGFR exon 19 deletion mutations. With the increasing likelihood of EGFR mutation abundance, the likelihood of mutations in exon 21 became less and deletion mutations in exon 19 were associated with an increase in EGFR mutation abundance as determined by a chi-square test derived trend ().
Table 6. The distribution of EGFR mutation sites and mutation abundance in 861 patients.
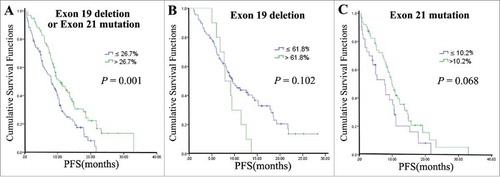
Furthermore, we analyzed the influence of high and low mutation abundance levels (26.7% as the cutoff value) on the median PFS, using the EGFR mutation site as a stratification factor (control factor) in a COX survival analysis model. The analysis revealed that the median PFS was significantly different between the high and low mutation abundance groups with an exon 19 deletion or an exon 21 mutation (P = 0.001). However, this difference was not apparent between the high and low mutation abundance groups with an exon 19 deletion (P = 0.102) and an exon 21 mutation (P = 0.068) ( , , ).
Discussion
Identifying EGFR gene mutations is becoming a routine test for every NSCLC patient, since EGFR-TKIs have significant effects only in patients with sensitive mutationsCitation19,Citation23 while exon 19 deletion and a single point mutation in exon 21 of the EGFR gene account for about 85% of all EGFR mutations in NSCLC patients.Citation15
Although direct sequencing is thought to be a classic method for EGFR mutation testing,Citation20,Citation25 ARMS has been demonstrated to have a high sensitivity of 0.1% to 1% and wide eligibility for different sample types, especially samples with only a few tumor cellsCitation7,Citation16 or pleural effusion fluid.Citation35 An alternative approach for detecting circulating tumor DNA (ctDNA) in patients with advanced lung adenocarcinoma has been droplet digital PCR (ddPCR).Citation32
The results of our study showed that the patients' median PFS was extended with an increase of EGFR mutation abundance up to 40%, but then decreased again; this phenomenon has not been reported in previous studies. In order to establish an accurate cutoff value for distinguishing EGFR mutant abundance and thus providing useful evidence for clinical practice, stratified analyses were further conducted by combining the median PFS data of patients given EGFR-TKI orally. The data in this study showed that 26.7% was the best cutoff value to separate the low and high EGFR mutant abundance. We found that after EGFR-TKI therapy, the ORR and PFS in patients with an exon 19 deletion were significantly better than those with an exon 21 mutation, which was consistent with data reported in previous studies,Citation12,Citation13,Citation17,Citation26,Citation27 although another study noted that though exon 19 mutations were predictive for longer PFS after EGF-TKI therapy the ORR and OS between patients with the two mutation sites showed no significant difference,Citation30 which might be explained by the relative small sample size and the different population included in the former study. However, other studies did not find any difference in the clinical efficacy of gefitinib regarding exon 19 and exon 21 EGFR mutationsCitation11,Citation22,Citation24 and it has been reported that the disease control rate and the median PFS of patients with exon 19 deletion and exon 21 mutations were not significantly different (82.8% vs 67.3%, 11.5 months vs 10.8 months).Citation21
One reason for the missing PFS difference might have been that the therapeutic efficacy of EGFR-TKI was affected by first-line treatment or non-first-line treatments. The first-line EGFR-TKI treatment PFSs of patients with abundant EGFR mutations were longer than non-first-line treatments (second line treatment or others) in our study (), which is supported by a previous report.Citation18 This difference may be due to the presence of induced tumor heterogeneity, leading to the coexistence of mutant and wild type tumor cells within the same tumor sample, as well as changes to EGFR-independent signaling, since another study found that chemotherapy could cause changes in EGFR mutation rates, which mainly originate from switches of the mutant to the wild type EGFR genotype. Therefore, the proportion of patients with mutations may be reduced after first-line platinum-based chemotherapy, leading to a lower efficacy rate and a shorter median PFS of second-line or other lines of EGFR-TKI treatments than first-line EGFR-TKI treatments.Citation1,Citation3
However, it is possible that the difference in the median PFS between the two mutation sites may be due to some differences in spatial conformation between the two mutations,Citation9 since these two mutations cause different phosphorylations of EGFR thus resulting in differences in downstream signaling.Citation28
In the present study, we found that the proportion of patients with a high mutation abundance was higher in the exon 19 deletion group and that there was a very low percentage (27/59, 46%) of first-line EGFR-TKI treatment. However, the proportion of patients with a high mutation abundance was lower in the exon 21 mutation group and there was a high percentage (14/25, 56%) of first-line EGFR-TKI treatment. Therefore, the distribution of mutation abundance and first-line treatment or non-first-line treatment of EGFR-TKI in patients with an exon19 deletion and an exon 21 mutation were different. This may have diluted the impact of mutational sites on the median PFS of patients.
In general, studies on the influence of EGFR gene mutation abundance and mutational sites on the therapeutic efficacy of EGFR-TKI in patients with advanced lung adenocarcinoma are mostly retrospective analyses. The conclusions from the various studies are however not consistent, therefore prospective trials with rigorous design are necessary to verify the purported conclusions. To the best of our knowledge, there are no OS data from studies that used EGFR mutation sites as the primary research objective. Hence, patients with exon 19 deletions or exon 21 mutations should be separated as two different groups to be studied in future investigations. Based on the current data and clinical guidelines, the availability and cost-effectiveness of EGFR-TKI for Chinese patients, the first generation of EGFR-TKIs were effective for patients with sensitive mutations, but because Osimertinib, a third-generation EGFR-TKI, which is approved in China since March 2017 showed a superior efficacy against NSCLC than standard EGFR-TKIs, it should be considered as alternative treatment particularly for EGFR mutation–positive cases.Citation29
A limitation of the present study is that according to existing literature the ARMS technology has in contrast to more sensitive methods the limitation to differentiate effectively between wild-type and mutant DNACitation5 with a sensitivity of 0.1% to 1%, which is less than 0.04% achieved by ddPCR.Citation32
Conclusions
Our study suggests that the PFS of patients was closely related with the mutation abundance of the EGFR gene after treatment with EGFR-TKI. First-line treatment with EGFR-TKI in patients with high EGFR mutation abundance achieved more benefits in terms of PFS, while 26.7% was the best cutoff value to separate the low and high EGFR mutant abundance. Therefore, EGFR mutation abundance might be used as a predictor for the extent of the response to EGFR-TKI therapy.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
2017CBT10825R-s02.doc
Download MS Word (30.5 KB)Acknowledgments
This work was supported by the Wu Jieping Medical Foundation.
References
- Bai H, Wang Z, Chen K, Zhao J, Lee JJ, Wang S, Zhou Q, Zhuo M, Mao L, An T, et al. Influence of chemotherapy on EGFR mutation status among patients with non-small-cell lung cancer. J Clin Oncol. 2012;30:3077–83. doi:10.1200/JCO.2011.39.3744. PMID:22826274.
- Beltran H, Rickman DS, Park K, Chae SS, Sboner A, MacDonald TY, Wang Y, Sheikh KL, Terry S, Tagawa ST, et al. Molecular characterization of neuroendocrine prostate cancer and identification of new drug targets. Cancer discovery. 2011;1:487–95. doi:10.1158/2159-8290.CD-11-0130. PMID:22389870.
- Chin TM, Quinlan MP, Singh A, Sequist LV, Lynch TJ, Haber DA, Sharma SV, Settleman J. Reduced Erlotinib sensitivity of epidermal growth factor receptor-mutant non-small cell lung cancer following cisplatin exposure: a cell culture model of second-line erlotinib treatment. Clin Cancer Res. 2008;14:6867–76. doi:10.1158/1078-0432.CCR-08-0093. PMID:18980981.
- Cox JD, Scott CB, Byhardt RW, Emami B, Russell AH, Fu KK, Parliament MB, Komaki R, Gaspar LE. Addition of chemotherapy to radiation therapy alters failure patterns by cell type within non-small cell carcinoma of lung (NSCCL): analysis of radiation therapy oncology group (RTOG) trials. Int J Radiat Oncol Biol Phys. 1999;43:505–9. doi:10.1016/S0360-3016(98)00429-5. PMID:10078629.
- Dang RK, Anthony RS, Craig JI, Leonard RC, Parker AC. Limitations of the use of single base changes in the p53 gene to detect minimal residual disease of breast cancer. Mol Pathol. 2002;55:177–81. doi:10.1136/mp.55.3.177. PMID:12032228.
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47. doi:10.1016/j.ejca.2008.10.026. PMID:19097774.
- Ellison G, Donald E, McWalter G, Knight L, Fletcher L, Sherwood J, Cantarini M, Orr M, Speake G. A comparison of ARMS and DNA sequencing for mutation analysis in clinical biopsy samples. J Exp Clin Cancer Res. 2010;29:132. doi:10.1186/1756-9966-29-132.
- Fukuoka M, Wu YL, Thongprasert S, Sunpaweravong P, Leong SS, Sriuranpong V, Chao TY, Nakagawa K, Chu DT, Saijo N et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J ClinOncol. 2011;29:2866–74. doi:10.1200/JCO.2010.33.4235. PMID:21670455.
- Gazdar AF, Minna JD. Inhibition of EGFR signaling: all mutations are not created equal. PLoS medicine. 2005;2:e377. doi:10.1371/journal.pmed.0020377. PMID:16288556.
- Han JY, Park K, Kim SW, Lee DH, Kim HY, Kim HT, Ahn MJ, Yun T, Ahn JS, Suh C et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol. 2012;30:1122–8. doi:10.1200/JCO.2011.36.8456. PMID:22370314.
- Igawa S, Kasajima M, Ishihara M, Kimura M, Hiyoshi Y, Asakuma M, Otani S, Katono K, Sasaki J, Masuda N. Comparison of the efficacy of gefitinib in patients with non-small cell lung cancer according to the type of epidermal growth factor receptor mutation. Oncology. 2014;87:215–23. doi:10.1159/000362603. PMID:25034225.
- Jackman DM, Miller VA, Cioffredi LA, Yeap BY, Janne PA, Riely GJ, Ruiz MG, Giaccone G, Sequist LV, Johnson BE. Impact of epidermal growth factor receptor and KRAS mutations on clinical outcomes in previously untreated non-small cell lung cancer patients: results of an online tumor registry of clinical trials. Clin Cancer Res. 2009;15:5267–73. doi:10.1158/1078-0432.CCR-09-0888. PMID:19671843.
- Jackman DM, Yeap BY, Sequist LV, Lindeman N, Holmes AJ, Joshi VA, Bell DW, Huberman MS, Halmos B, Rabin MS et al. Exon 19 deletion mutations of epidermal growth factor receptor are associated with prolonged survival in non-small cell lung cancer patients treated with gefitinib or erlotinib. Clin Cancer Res. 2006;12:3908–14. doi:10.1158/1078-0432.CCR-06-0462. PMID:16818686.
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. PMID:21296855.
- Jiang J, Wang C, Yu X, Sheng D, Zuo C, Ren M, Wu Y, Shen J, Jin M, Xu S. PCR-sequencing is a complementary method to amplification refractory mutation system for EGFR gene mutation analysis in FFPE samples. Exp Mol Pathol. 2015;99:581–9. doi:10.1016/j.yexmp.2015.10.002. PMID:26477713.
- Kimura H, Fujiwara Y, Sone T, Kunitoh H, Tamura T, Kasahara K, Nishio K. High sensitivity detection of epidermal growth factor receptor mutations in the pleural effusion of non-small cell lung cancer patients. Cancer Sci. 2006;97:642–8. doi:10.1111/j.1349-7006.2006.00216.x. PMID:16827805.
- Lee CK, Wu YL, Ding PN, Lord SJ, Inoue A, Zhou C, Mitsudomi T, Rosell R, Pavlakis N, Links M et al. Impact of Specific Epidermal Growth Factor Receptor (EGFR) mutations and clinical characteristics on outcomes after treatment With EGFR tyrosine kinase inhibitors versus chemotherapy in EGFR-Mutant lung cancer: A meta-analysis. J Clin Oncol. 2015;33:1958–65. doi:10.1200/JCO.2014.58.1736. PMID:25897154.
- Liang Y, Ma R. Comparison of therapeutic efficacy of gefitinib as first – and second- line therapies for patients with EGFR mutated advanced non – small cell lung cancer. J. 2014;22:2091–4.
- Linardou H, Dahabreh IJ, Bafaloukos D, Kosmidis P, Murray S. Somatic EGFR mutations and efficacy of tyrosine kinase inhibitors in NSCLC. Nat Rev Clin Oncol. 2009;6:352–66. doi:10.1038/nrclinonc.2009.62. PMID:19483740.
- Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39. doi:10.1056/NEJMoa040938. PMID:15118073.
- Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–8. doi:10.1056/NEJMoa0909530. PMID:20573926.
- Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–8. doi:10.1016/S1470-2045(09)70364-X. PMID:20022809.
- Mok T, Yang JJ, Lam KC. Treating patients with EGFR-sensitizing mutations: first line or second line–is there a difference? J Clin Oncol. 2013;31:1081–8. doi:10.1200/JCO.2012.43.0652. PMID:23401448.
- Morita S, Okamoto I, Kobayashi K, Yamazaki K, Asahina H, Inoue A, Hagiwara K, Sunaga N, Yanagitani N, Hida T et al. Combined survival analysis of prospective clinical trials of gefitinib for non-small cell lung cancer with EGFR mutations. Clin Cancer Res. 2009;15:4493–8. doi:10.1158/1078-0432.CCR-09-0391. PMID:19531624.
- Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–500. doi:10.1126/science.1099314. PMID:15118125.
- Riely GJ, Pao W, Pham D, Li AR, Rizvi N, Venkatraman ES, Zakowski MF, Kris MG, Ladanyi M, Miller VA. Clinical course of patients with non-small cell lung cancer and epidermal growth factor receptor exon 19 and exon 21 mutations treated with gefitinib or erlotinib. Clin Cancer Res. 2006;12:839–44. doi:10.1158/1078-0432.CCR-05-1846. PMID:16467097.
- Rosell R, Moran T, Queralt C, Porta R, Cardenal F, Camps C, Majem M, Lopez-Vivanco G, Isla D, Provencio M et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361:958–67. doi:10.1056/NEJMoa0904554. PMID:19692684.
- Sordella R, Bell DW, Haber DA, Settleman J. Gefitinib-sensitizing EGFR mutations in lung cancer activate anti-apoptotic pathways. Science. 2004;305:1163–7. doi:10.1126/science.1101637. PMID:15284455.
- Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, Dechaphunkul A, Imamura F, Nogami N, Kurata T et al. osimertinib in untreated egfr-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378:113–25. doi:10.1056/NEJMoa1713137. PMID:29151359.
- Won YW, Han JY, Lee GK, Park SY, Lim KY, Yoon KA, Yun T, Kim HT, Lee JS. Comparison of clinical outcome of patients with non-small-cell lung cancer harbouring epidermal growth factor receptor exon 19 or exon 21 mutations. J. Clin. Pathol. 2011;64:947–52. doi:10.1136/jclinpath-2011-200169. PMID:21725039.
- Yang JC, Wu YL, Schuler M, Sebastian M, Popat S, Yamamoto N, Zhou C, Hu CP, O'Byrne K, Feng J et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 2015;16:141–51. doi:10.1016/S1470-2045(14)71173-8. PMID:25589191.
- Yang X, Zhuo M, Ye X, Bai H, Wang Z, Sun Y, Zhao J, An T, Duan J, Wu M et al. Quantification of mutant alleles in circulating tumor DNA can predict survival in lung cancer. Oncotarget. 7;2016:20810–24. PMID:26989078.
- Zhao ZR, Wang JF, Lin YB, Wang F, Fu S, Zhang SL, Su XD, Jiang L, Zhang YG, Shao JY et al. Mutation abundance affects the efficacy of EGFR tyrosine kinase inhibitor readministration in non-small-cell lung cancer with acquired resistance. Med. Oncol. 2014;31:810. doi:10.1007/s12032-013-0810-6. PMID:24338271.
- Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, Zhang S, Wang J, Zhou S, Ren S et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011a;12:735–42. doi:10.1016/S1470-2045(11)70184-X.
- Zhou Q, Zhang XC, Chen ZH, Yin XL, Yang JJ, Xu CR, Yan HH, Chen HJ, Su J, Zhong WZ et al. Relative abundance of EGFR mutations predicts benefit from gefitinib treatment for advanced non-small-cell lung cancer. J Clin Oncol. 2011b;29:3316–21. doi:10.1200/JCO.2010.33.3757.