ABSTRACT
Background: Regulatory T cells(Tregs) and myeloid-derived suppressor cells(MDSCs) represent two immunosuppressive cell populations that are important in the establishment and maintenance of cancer immune tolerance. MDSCs can express IDO and promote immune tolerance via expansion of Treg cell.Method: We use needle biopsy breast cancer tissues prior to neoadjuvant chemotherapy(NCT) staining for CD33, Foxp3 and IDO by immunohistochemistry to evaluate whether they were correlated with subsequent treatment responses in breast cancer.Results: Expressions of IDO, CD33+MDSCs and Foxp3+Tregs were correlated with each other. Immunohistochemical double staining revealed that IDO expression in CD33+MDSCs was positively correlated with Foxp3+Tregs (P < 0.05). CD33+MDSCs, Foxp3+Tregs, and IDO expression in tumor tissues were associated with advanced clinical stage prior to NCT (P < 0.05). CD33+MDSCs, Foxp3+Tregs, IDO expression, IDO expression in CD33+MDSCs and clinical T3–T4 stage prior to NCT, pathological T3–T4 stage, ER(+), luminal type were correlated with clinical responses of PD+SD (P < 0.05). Multivariate analysis showed that CD33+MDSCs, IDO expression, IDO expression in CD33+MDSCs, and advanced pathological T stage were risk factors for PD+SD. Focusing on the pCR of NCT, only CD33+MDSCs, clinical T3–T4, and N1–N3 stage prior to NCT were associated with no-pCR (P < 0.05). The multivariate analysis showed that advanced clinical T stage and N stage were risk factors for no-pCR. Clinical stage prior to NCT were significantly correlated with progression free survival (P = 0.021), while Foxp3+Tregs and clinical T stage were significantly correlated with overall survival (P = 0.022 and P = 0.001, respectively). Foxp3+Treg was significant risk factor for overall survival after adjusting covariates by COX regression.Conclusion: Tumor-infiltrating MDSCs, Tregs, IDO expression and IDO expression in MDSCs were correlated with clinicopathological features, NCT response, and prognosis of breast cancer patients, suggesting that they might be potential markers for clinical outcomes of NCT and help clinical decision-making for improved therapies for breast cancer.
Introduction
The breast cancer treatment decision making depends on various factors, including disease stage, patient age hormone receptor etc. For patients with poor prognosis and high recurrence risk, more aggressive or radical treatments are proposed. Patients diagnosed with high-risk non-metastatic breast cancer, including locally advanced and inflammatory breast cancer, are usually treated with upfront chemotherapy, in the form of neoadjuvant chemotherapy (NCT) followed by surgery. This treatment has been demonstrated to decrease tumor burden and increase breast conservation rate.Citation1,Citation2 Simultaneously, as the response to NCT can be evaluated, there is possibility to modify regimens to increase rates of pathological complete response (pCR); thus, the primary tumor response serves as an in vivo chemosensitivity testCitation2; therefore, this treatment has wide applications.
Regulatory T cell (Tregs) and myeloid derived suppressor cells (MDSCs) represent two immunosuppressive cell populations that play an important role in the establishment and maintenance of tumor immune escape. MDSCs are heterogeneous cells composed of myeloid cells blocked at many steps in differentiation. These cells were observed to be enriched in the peripheral blood, bone marrow, tumor tissues, lymphatic drainage areas, and cancer metastases of cancer patients.Citation3-Citation6 These cells are characterized by their ability to inhibit both innate and adaptive immune reactions, thus exerting a suppressive effect on antitumor immunity. In humans, markers for MDSCs currently are not unified until now. However, these cells are proposed characterized by Lin−CD33+CD11b+HLA-DR− recently.Citation7 In our previous study, we found that the MDSCs characterized by CD45+CD13+CD33+CD14−CD15− were significantly increased in blood and tumor tissues,Citation8 whereas in vitro, MDSCs characterized by CD45+CD13+CD33+CD14−CD15− could be induced by MDA-MB-231 breast cancer cells when co-cultured with CD33+ myeloid cells from healthy donors.
Tumor-infiltrating Tregs stably express the transcription factor fork head box protein 3 (Foxp3) and down-regulate immune responses, which is often associated with poor clinical outcomes.Citation9 In patients with malignant tumors, increased level of peripheral Tregs before therapy has been reported to be correlated with shorter progression free survival (PFS), whereas elevated Treg in the blood and tumor tissues of patients with ovarian carcinoma, non-small cell lung carcinoma (NSCLC), and hepatocellular carcinoma are associated with poorer prognosis and an higher recurrence risk.Citation10-Citation12
The mechanisms by which tumors promote the expansion and/or function of these suppressive cells and cross-talks between them are remain unclear. Recent studies demonstrated a distinct correlation between indoleamine 2, 3-dioxygenase(IDO), a rate-limiting enzyme of tryptophan catabolism, and tumor-induced immunosuppression.Citation13,Citation14 It was reported that MDSCs could promote immunosuppression via Treg expansion which is induced by IDO expression in MDSCs.Citation15 It was reported that IDO exerts its suppressive effects through a reduction in local tryptophan and the generation of cytotoxic metabolites at tumors and lymphatic drainage areas to block the initiation of Ag-specific immune responses, the antitumor cytotoxicity of activated T cells, and to increase Tregs.Citation16-Citation18
In our previous study of breast cancer, we observed increased IDO expression in tumor tissues, which was associated with increased Foxp3+Treg cells in tumor situ and metastatic lymph nodes. Further, IDO was essential for the immunosuppressive effect of MDSCs on T cells; this effect could be abolished by IDO inhibitors or STAT3 antagonists.Citation8 Then, in study of 2014, we confirmed that breast cancer cell-induced MDSCs upregulated IDO expression via STAT3-stimulated activation of the non-canonical NF-κB pathway.Citation19
Chemotherapy can enhance antitumor immunity through a series of different mechanisms. Previous studies found that chemotherapy exerted antitumor effects through cytotoxic effects against tumor cells, in addition to the elimination or inactivation of immunosuppressive cells such as Tregs and MDSCs.Citation20,Citation21 McCoy et al. found that patients with low number of stromal Tregs were nearly five times more likely to achieve pCR.Citation22 Romano et al. reported that circulating MDSC subsets were higher in non-responders than responders in patients with Hodgkin lymphoma after chemotherapy.Citation23 Muller et al. reported that the combination of IDO inhibition and chemotherapy effectively promoted the shrinkage of refractory breast cancers.Citation24 An alternate hypothesis was suggested, whereby fewer suppressive immune cells in the local tumor environment prior to treatment allow effective chemotherapy via inducing antitumor immunity. In this study, we presented an evaluation of Foxp3+Treg, CD33+MDSC density and IDO expression in pre-treatment diagnostic biopsy samples, with the aim of determining whether pre-NCT infiltrating MDSC density, Treg cell density, IDO expression, and IDO expression in MDSCs were correlated with the subsequent treatment response and survival.
Methods
Patients
Fifty-four women with breast cancer were enrolled in this study. The inclusion criteria of patients were: (1) a diagnosis of advanced breast cancer from the Department of Breast Oncology of Tianjin Medical University Cancer Institute and Hospital between December 1, 2010, and June 30, 2011; (2) histological confirmed breast cancer for all patients and complete information on preoperative immunohistochemistry, including estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor-2 (Her-2), p53, and Ki67, were obtained by coarse needle biopsy and postoperative histological information, which were both reviewed based on the 7th AJCC Cancer Staging System; (3) patients were administered different cycles of planned-dose neoadjuvant chemotherapy in accordance with a multi-agent chemotherapy protocol; (4) patients were subjected to mastectomy or lumpectomy in conjunction with either biopsy of ipsilateral sentinel lymph node or axillary resection after the evaluation of the curative effect of NCT and reached R0 resection with a negative surgical margin.
We enrolled 54 patients with a solitary breast tumor in this study; representative formalin-fixed blocks from each coarse needle biopsy samples (at least three tissue cylinders) were selected for immunohistochemical staining. The clinical stages prior to NCT were clinical T1 (3 cases), T2 (20 cases), T3 (20 cases), T4 (11 cases), N0 (13 cases), N1 (16 cases), N2 (18 cases), and N3 (7 cases) and clinical stage II (14 cases), III (38 cases), and IV (2 cases). The pathological stages after surgical resection were pT0 (2 cases), T1 (28 cases), T2 (13 cases), T3 (6 cases), T4 (5 cases) and N0 (9 cases), N1 (16 cases), N2 (13 cases), N3 (16 cases), and stages I (2 cases), II (16 cases), III (34 cases), and IV (2 cases).
The Ethics Committee of Tianjin Cancer Institute and Hospital approved this research project and written consent was obtained from each patient.
Pathologic evaluation
Core biopsy samples and surgical specimens were evaluated in accordance with the Chinese Breast Cancer Guideline. The initial core biopsy samples of the primary tumor were evaluated by standard hematoxylin and eosin (H&E) staining, immunohistochemistry(IHC), and fluorescence or chromogenic in situ hybridization (FISH/CISH) (or both) to determine the histological subtype, the Ki67 index, and the status of ER, PR, and Her-2. The cut-off values for ER, PR, and Ki67 were 10%, 10%, and 20%, respectively.Citation25 Her-2 was scored for intensity and completeness (-, no staining; +, partial weak membranous staining in more than 10% of cancer cells; ++, moderate complete membrane staining in ≥10% cancer cells or strong complete membranous staining in ≤10% of cancer cells; +++, strong complete membranous staining in >10% of cancer cells). Staining at the +++ level was deemed as positive and fluorescence in situ hybridization (FISH) was applied in tumors with ++ staining.Citation26
Immunohistochemistry for IDO, CD33, and Foxp3
Fifty-four tissue samples from breast cancer patients were obtained by coarse needle biopsy prior to NCT, fixed in formaldehyde, and embedded in paraffin wax blocks. Serially cut sections of 4-μm thickness were generated. The first and last sections from each block were stained with H&E for pathological examination. Others were selected and subjected to immunohistochemical staining in accordance with manufacturer's instruction manuals, as previously reported.Citation27 Each tissue sample was analyzed in 5 sections at least. The tissue sections were incubated with mouse anti-human IDO (Millipore, Billerica, MA), Foxp3 (eBioscience, San Diego, CA), and rabbit anti-human CD33 (Abcam, San Francisco, CA) antibodies (Abs) overnight at 4 °C, and then with goat anti-mouse or anti-rabbit IgG secondary Abs conjugated with streptavidin-HRP (Santa Cruz). The tissue sections were developed by a diaminobenzidine staining kit (Maixin Biotechnology, Fuzhou, China) and observed by an Olympus BX51 microscope (Olympus, Tokyo, Japan). Mouse/rabbit isotype IgG1 was used as the negative controls.
For immunohistochemical double staining, primary Abs against CD33 and IDO stain were performed by the DouSP double-stain kit (Maxim-Bio, Fuzhou City, China), based on the instruction manual. The second Abs were HRP-conjugated anti-mouse IgG and alkaline phosphatase (ALP) conjugated anti-rabbit IgG Abs. IDO+ cells were stained red in the cytoplasm by 3-amino-9-ethylcarbazole as substrates for HRP. CD33+ cells were stained blue on membrane by 5-bromo-4-chloro-3-indolyl phosphate/NBT as substrate for ALP. The sections were then counterstained with hematoxylin. All sections were observed under an Olympus BX51 microscope.
The levels of positive staining were evaluated by both staining rate (SR) and index (SI). The SR was the percentage of positive cells and defined as an expression score between 0 and 3 (0, <5% of tumor cells were stained; 1, 5–30% were stained; 2, 30–70% were stained; 3, >70% were stained). The SI was accessed based on average of ≥5 high-powered fields (400 × magnification) and scored between 0 and 3 (0, no staining; 1, mild staining; 2, moderate staining; 3, strong staining). The sum of these two parts was designated as the last score. Negative (−) and positive (+) expression was defined as the last score ≤3 and last score >3, respectively. All samples were reviewed by experienced pathologists who were blinded to the identity of all specimens.
Response to NCT
The clinical response was evaluated by physical examination, mammography, and ultrasonography, based on the Response Evaluation Criteria in Solid Tumors (RECIST 1.1). The clinical complete response (CR) was the disappearance of all known lesions; a clinical partial response (PR) was shrinkage of ≥30% in the sum of the longest diameter of the lesion; progressive disease (PD) was defined as a ≥20% rise in the sum of the longest diameter of the primary lesion; and stable disease (SD) was defined as insufficient shrinkage to PR or no rise to PD.Citation28
In line with the criteria of the National Surgical Adjuvant Breast and Bowel Project B-18,Citation29 pCR was no invasive carcinoma in the breast at the time of surgery.
Statistical analyses
For categorical variables, the Chi-squared and Fisher's exact tests were used to compare the differences; Spearman's rank correlation test was used to analyze the correlations. Chi-squared and Fisher's exact tests were also applied for the univariate analysis of the factors with significant difference among different responders; the factors that had significant difference in univariate analysis were included in the multivariate logistic regression analysis. The survival curves were analyzed and compared by Kaplan-Meier method and Log-rank test. COX regression was applied to get adjusted hazard ratios for prognosis. P<0.05 were considered statistically significant. All statistical analyses were computed by using SPSS 17.0 software.
Results
The association between tumor-infiltrating CD33+MDSCs, Foxp3+Tregs, IDO expression, and IDO expression in CD33+MDSCs
Foxp3 infiltration staining to quantify Tregs and CD33+ staining to quantify infiltrating MDSCs were used because, in our previous study, we demonstrated that the majority (82.3 ± 3.1%) of CD33+ myeloid cells isolated from breast tumor tissues displayed the characteristic phenotype of CD45+CD13+CD33+CD14+CD15+.Citation8 The expression of CD33 (), IDO (), Foxp3 () in tumor tissues was shown in , in which the negative staining of normal breast tissues was considered to the negative control (NC). In , CD33 expression was dispersed on membrane of normal cells between cancer cell nests and few in cytoplasm; showed IDO in cancer tissues, which was mainly expressed in tumor cells and some dispersed in stroma, with brownish yellow staining in cytoplasm; showed that Foxp3 was expressed in lymphocytes and dispersed in stroma with brownish dark yellow staining in nucleus. The co-expression of IDO and CD33 in tumor tissues, as determined by a double-staining method, was shown in . The double-positive cells (IDO+CD33+MDSCs) were red on membrane (CD33) and purple in cytoplasm (IDO).
Figure 1. The association between tumor-infiltrating CD33+MDSC, Foxp3+Treg, IDO expression, and IDO expression in CD33+MDSCs. (a1) CD33 in cancer tissues was expressing in normal cells between cancer cell nests dispersed on membrane and few in cytoplasm in cancer tissues (× 400, red arrows); (a2) negative expression of CD33 in breast cancer tissues (× 400); (a3) negative expression of CD33 in normal breast tissues, which were set as the negative control (NC); (b1) IDO in cancer tissues mainly expressed in tumor cells and some karyocytes dispersed in stroma with brownish yellow staining in cytoplasm (× 400, red arrows); (b2) negative expression of IDO in breast cancer tissues (× 400); (b3) negative control; (c1) in breast cancer tissues, Foxp3 expression in lymphocytes dispersed in stroma with brownish dark yellow staining in nucleus (× 400, red arrows); (c2) negative expression of Foxp3 in breast cancer tissues (× 400); (c3) negative control; (d) co-expression of IDO and CD33 in tumor tissues was determined using immunohistochemical double-staining method; (d1) IDO and CD33 negative staining of breast cancer tissues; (d2) CD33 positive and IDO negative cells (IDO−CD33+) were stained red on membrane (red arrows); (d3) IDO positive and CD33 negative cells (IDO+CD33−) were stained purple in cytoplasm (red arrows); (d4) IDO+CD33+MDSCs were stained red on membrane and purple in cytoplasm (× 400, red arrows); (e1) from the total of 54 patients, 33 patients were positive for CD33+MDSCs, 27 patients were positive for IDO expression (IDO positive, orange; negative, blue), and 22 patients were both positive for CD33+MDSCs and IDO expression. By Spearman's rank correlation test, it was found that CD33+MDSCs were positively correlated with IDO expression (r2 = 0.418, P = 0.002); (e2) 31 patients were positive for Foxp3+Tregs (Foxp3+Treg positive, orange; negative, blue), 33 patients were positive for CD33+MDSCs, 24 patients were positive for both CD33+MDSCs and Foxp3+Tregs. By Spearman's rank correlation test, it was found that CD33+MDSCs were positively correlated with Foxp3+Tregs (r2 = 0.388, P = 0.004); (e3) 31 patients were positive for Foxp3+Tregs, 27 patients were positive for IDO expression, 22 patients were positive for both Foxp3+Treg and IDO expression (IDO positive, orange; negative, blue). By Spearman's rank correlation test, it was found that IDO expression was positively correlated with Foxp3+Tregs (r2 = 0.487, P = 0.000); (f) 31 patients were positive for Foxp3+Tregs, 22 patients were positive for IDO expression in CD33+MDSCs, 20 patients were positive for both Foxp3+Tregs and IDO expression in CD33+MDSCs. By Spearman's rank correlation test, IDO+CD33+MDSCs were positively correlated with Foxp3+Tregs (r2 = 0.528, P = 0.000).
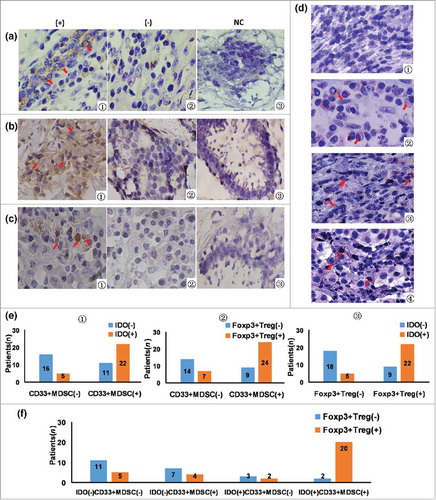
As shown in , IDO expression, CD33+MDSCs, and Foxp3+Tregs were correlated with each other. CD33+MDSCs in tumor tissues were positively correlated with IDO expression (r2 = 0.418, P = 0.002) and Foxp3+Tregs (r2 = 0.388, P = 0.004). IDO expression was positively correlated with Foxp3+Tregs (r2 = 0.487, P = 0.000). showed that IDO+CD33+MDSCs were positively correlated with Foxp3+Tregs (r2 = 0.528, P = 0.000).
The association between tumor-infiltrating CD33+MDSC, Foxp3+Treg, IDO expression, IDO expression in CD33+MDSC, and clinicopathological features
We analyzed the association between tumor-infiltrating CD33+MDSCs, Foxp3+Tregs, IDO expression, and the clinicopathological features of breast cancer patients. CD33+MDSCs (P = 0.000), Foxp3+Tregs (P = 0.001), and IDO expression (P = 0.005) were associated with advanced clinical T stage. The positivity of IDO expression and Foxp3+Treg were more frequently expressed in patients with breast cancer at the N1–N3 stage (P = 0.051 and P = 0.054), as shown in .
Table 1. The association between tumor-infiltrating CD33+MDSC, Foxp3+Treg, IDO expression and clinicopathological features prior NCT.
We analyzed IDO/CD33 co-expression to confirm the association between IDO expression in CD33+MDSCs and the clinicopathological features prior to NCT. The IDO+ expression in CD33+MDSCs was correlated with advanced clinical T stage (P = 0.000) and ER(+) (P = 0.031, ).
Table 2. The association between co-expression of IDO/CD33 and clinicopathological features prior NCT.
The association between tumor-infiltrating CD33+MDSC, Foxp3+Treg, IDO expression, IDO expression in CD33+MDSC, and response to NCT
In and, the univariate analysis of clinicopathological features with respect to the patients’ responses to NCT was shown, whereas showed the univariate analysis of immunological features with respect to the patients’ responses to NCT.
Table 3. Univariate analysis of clinicopathological features with respect to breast cancer patients’ response to NCT.
Table 4. Univariate analysis of immunological features with respect to breast cancer patients’ response to NCT.
Positive results for tumor infiltrating Foxp3+Tregs (P = 0.047), CD33+MDSCs (P = 0.023), IDO expression (P = 0.024), IDO expression in CD33+MDSCs (P = 0.014) and clinical T3–T4 stage (P = 0.047), pathological T3–T4 stage (P = 0.015), ER positivity (P = 0.015), and luminal type or Her-2 and Basal-like (P = 0.044) were correlated with PD+SD in univariate analysis. In this study, P < 0.05 was considered to be statistically significant; however, the statistical significance of Foxp3+Treg (P = 0.044), clinical T stage (P = 0.047), and luminal type (P = 0.047) were borderline. Therefore, we excluded these three factors in the multivariate analysis. Finally, we included CD33+MDSCs, IDO expression, IDO expression in CD33+MDSCs, pathological stage, and ER(+) in multivariate logistic regression analysis. These results showed that CD33+MDSCs [OR: 56.843(3.000–1077.076), P = 0.007], IDO expression [OR: 26.743(1.365–523.973), P = 0.030], IDO expression in CD33+MDSCs [OR: 145.553(2.831–7483.474), P = 0.013], and advanced pathological T stage [OR: 14.287(1.933–105.574), P = 0.009] were risk factors for PD+SD ().
Table 5. Multivariate analysis of immunological and clinicopathological features with respect to breast cancer patients’ clinical response to NCT.
When we concentrated on the pCR of NCT, only the positive expression of CD33+MDSCs (P = 0.035), clinical T3–T4 stage (P = 0.012), and clinical N1–N3 stage (P = 0.048) were associated with no-pCR in the univariate analyses ( and ). These significant features were then included in the multivariate logistic regression analysis, which showed that advanced clinical T stage [OR: 10.322(1.109–96.078), P = 0.040] and clinical N stage [OR: 7.366(1.111–48.852), P = 0.039] were significant risk factors for no-pCR ().
Table 6. Multivariate analysis of immunological and clinicopathological features with respect to breast cancer patients’ pathological response to NCT.
Prognostic analysis
In the survival analysis, clinical stage prior NCT was significantly correlated with PFS (P = 0.021, ), clinical T3–T4 stage() and Foxp3+Treg ( and ) were significantly correlated with shorter overall survival (OS, P = 0.001 and P = 0.022, respectively). However, patients positive for CD33+MDSCs ( and ), IDO expression ( and ), and IDO expression in CD33+MDSCs ( and ) inclined to poorer prognosis. The 1, 3 and 5 year survival rates of breast cancer patients according to all immunological features, clinicopathological features and therapies were shown in supplementary . In , we used COX regression to adjust covariates, including Clinical T stage, Clinical N stage, Clinical stage, Pathological T stage, Pathological N stage, Pathological stage, Histological type, ER status, PR status, Her-2, Luminal type, Clinical response, pathological response, surgery method, post-operative chemotherapy, post-operative radiation therapy, post-operative hormone therapy, to get adjusted hazard ratios for prognosis. Foxp3+Treg was significant risk factors for overall survival [HR: 34107.438(17.192∼664331.905), P = 0.007].
Figure 2. The survival curves for breast cancer patients treated by NCT. (a) PFS curves arranged by clinical stage (P = 0.021); (b) OS curves arranged by clinical T stage (P = 0.001); (c) PFS curves arranged by tumor-infiltrating CD33+MDSCs (P = 0.225); (d) OS curves arranged by tumor-infiltrating CD33+MDSCs (P = 0.121); (e) PFS curves arranged by IDO expression (P = 0.378); (f) OS curves arranged by IDO expression (P = 0.508); (g) PFS curves arranged by tumor-infiltrating Foxp3+Tregs (P = 0.979); (h) OS curves arranged by tumor-infiltrating Foxp3+Tregs (P = 0.022); (i) PFS curves arranged by IDO expression in CD33+MDSCs (P = 0.354); (j) OS curves arranged by IDO expression in CD33+MDSCs (P = 0.523).
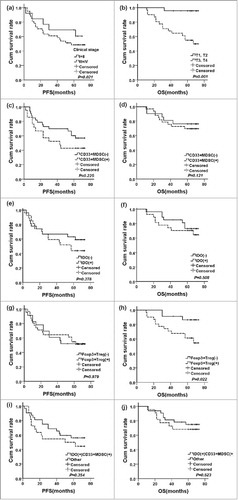
Table 7. COX regression for prognostic significance of immunological features for breast cancer patients.
Discussion
MDSCs and Tregs play a key role in immunosuppressive networks and contribute to tumor immune toleration. Owing to the multiple factors and the multiple steps of immunoregulation that contribute to the complicate immunoregulatory mechanisms in the development of cancer, each patient had different immune characteristics based on different pathologies, including tumor progression, stage, and basic disease. However, previous studies had demonstrated that elevated MDSCs and Tregs were associated with advanced disease stage and poor prognosis in various malignancies.Citation4-Citation6,Citation30,Citation31 Both MDSCs and Tregs were also confirmed to be correlated with chemotherapy outcomes in various malignant cancers.Citation20
Mizukoshi et al. found that MDSCs prior to treatment were significantly lower in patients who achieved CR and PR by hepatic arterial infusion chemotherapy than in those SD or PD.Citation32 MDSCs <30.5% was an important prognostic factor for longer survival after hepatic arterial infusion chemotherapy. In the study of Kawano et al., the G-CSF-induced increase of MDSCs in situ contributed to the chemoresistance of cervical cancer with G-CSF producing tumors,Citation33 and the elimination of MDSCs sensitized cervical cancers to cisplatin. Romano et al. showed that all MDSC subsets were elevated in patients with Hodgkin lymphoma compared with healthy controls and were higher in non-responders treated upfront.Citation23 In our study, tumor-infiltrating CD33+MDSCs were lower in patients who achieved CR+PR and pCR, lower CD33+MDSCs was also related to longer PFS and OS.
In a recent study that focused on Tregs, McCoy et al. found that tumor-infiltrating Tregs prior to NCT for rectal tumors were not correlated with response.Citation34 However, in their previous study in 2016, a low density of Foxp3+ Tregs within the tumor stroma after NCT was shown to be strongly associated with pCR.Citation22 Baras et al. reported that the ratio of CD8+T cells to Tregs in situ was significantly related to a response to NCT in patients with muscle invasive urothelial cancer of the bladder.Citation35 Roselli et al. showed the frequency of Tregs prior to therapy associated with PFS. The Treg of responders was reduced more obviously after first-line FOLFIRI plus bevacizumab therapy in comparison with non-responders.Citation36 Kotsakis et al. found that CD4+Tregs were increased in NSCLC and correlated with the response and prognosis; patients at stage IV who achieved PD in chemotherapy had a significantly elevated initial naive Treg ratio.Citation11 Kim et al. found that, in vivo and vitro, the combination of cisplatin and inhibiting Treg cell migration strengthened the anti-cancer outcome in comparison with a single treatment.Citation37 In our study, Foxp3+Tregs were also associated with CR+PR in univariate analysis, which was in agreement with the studies mentioned above. However, in multivariate logistic regression analysis, this association was not confirmed. In prognostic analysis, the Foxp3+Tregs were significantly correlated with a shorter OS.
Limagne et al. reported that both Treg and MDSC accumulation in metastatic colorectal cancer were also correlated with a poor outcome.Citation7 Previous reports have suggested that MDSCs may contribute to Treg induction in cancer.Citation38 In a study of human fibrocytic MDSCs, Zoso et al. found that MDSCs that expressed IDO and enhanced immune suppression by Treg cell expansion.Citation15 IDO is an important mechanism by which effector T cells can be induced and converted to Tregs. The IDO-mediated depletion of tryptophan and subsequent production of immunosuppressive products such as kynurenine may also lead to T cell suppression through the down-regulation of the TCR-CD3-ζ chain.Citation13,Citation14,Citation16 So, IDO exerts its suppressive effects via blocking the initiation of Ag-specific immune response and antitumor cytotoxicity of activated T cells, and to increase Tregs.Citation17,Citation18 In our previous study, we also found that IDO expression was significantly up-regulated in MDSCs and this higher expression was associated with increased Foxp3+Tregs. Breast cancer-induced MDSCs can up-regulate IDO expression via STAT3-stimulated activation of the non-canonical NF-κB pathway.Citation19 The inhibition of IDO by 1-MT can significantly attenuate MDSC-regulated immunosuppression of T cell proliferation and Th1 polarization, which suggests that the immunosuppressive activity of MDSCs was IDO-dependent.Citation8 In the cancers that highly express IDO, MDSCs are an important cell group in promoting cancer growth and immunotherapy resistance.Citation39 In this study, we found that CD33+MDSCs and IDO expression were positively correlated with Foxp3+Treg, respectively. IDO expression in CD33+MDSCs showed a significant positive correlation with Foxp3+Tregs. When focused on the clinical aspects, higher IDO expression in MDSCs were correlated with advanced clinical T stage and ER(+), poorer survival, and more frequent achievement of patients in PD+SD.
These findings had many limitations: first, this was a relatively small patient cohort from a single center; thus, these results must be confirmed in larger sample and multi-center studies; second, we applied Foxp3 for labeling Treg, which is commonly used, and used CD33 staining to quantify infiltrating MDSCs based on our previous study. However, we also recognized that Foxp3 and CD33 could also found in activated CD4+T cells and other subgroups of myeloid cells in humans. Additionally, these data are only correlative analyses and further experimental study is required to explore the underlying mechanisms of these correlations.
Based on our study, these positive immunosuppressive features in preoperative evaluation may be suggestive for the need of more aggressive surgical resection and chemotherapy to achieve better therapeutic outcome and survival in cancer treatment. Recently, the inhibition of IDO was applied in a phase II-II clinical trial,Citation24,Citation39 and the immunotherapy target to key checkpoints which also inhibit Tregs and MDSCs had also been entered into clinical trials.Citation40 Therefore, the combination of immunotherapy and chemotherapy may improve the clinical efficacy of chemotherapy. In particular, for patients with these immunosuppressive features, the use of targeted immunotherapy as an adjuvant therapy may be an effective choice to prolong the survival time.
In conclusion, this study proved that tumor-infiltrating Tregs, MDSCs, and IDO expression, and IDO expression in MDSCs were correlated with each other and with clinicopathological features. The tumor-infiltrating Treg was a significant factor for poor OS. These findings suggested that tumor-infiltrating Tregs, MDSCs, and IDO expression were predictable markers for the outcome of NCT and might help clinical decision-making for the delivery of improved therapies for breast cancer.
Declarations
– Ethics approval and consent to participate: This research project was approved by the Ethics Committee of Tianjin Cancer Institute and Hospital. Written consents were obtained from each patient.
– Consent to publish: Consent to publish from the participant to report individual patient data were obtained from each patient.
– Competing interests: The authors have declared that no competing interests exist.
– Funding: The work was supported by National Natural Science Fund of China (81502309).
– Authors' Contributions: Fangxuan Li, Juntian Liu and Lijuan Wei analyzed and interpreted the patient data. Fangxuan Li and Yang Zhao performed the IHC and histological examination of the cancer tissues, and Fangxuan Li was a major contributor in writing the manuscript. Yang Zhao and Shixia Li was analyzed and interpreted and revised the manuscript. All authors read and approved the final manuscript.
2017CBT10826R-s02.docx
Download MS Word (30.9 KB)Additional information
Funding
References
- Mieog JS, van der Hage JA, van de Velde CJ. Preoperative chemotherapy for women with operable breast cancer. Cochrane Database Syst Rev. 2007; 18(2):CD005002. PMID:17443564.
- Kaufmann M, von Minckwitz G, Smith R, Valero V, Gianni L, Eiermann W, Howell A, Costa SD, Beuzeboc P, Untch M, et al. International expert panel on the use of primary (preoperative) systemic treatment of operable breast cancer: review and recommendations. J Clin Oncol. 2003;21(13):2600–8. doi:10.1200/JCO.2003.01.136. PMID:12829681.
- Marvel D, Gabrilovich DI. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J Clin Invest. 2015;125(9):3356–64. doi:10.1172/JCI80005. PMID:26168215.
- Shoji H, Tada K, Kitano S, Nishimura T, Shimada Y, Nagashima K, Aoki K, Hiraoka N, Honma Y, Iwasa S, et al. The peripheral immune status of granulocytic myeloid-derived suppressor cells correlates the survival in advanced gastric cancer patients receiving cisplatin-based chemotherapy. Oncotarget. 2017;8(56):95083–95094. doi:10.18632/oncotarget.18297. PMID:29221113.
- Pergamo M, Miller G. Myeloid-derived suppressor cells and their role in pancreatic cancer. Cancer Gene Ther. 2017;24(3):100–5. doi:10.1038/cgt.2016.65. PMID:27910857.
- Yang G, Shen W, Zhang Y, Liu M, Zhang L, Liu Q, Lu HH, Bo J. Accumulation of myeloid-derived suppressor cells (MDSCs) induced by low levels of IL-6 correlates with poor prognosis in bladder cancer. Oncotarget. 2017;8(24):38378–88. doi:10.18632/oncotarget.16386. PMID:28418913.
- Limagne E, Euvrard R, Thibaudin M, Rébé C, Derangère V, Chevriaux A, Boidot R, Végran F, Bonnefoy N, Vincent J, et al. Accumulation of MDSC and Th17 Cells in Patients with Metastatic Colorectal Cancer Predicts the Efficacy of a FOLFOX-Bevacizumab Drug Treatment Regimen. Cancer Res. 2016;76(18):5241–52. doi:10.1158/0008-5472.CAN-15-3164. PMID:27496709.
- Yu J, Du W, Yan F, Wang Y, Li H, Cao S, Yu W, Shen C, Liu J, Ren X. Myeloid-derived suppressor cells suppress antitumor immune responses through IDO expression and correlate with lymph node metastasis in patients with breast cancer. J Immunol. 2013;190(7):3783–97. doi:10.4049/jimmunol.1201449. PMID:23440412.
- Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6(4):295–307. doi:10.1038/nri1806. PMID:16557261.
- Woo EY, Chu CS, Goletz TJ, Schlienger K, Yeh H, Coukos G, Rubin SC, Kaiser LR, June CH. Regulatory CD4(+)CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61(12):4766–72. PMID:11406550.
- Kotsakis A, Koinis F, Katsarou A, Gioulbasani M, Aggouraki D, Kentepozidis N, Georgoulias V, Vetsika EK. Prognostic value of circulating regulatory T cell subsets in untreated non-small cell lung cancer patients. Sci Rep. 2016;6:39247. doi:10.1038/srep39247. PMID:27976733.
- Li X, Peng J, Pang Y, Yu S, Yu X, Chen P, Wang W, Han W, Zhang J, Yin Y, et al. Identification of a FOXP3(+)CD3(+)CD56(+) population with immunosuppressive function in cancer tissues of human hepatocellular carcinoma. Sci Rep. 2015;5:14757. doi:10.1038/srep14757. PMID:26437631.
- Friberg M, Jennings R, Alsarraj M, Dessureault S, Cantor A, Extermann M, Mellor AL, Munn DH, Antonia SJ. Indoleamine 2,3-dioxygenase contributes to tumor cell evasion of T cell-mediated rejection. Int J Cancer. 2002;101(2):151–5. doi:10.1002/ijc.10645. PMID:12209992.
- Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4(10):762–74. doi:10.1038/nri1457. PMID:15459668.
- Zoso A, Mazza EM, Bicciato S, Mandruzzato S, Bronte V, Serafini P, Inverardi L. Human fibrocytic myeloid-derived suppressor cells express IDO and promote tolerance via Treg-cell expansion. Eur J Immunol. 2014;44(11):3307–19. doi:10.1002/eji.201444522. PMID:25113564.
- Lee GK, Park HJ, Macleod M, Chandler P, Munn DH, Mellor AL. Tryptophan deprivation sensitizes activated T cells to apoptosis prior to cell division. Immunology. 2002;107(4):452–60. doi:10.1046/j.1365-2567.2002.01526.x. PMID:12460190.
- Sharma MD, Baban B, Chandler P, Hou DY, Singh N, Yagita H, Azuma M, Blazar BR, Mellor AL, Munn DH. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J Clin Invest. 2007;117(9):2570–82. doi:10.1172/JCI31911. PMID:17710230.
- Curti A, Pandolfi S, Valzasina B, Aluigi M, Isidori A, Ferri E, Salvestrini V, Bonanno G, Rutella S, Durelli I, et al. Modulation of tryptophan catabolism by human leukemic cells results in the conversion of CD25- into CD25+ T regulatory cells. Blood. 2007;109(7):2871–7. PMID:17164341.
- Yu J, Wang Y, Yan F, Zhang P, Li H, Zhao H, Yan C, Yan F, Ren X. Noncanonical NF-kappaB activation mediates STAT3-stimulated IDO upregulation in myeloid-derived suppressor cells in breast cancer. J Immunol. 2014;193(5):2574–86. doi:10.4049/jimmunol.1400833. PMID:25063873.
- Alizadeh D, Larmonier N. Chemotherapeutic targeting of cancer-induced immunosuppressive cells. Cancer Res. 2014;74(10):2663–8. doi:10.1158/0008-5472.CAN-14-0301. PMID:24778417.
- Zheng Y, et al. Using chemo-drugs or irradiation to break immune tolerance and facilitate immunotherapy in solid cancer. Cell Immunol. 2015;294(1):54–9. doi:10.1016/j.cellimm.2015.02.003. PMID:25687508.
- McCoy MJ, Hemmings C, Miller TJ, Austin SJ, Bulsara MK, Zeps N, Nowak AK, Lake RA, Platell CF. Low stromal Foxp3+ regulatory T-cell density is associated with complete response to neoadjuvant chemoradiotherapy in rectal cancer. Br J Cancer. 2015;113(12):1677–86. doi:10.1038/bjc.2015.427. PMID:26645238.
- Romano A, Parrinello NL, Vetro C, Forte S, Chiarenza A, Figuera A, Motta G, Palumbo GA, Ippolito M, Consoli U, et al. Circulating myeloid-derived suppressor cells correlate with clinical outcome in Hodgkin Lymphoma patients treated up-front with a risk-adapted strategy. Br J Haematol. 2015;168(5):689–700. doi:10.1111/bjh.13198. PMID:25376846.
- Muller AJ, DuHadaway JB, Donover PS, Sutanto-Ward E, Prendergast GC. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat Med. 2005;11(3):312–9. doi:10.1038/nm1196. PMID:15711557.
- Ignatiadis M, Buyse M, Sotiriou C. St Gallen International Expert Consensus on the primary therapy of early breast cancer: an invaluable tool for physicians and scientists. Ann Oncol. 2015;26(8):1519–20. doi:10.1093/annonc/mdv259. PMID:26063634.
- Bianchi S, Caini S, Paglierani M, Saieva C, Vezzosi V, Baroni G, Simoni A, Palli D, Tuscany Breast Cancer Study Group. Accuracy and Reproducibility of HER2 Status in Breast Cancer Using Immunohistochemistry: A Quality Control Study in Tuscany Evaluating the Impact of Updated 2013 ASCO/CAP Recommendations. Pathol Oncol Res. 2015;21(2):477–85. doi:10.1007/s12253-014-9852-0. PMID:25367072.
- Yu J, Sun J, Wang SE, Li H, Cao S, Cong Y, Liu J, Ren X. Upregulated expression of indoleamine 2, 3-dioxygenase in primary breast cancer correlates with increase of infiltrated regulatory T cells in situ and lymph node metastasis. Clin Dev Immunol. 2011;2011:469135. doi:10.1155/2011/469135. PMID:22110525.
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47. doi:10.1016/j.ejca.2008.10.026. PMID:19097774.
- Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr. 2001(30):96–102. doi:10.1093/oxfordjournals.jncimonographs.a003469. PMID:11773300.
- Mougiakakos D, Johansson CC, Trocme E, All-Ericsson C, Economou MA, Larsson O, Seregard S, Kiessling R. Intratumoral forkhead box P3-positive regulatory T cells predict poor survival in cyclooxygenase-2-positive uveal melanoma. Cancer. 2010;116(9):2224–33. PMID:20209608.
- Bates GJ, Fox SB, Han C, Leek RD, Garcia JF, Harris AL, Banham AH. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol. 2006;24(34):5373–80. doi:10.1200/JCO.2006.05.9584. PMID:17135638.
- Mizukoshi E, Yamashita T, Arai K, Terashima T, Kitahara M, Nakagawa H, Iida N, Fushimi K, Kaneko S. Myeloid-derived suppressor cells correlate with patient outcomes in hepatic arterial infusion chemotherapy for hepatocellular carcinoma. Cancer Immunol Immunother. 2016;65(6):715–25. doi:10.1007/s00262-016-1837-2. PMID:27083166.
- Kawano M, Mabuchi S, Matsumoto Y, Sasano T, Takahashi R, Kuroda H, Kozasa K, Hashimoto K, Isobe A, Sawada K, et al. The significance of G-CSF expression and myeloid-derived suppressor cells in the chemoresistance of uterine cervical cancer. Sci Rep. 2015;5:18217. doi:10.1038/srep18217. PMID:26666576.
- McCoy MJ, Hemmings C, Anyaegbu CC, Austin SJ, Lee-Pullen TF, Miller TJ, Bulsara MK, Zeps N, Nowak AK, Lake RA, et al. Tumour-infiltrating regulatory T cell density before neoadjuvant chemoradiotherapy for rectal cancer does not predict treatment response. Oncotarget. 2017;8(12):19803–13. doi:10.18632/oncotarget.15048. PMID:28177891.
- Baras AS, Drake C, Liu JJ, Gandhi N, Kates M, Hoque MO, Meeker A, Hahn N, Taube JM, Schoenberg MP, et al. The ratio of CD8 to Treg tumor-infiltrating lymphocytes is associated with response to cisplatin-based neoadjuvant chemotherapy in patients with muscle invasive urothelial carcinoma of the bladder. Oncoimmunology. 2016;5(5):e1134412. doi:10.1080/2162402X.2015.1134412. PMID:27467953.
- Roselli M, Formica V, Cereda V, Jochems C, Richards J, Grenga I, Orlandi A, Ferroni P, Guadagni F, Schlom J. The association of clinical outcome and peripheral T-cell subsets in metastatic colorectal cancer patients receiving first-line FOLFIRI plus bevacizumab therapy. Oncoimmunology. 2016;5(7):e1188243. doi:10.1080/2162402X.2016.1188243. PMID:27622042.
- Kim H, Lee G, Sohn SH, Lee C, Kwak JW, Bae H. Immunotherapy with methyl gallate, an inhibitor of Treg cell migration, enhances the anti-cancer effect of cisplatin therapy. Korean J Physiol Pharmacol. 2016;20(3):261–8. doi:10.4196/kjpp.2016.20.3.261. PMID:27162480.
- Pan PY, Ma G, Weber KJ, Ozao-Choy J, Wang G, Yin B, Divino CM, Chen SH. Immune stimulatory receptor CD40 is required for T-cell suppression and T regulatory cell activation mediated by myeloid-derived suppressor cells in cancer. Cancer Res. 2010;70(1):99–108. doi:10.1158/0008-5472.CAN-09-1882. PMID:19996287.
- Holmgaard RB, Zamarin D, Lesokhin A, Merghoub T, Wolchok JD. Targeting myeloid-derived suppressor cells with colony stimulating factor-1 receptor blockade can reverse immune resistance to immunotherapy in indoleamine 2,3-dioxygenase-expressing tumors. EBioMedicine. 2016;6:50–8. doi:10.1016/j.ebiom.2016.02.024. PMID:27211548.
- Chen D, Zhang X. Cellular immunity augmentation in mainstream oncologic therapy. Cancer Biol Med. 2017 May;14(2):121–8 doi:10.20892/j.issn.2095-3941.2017.0022.
