ABSTRACT
Background: Dysgerminoma is an uncommon malignant tumor arising from the germ cells of the ovary. Its association with pregnancy is extremely rare; the incidence is about 0.2–1 per 100,000 pregnancies. Because of its infrequency, there are few recommendations regarding its management in pregnancy; therefore, it is important to discuss and summarize the treatment strategy.
Case: We presented a case of a 23-year-old pregnant woman with a large dysgerminoma originated from the right ovary, which had the unusual coincidence of being associated with an abdominal desmoid tumor simultaneously. We did not find any similar cases published in the PubMed database after 1947. A cesarean section was performed at 34 + 6 weeks gestation secondary to her abdominal pain worsening. The patient delivered a healthy boy and had fertility-preserving surgery, followed by 6 cycles of chemotherapy. This case is compared with 21 other reported cases of pure ovarian dysgerminoma in the literature to evaluate the clinical characteristics, feto-maternal compromise, treatment, long-term survival, and fertility outcome.
Conclusion: The treatment strategy in women with ovarian dysgerminoma should be discussed and structured on an individual basis. If pregnancy is desired, surgical intervention undertaken in the second trimester seems to be the first choice. When chemotherapy is indicated, unless delivery can be accomplished within a few weeks of diagnosis, it should not necessarily be delayed until after delivery. Good reproductive function and high survival rate can be achieved in patients treated with conservative surgery and adjuvant chemotherapy.
Introduction
Malignant germ cell tumor (MGCT) is a relatively uncommon subtype of ovarian cancer accounting for less than 5% of all ovarian cancers. The incidence of any type of ovarian cancer is 2.8–11 per 100,000 pregnancies.Citation1-Citation3 Because of its preponderance for reproductive aged women, MGCT accounts for 18–26% of all ovarian cancers complicating pregnancy.Citation2,Citation4 The most common type of MGCT was dysgerminoma (38.2%) followed by yolk sac tumor (30.4%), and immature teratoma (15.7%).Citation5 In other words, the incidence of ovarian dysgerminoma is approximately 0.2–1 per 100,000 pregnancies; therefore, ovarian dysgerminoma in pregnancy is an extremely rare clinical condition. However, the previous literature only showed sporadic case reports; further studies are needed in order to confirm the best management of these patients. The aim of this study was to present our case as well as to review the available literature describing the clinical characteristics, feto-maternal outcomes, oncologic outcomes, and management of pregnancies complicated by ovarian dysgerminoma.
Case report
A 23-year-old patient, gravida 2, para 1, presented to our emergency room with intermittent abdominal pain for 40 days and a fever for 12 hours at 34 + 6 weeks of gestation. She had a history of previous cesarean section (CS) done 5 years ago for a failed induction. At her initial prenatal examination (at 7 weeks gestation), the ultrasound revealed bilateral hypoechoic lesions, one 9.6 × 9.5 × 9.1 cm just posterior to the uterus and the other 5.2 × 5.4 × 4.9 cm at the left iliac fossa, a finding consistent with a leiomyoma. At 29 weeks gestation, because she started to complain of intermittent abdominal pain, another ultrasound was performed that showed the masses had grown to the size of 13.8 × 12.1 × 11.2 cm and 8.1 × 7.1 × 6.7 cm, respectively. All but one of her tumor markers were negative. Cancer antigen (CA)-125 was 11.8 U/mL (0 to 35 U/mL), CA-199 was 13.78 U/mL (0 to 37 U/mL), human epididymal protein (HE)−4 was 33.5 pmol/L (0 to 150 pmol/L), and carcinoembryonic antigen (CEA) was 0.51 ng/mL (0 to 5 ng/mL). Conversely, the α-fetoprotein (AFP) was elevated to 140.8 ng/mL (0 to 10 ng/mL). Considering there was a possibility of premature delivery, the patient was administered ritodrine and dexamethasone. At 33 + 4 weeks of gestation, a magnetic resonance imaging (MRI) was performed and showed the mass in the Douglas cul-de-sac had reached 12 × 11.8 × 16.2 cm in size (), whereas the mass at the left side of the uterus reached 6.7 × 9.4 × 9 cm (). The interpretation of the MRI was reported as subserosal fibroids with degeneration. After 2 days, the patient did not respond to conservative therapy and was transferred to our hospital for further management.
Figure 1. MRI and gross appearance of tumors. (A) MRI showing the dysgerminoma occupying the whole Douglas cul-de-sac. (B) MRI showing the desmoid tumor at the left side of uterus. (C) The right ovarian mass and fallopian tube after excision. (D) The retroperitoneal mass after excision.
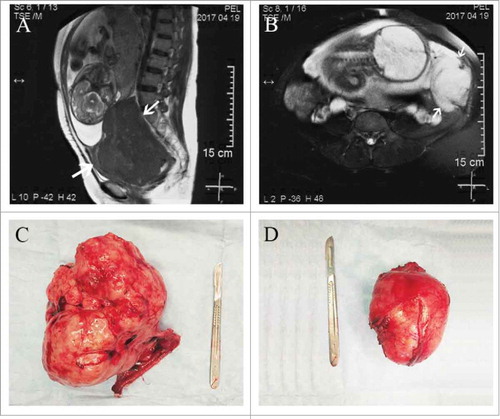
On abdominal examination, the uterine fundal height was 34 cm and the abdominal circumference was 100 cm with cephalic presentation. A firm lump was felt adjacent to the uterus on the left side with restricted mobility. Her uterine contractions were irregular and the fetal heart rate was 145/min. Her laboratory results after admission revealed mild anemia (hemoglobin 9.3 g/dl), elevated granulocytes (N 88.8%), and elevated lactate dehydrogenase (LDH) 385 U/L.
A decision for termination of pregnancy was taken in view of her abdominal pain being worsen. She delivered a 2,200-g healthy boy with a one-minute Apgar score of 10 by CS. Intraoperatively, a large solid mass of 18 × 13 × 10 cm originated from the right ovary was seen in the cul-de-sac. The mass with a lobulated surface was closely adherent to the posterior wall of the uterus and the anterior wall of the rectum. The homolateral tube was congested, edematous, and adherent over the mass. No abnormalities were found in the left adnexa. Another mass of 11 × 8.5 × 7 cm was found retroperitoneally and originated from the left abdominal wall. The right adnexa () and the retroperitoneal mass () were removed with careful dissection. There was no ascites and no enlarged para-aortic or retroperitoneal lymph nodes were appreciated. All other structures in the abdomen and pelvis were grossly normal. On cut section, the masses were homogeneous, grayish white, without cystic changes or hemorrhage.
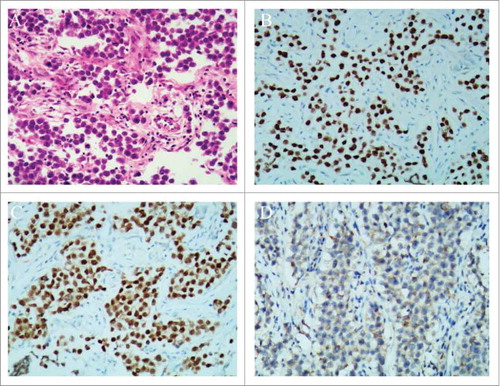
Postoperatively, histopathology confirmed that the right ovarian mass was pure dysgerminoma, showing sheets of tumor cells separated by fibrous septa (). Immunohistochemical staining markers SALL-4 () and Oct-4 () were positive, and CK was weakly positive (). The retroperitoneal mass proved to be a desmoid tumor. A Positron Emission Tomography (PET) scan was done and found to be normal. She then completed 6 cycles of chemotherapy with paclitaxel liposome and carboplatin. Her postoperative period was uneventful.
Figure 2. Histopathological results of the ovarian dysgerminoma and immunohistochemical staining. (A) Histopathology showing sheets of tumor cells, separated by fibrous septa (H & E stain, × 100). (B) SALL-4 staining was positive (SALL-4, × 100). (C) Oct-4 staining was positive (Oct-4, × 100). (D) CK staining was weekly positive (CK, × 100).
Discussion
Our dysgerminoma case had the unusual coincidence of being associated with an abdominal desmoid tumor simultaneously. We did not find any similar cases published in the PubMed database after 1947. However, we will be discussing the ovarian dysgerminoma during pregnancy, rather than the desmoid tumor in this study. Therefore, a systematic search was performed in the PubMed database from 1947 to July 2017. The search was limited to the literature published in English: “(gestation [TI/AB] OR gestational [TI/AB] OR pregnant [TI/AB] OR pregnancy [TI/AB]) AND (dysgerminoma [TI/AB] OR dysgerminomas [TI/AB]).” Finally, only 21 cases in 17 articles met our criteria of having an adequate description for analysis; our case is the 22th. All the cases were pure dysgerminoma; no ovarian mixed germ cell tumors were included in this review.
Clinical characteristics
Ovarian dysgerminoma occurs in reproductive age usually in women under 30 years. The pure dysgerminoma accounts for 0.6% of all ovarian cancers diagnosed in North America, predominantly affecting younger women, with 85% of patients being less than 30 years of age at the time of diagnosis.Citation6 In our series, the age of patients ranged from 17 years to 33 years () with a median of 24 ± 4.4 years, with 86.4% of them being below age 30 (), a finding similar to non-pregnancy. Eleven patients (50%) were primigravid, 7 were in their second pregnancy and 4 were pregnant for the third time ().
Table 1. Clinical features in 22 cases of ovarian dysgerminoma in pregnancy.
Table 2. Tumor characteristics.
Symptoms and diagnosis
The most common symptoms of MGCT in pregnancy were abdominal pain (35.3%), abdominal distention (19.6%), a growing mass (19.6%), multiple symptoms (18.6%), and no symptoms (21.6%).Citation5 In our series, more than half of the cases were asymptomatic (52.4%), followed by abdominal pain (28.5%), abdominal distention (9.5%), and obstructed labor (9.5%). Of the 11 asymptomatic patients, abdominal masses were detected in 8 cases (4 were diagnosed on clinical palpation, 4 were discovered on prenatal ultrasound or MRI), and an incidental finding during CS were in 3 cases (). The detection of an ovarian tumor during gestation is difficult, as the growing uterus interferes with an adequate abdominal or pelvic examination. With increasing use of sonography in prenatal care, ovarian dysgerminomas are being detected more frequently than they were in the 20th century. As a result, all tumors revealed by ultrasound or MRI were reported after 1992; additionally, obstructed labor no longer occurred after that. Pelvic ultrasound imaging seems to be an essential and reliable technique which can assist in making the diagnosis. The ultrasound finding of a highly vascularized, large, solid, lobulated adnexal mass with irregular internal echogenicity in a woman 20–30 years old should raise the suspicion of ovarian dysgerminoma. However, the preoperative diagnostic rate of ovarian dysgerminoma made by the original ultrasound examiner was only 59%.Citation7 As approximately 10% of masses are complex, a second diagnostic test should be performed by a fully trained sonographer. And it is suggested pelvic MRI is the second line examination, which should only be performed during pregnancy to confirm the diagnosis or to provide additional information if the ultrasound examination is not sufficient for the assessment of ovarian cancer;Citation8 pelvic CT scanning is not indicated during pregnancy. On MRI, the tumor has been typically described as a multi-lobulated solid mass with lobules divided by fibrovascular septa.Citation9 In our case, the bilateral solid masses on MRI showed neither cystic degeneration, thick septations nor ascites may have misled us to the diagnosis of subserosal fibroids. Tumor markers can also assist in diagnosis; however, tumor maker values should be interpreted with caution during pregnancy as there are wide variations in results and poor specificity due to physiological changes in pregnancy. Abnormal AFP (80.8%) and LDH (85.7%) were frequently reported in ovarian MGCT in pregnancy.Citation5 In the present series, only 6 cases had detailed results of the tumor markers, which were LDH, AFP, and CA-125 elevated in 5 (83.3%), 3 (50%), and 2 (33.3%) cases, respectively. Furthermore, LDH also proved to be a reliable tumor marker in predicting the response of a dysgerminoma to chemotherapy.Citation10 Thus, LDH may be more sensitive than other tumor markers in patients with dysgerminoma.
Feto-maternal compromise
Dysgerminomas can affect conception, and if pregnancy occurs, it can lead to feto-maternal compromise. In our series, there were 2 cases of tumor torsion that occurred in the puerperium and second trimester, respectively; there were 2 cases of tumor incarceration found because of obstructed labor; there were another 2 cases of tumor rupture diagnosed during the surgical exploration. Thus, irrespective of the term of the pregnancy, increased risk of torsion, incarceration, rupture, and hemorrhage can occur during pregnancy, vaginal delivery, or the puerperium. Intrauterine growth restriction (IUGR) is the most frequent adverse event (22.8%) in live births of maternal MGCT; other pregnancy outcomes include elective termination, intrauterine fetal demise (IUFD), ectopic pregnancy, and spontaneous abortion.Citation5 Details of the pregnancy outcomes of our series are shown in . Gestational age at termination was mainly in the third trimester (1st, 2nd, 3rd trimester: 4.5%, 9.1%, 86.4%, respectively). The majority of cases resulted in live birth (85.7%) via cesarean section (66.7%). The mean weight of the live births was 2,879 ± 370 g at term (61%), 2,300 ± 429 g preterm, and IUGR was common (11.1%). The pregnant losses were 1 case of ectopic pregnancy, 1 case of spontaneous abortion, and 1 patient underwent the radical surgery at 15 weeks gestation, respectively.
Oncologic characteristics
Most of the ovarian dysgerminomas in pregnancy are unilateral. In our study, the majority of tumors (95%) were located unilaterally, whereas only 1 case was bilateral (). Some authors reported that bilateral dysgerminomas were found in 12–20% of pregnant cases,Citation11,Citation12 which is a much higher rate than ours (5%). In our study, unilateral dysgerminomas occurred predominantly on the right side (more than twice of the left side [65% versus 30%]) (). The mean diameter of the ovarian dysgerminomas in our study was 14.7 ± 7.8 cm (range 4–30 cm), with 20% measuring ≥ 20 cm (). More than half of the patients were free of ascites, 23.8% patients had a little ascites, whereas 14.3% had remarkable ascites. All the patients with remarkable ascites had abdominal distention, but it seemed there was no positive correlation with tumor stage, for 2 cases were at early stage and 1 was at advanced stage, unlike epithelial ovarian cancer. Vicus observed 65 non-pregnant patients with pure dysgerminomas, 75.4% presented with stage I and II.Citation6 In our study, the dysgerminomas among patients who were pregnant were detected in FIGO stage I + II (81.8%), stage III + IV (18.2%) (). By comparison, the majority of ovarian dysgerminomas associated with pregnancy are diagnosed at an earlier stage.
Treatment
The management of ovarian cancer in pregnancy is complicated, as there are 3 separate but interactive parts, i.e., mother, fetus, and malignancy, which must be managed simultaneously. Therefore, the decisions regarding each case should be on an individual basis, taking into consideration the patient's age, parity, desire for present pregnancy, future fertility, stage of the tumor, and duration of gestation.
If pregnancy is desired, in general, abdominal surgery should be undertaken in the second trimester because the risk of miscarriage is decreased and the size of uterus still allows a certain degree of access.Citation2,Citation13-Citation16 Firstly, according to committee opinion of the American Society of Anesthesiologists, no currently used anesthetic agents have been shown to have any teratogenic effects in humans when using standard concentrations at any gestational age.Citation13 Secondly, multiple studies have reported surgery for adnexal masses in pregnancy by experienced practitioners is safe and feasible.Citation14,Citation15,Citation17-Citation20 However, surgical intervention in the first trimester is still controversial. Although several studies reported the safety of surgery during the first trimester,Citation21-Citation23 but some authors found that there was an increased rates of abortionCitation15,Citation24,Citation25 and neural tube defectsCitation26 among women undergoing surgery in early pregnancy. Meanwhile, surgical exploration during the third trimester is reported to be associated with premature labor.Citation27,Citation28 In the present series, the initial surgeries were performed during CS in 12 cases (54.5%), whereas 8 cases (36.3%) were during pregnancy. The majority of fetal-preserving surgeries were performed during the second trimester. Only 1 woman resulted in spontaneous abortion and the remaining 5 (83.3%) delivered vaginally at term. Therefore, we presume the second trimester is the safest time to perform adnexal surgery with utmost care. Thirdly, pregnancy is no longer a contraindication for laparoscopic surgery (< 28 weeks). Laparoscopic management of adnexal masses in pregnancy by an experienced team is a safe and effective procedure that allows a shorter hospital stay and a reduced rate of post-operative complications when comparing with laparotomy.Citation19,Citation20,Citation29 Furthermore, pregnant outcomes following laparoscopy in pregnancy have improved significantly over the past 20 years. There was no difference in post-operative spontaneous abortion, vaginal bleeding, IUFD, congenital malformations and neonatal deaths between laparoscopy and laparotomy.Citation20,Citation29-Citation31 What is more, some authors reported the rate of premature labor even lower in laparoscopic management.Citation32,Citation33 Remarkably, as recommended by the European Society of Gynecological Oncology (ESGO), these are 4 prerequisites for surgical interventions during pregnancy as follows: a maximal laparoscopic procedure time of 90 minutes, a pneumoperitoneum with a maximal intraabdominal pressure of 10 to 13 mm Hg, open introduction, and an experienced surgeon.Citation16 No fetal-preserving surgery in our series was performed by laparoscopy, which was probably because gynecologists did not have enough experience in the last century.
Another option is chemotherapy treatment during pregnancy. In the present series, 2 (9.5%) patients were administered the chemotherapy during gestation (). The 2 patients were both characterized by rapidly enlarging tumors, elevated tumor markers, accompanying remarkable ascites, as well as confirmation of histologic diagnosis (after limited staging surgery and transvaginal biopsy, respectively). Therefore, if there is histologic evidence, adjuvant chemotherapy is indicated, especially when there is rapidly increasing ascites, large tumor size, advanced tumor stage, the possibility of a mixed epithelial and germ cell tumor, and foreseeable risks of the surgery to the pregnancy are present. Multiple studies have shown that chemotherapy administered during the first trimester increases the risk of spontaneous abortion, fetal death, and major malformations.Citation34-Citation37 A review of 217 pregnant women treated with cytotoxic therapies for a variety of malignancies reported 18 newborns with congenital abnormalities, 2 with chromosomal abnormalities, 4 were stillborn and 15 were spontaneous abortions. The majority of stillborn infants and infants with chromosomal or congenital abnormalities had mothers who were given chemotherapy in the first trimester.Citation38 The risk of malformations is approximately 7% to 17% when a single agent treatment is used and increases to 25% in cases of combination therapy from 4 weeks to the end of first trimester.Citation39 As further evidence accumulated, the recent information supports that chemotherapy after the first trimester is not associated with increased rates of birth defects above the rate in the general population (3%).Citation36,Citation40-Citation42 However, the main complications of chemotherapy exposure during the second and third trimesters are IUGR and low birthweight, followed by preterm delivery, fetal toxicities (eg, transient myelosuppression, ototoxicity), fetal and neonatal death.Citation34,Citation39-Citation44 Most fetal and neonatal deaths were related to maternal hematological malignancies. Moreover, administration of chemotherapy within 3 weeks of anticipated delivery or beyond 35 weeks of gestation is not recommended to avoid transient neonatal myelosuppression and potential complications of bleeding, sepsis at the time of delivery.Citation37 Therefore, the risks to the fetus must be weighed against the impact of delayed treatment on maternal survival.
Platinum-based chemotherapy is highly effective in all cases of germ-cell tumors, and dysgerminoma, even more than other MGCTs, seems to be exquisitely chemosensitive. Cisplatin has been more extensively investigated in human pregnancy than any other chemotherapeutic agents. Cardonick reported that 28 fetuses have been exposed to cisplatin in utero, with 23 normal outcomes and 5 complicated by IUGR, IUFD, hearing loss, and ventriculomegaly.Citation34 There are more case reports showed that no abnormalities was observed in infant after the administration of cisplatin in women with cervical cancer.Citation45-Citation48 Cisplatin therapy seems the most reliable form of chemotherapy, permitting a good outcome for most patients. It is reported that paclitaxel and carboplatin were used in pregnancy after organogenesis without apparent fetal effects, though the evidence was mainly based on case reports.Citation10,Citation42,Citation49-Citation53 In nonpregnant patients, the combination of bleomycin-etoposide-cisplatin (BEP) is used for nonepithelial ovarian tumors. The patient who was administered etoposide and cisplatin in our selected case carried her pregnancy to term without congenital defects.Citation54 However, compared with the platinum-based regimen, etoposide has more adverse advents reported, such as fetal ventriculomegaly with cerebral atrophy, plagiocephaly, syndactyly, and hearing loss.Citation42,Citation55-Citation57 Therefore, as stated by ESGO, paclitaxel-carboplatin or cisplatin-vinblastin-bleomycin chemotherapy can be used instead of BEP for nonepithelial ovarian cancer in pregnancy.Citation16
If suspicious of malignancy, termination can be offered to women not desirous of pregnancy. In our series, the type of surgery ranged from biopsy to radical surgery. The most common procedures were adnexectomy (45.5%), followed by radical surgery (18.2%), stage laparotomy (13.6%) and oophorectomy(13.6%) (). There is evidence showing that conservative treatment for clinical stage IA ovarian dysgerminoma is safe with a 10-year survival of 91% in the pre-chemotherapy era.Citation58 In our study, 12 (92.3%) patients with stage IA tumors underwent conservative surgeries, including unilateral oophorectomy or adnexectomy (2 cases with staging laparotomy). None of the 12 patients received any other adjuvant treatment and had no recurrence within an average of 4.4 years of follow-up. Moreover, 2 patients became pregnant and delivered healthy babies after surgery. Therefore, young patients found to have stage IA dysgerminoma could be treated with fertility-sparing surgery without adjuvant chemotherapy or radiotherapy. In incompletely staged tumors, there should be close follow-up, for the recurrence rate is approximately 10–20% in patients who have unstaged tumors.Citation59 Vicus reported 13.1% of recurrence rate in dysgerminoma patients with stage IA and in 5 of them (occurred after performing unstaged unilateral adnexectomy) were successfully salvaged by chemotherapy or radiation.Citation6 It is reported that the 5-year survival rate for ovarian dysgerminoma stage IA can attain 100%.Citation60 In our series, all patients with stage IA tumors remained disease free at the end of follow-up with an average duration of 4 years, similar to those who were not pregnant. Therefore, treating stage IA tumors with surgery alone is likely sufficient. Furthermore, fertility-sparing surgery can be done even in bilateral dysgerminomas if the patient is desirous of future pregnancy. From a single institutional experience of 65 patients with dysgerminomas over 34 years, there was no difference in disease-free survival (DFS) and overall survival (OS) between fertility sparing and non-conservative surgery (hysterectomy and/or bilateral oophorectomy).Citation61 Adjuvant chemotherapy was independently a better prognostic factor for DFS (HR = 0.09, P = 0.034). Additionally, of the 50 patients (77%) treated with fertility-sparing surgery, 16 patients (32%) achieved pregnancy with 14 live births without congenital defects, and 10 out of the 16 women who conceived were treated with adjuvant chemotherapy. Therefore, complete surgical staging may not play a significant role in the outcome of ovarian dysgerminoma and patients can be treated safely with fertility-sparing surgery and can expect good reproductive outcomes.
In this series, second-look operations were performed in 3 patients who underwent unilateral adnexectomy. One was found to be stage IB and the other 2 were negative (). Though the recurrence rates had been reported more in unstaged patients, these tumors are highly chemosensitive and the survival rate was greater than 95% for tumors confined to the ovary and about 60 to 80% for advanced staged tumors. Therefore, second look surgery is not required for dysgerminoma and should only be considered if tumor markers are persistently elevated, especially if abnormal findings are seen on post-treatment imaging. For more-advanced tumors (stages III–IV), it seems preferable to consider termination of the pregnancy before week 24 of pregnancy and to perform routine surgical treatment followed by chemotherapy. If these tumors are found incidentally during CS, tumor markers, CT, or PET scan should be done postoperatively to plan optimal treatment.
Conclusion
In conclusion, we believe that our study shows that the long-term outcome of patients with ovarian dysgerminoma during pregnancy is excellent. The treatment strategy should be discussed and structured on an individual basis. If termination is not desired, it seems that operative intervention is the first choice. Fertility-preserving surgery can be done safely with a favorable outcome in the early stage in pregnancy; it especially seems adequate for stage IA tumors treated with unilateral adnexectomy without adjuvant therapy. When chemotherapy is indicated, unless delivery can be accomplished within a few weeks of diagnosis, chemotherapy should not necessarily be delayed until after delivery. Though the experience of chemotherapy in humans in utero is limited, the platinum-based regimen seems to be the best choice after the first trimester based on available evidence. Good reproductive function and high survival rates can be achieved in patients treated with conservative surgery and adjuvant chemotherapy.
Informed consent
We have obtained the informed consent from the participant included in the cases report.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- Salani R, Billingsley CC, Crafton SM. Cancer and pregnancy: an overview for obstetricians and gynecologists [J]. Am J Obstet Gynecol. 2014;211(1):7–14. doi:10.1016/j.ajog.2013.12.002. PMID:24316272
- Gezginc K, Karatayli R, Yazici F, Acar A, Celik C, Capar M. Ovarian cancer during pregnancy [J]. Int J Gynaecol Obstet. 2011;115 (2):140–3. doi:10.1016/j.ijgo.2011.05.025. PMID:21872237
- Sayedur RM, Al-Sibai MH, Rahman J, Al-Suleiman SA, El-Yahia AR, Al-Mulhim AA, Al-Jama F. Ovarian carcinoma associated with pregnancy. A review of 9 cases [J]. Acta Obstet Gynecol Scand. 2002;81(3):260–4.
- Aggarwal P, Kehoe S. Ovarian tumours in pregnancy: a literature review [J]. Eur J Obstet Gynecol Reprod Biol. 2011;155(2):119–24. doi:10.1016/j.ejogrb.2010.11.023. PMID:21194826
- Kodama M, Grubbs BH, Blake EA, Cahoon SS, Murakami R, Kimura T, Matsuo K. Feto-maternal outcomes of pregnancy complicated by ovarian malignant germ cell tumor: a systematic review of literature [J]. Eur J Obstet Gynecol Reprod Biol. 2014;181:145–56. doi:10.1016/j.ejogrb.2014.07.047. PMID:25150953
- Vicus D, Beiner ME, Klachook S, Le LW, Laframboise S, Mackay H. Pure dysgerminoma of the ovary 35 years on: a single institutional experience [J]. Gynecol Oncol. 2010;117(1):23–26. doi:10.1016/j.ygyno.2009.12.024. PMID:20097412
- Guerriero S, Testa AC, Timmerman D, Van Holsbeke C, Ajossa S, Fischerova D, Franchi D, Leone FP, Domali E, Alcazar JL, et al. Imaging of gynecological disease (6): clinical and ultrasound characteristics of ovarian dysgerminoma [J]. Ultrasound Obstet Gynecol. 2011;37(5):596–602. doi:10.1002/uog.8958. PMID:21305635
- Marret H, Lhomme C, Lecuru F, Canis M, Leveque J, Golfier F, Morice P. Guidelines for the management of ovarian cancer during pregnancy [J]. Eur J Obstet Gynecol Reprod Biol. 2010;149(1):18–21. doi:10.1016/j.ejogrb.2009.12.001. PMID:20042265
- Kim SH, Kang SB. Ovarian dysgerminoma: color Doppler ultrasonographic findings and comparison with CT and MR imaging findings [J]. J Ultrasound Med. 1995;14(11):843–8. doi:10.7863/jum.1995.14.11.843. PMID:8551550
- Hubalek M, Smekal-Schindelwig C, Zeimet AG, Sergi C, Brezinka C, Mueller-Holzner E, Marth C. Chemotherapeutic treatment of a pregnant patient with ovarian dysgerminoma [J]. Arch Gynecol Obstet. 2007;276(2):179–83. doi:10.1007/s00404-007-0328-2. PMID:17342499
- Montesinos L, Acien P, Martinez-Beltran M, Mayol M-J. Ovarian dysgerminoma and synchronic contralateral tubal pregnancy followed by normal intra-uterine gestation: a case report [J]. J Med Case Rep. 2012;6:399. doi:10.1186/1752-1947-6-399. PMID:23176153
- Gupta M, Jindal R, Saini V. An Incidental Finding of Bilateral Dysgerminoma During Cesarean Section: Dilemmas in Management [J]. J Clin Diagn Res. 2016;10(8):D4–D5. doi:10.7860/JCDR/2016/20163.8319.
- Committee Opinion No. 696: Nonobstetric Surgery During Pregnancy[J]. Obstet Gynecol. 2017;129(4):777–8. doi:10.1097/AOG.0000000000002014. PMID:28333816
- Yuen PM, Ng PS, Leung PL, Rogers MS. Outcome in laparoscopic management of persistent adnexal mass during the second trimester of pregnancy[J]. Surg Endosc. 2004;18(9):1354–7. doi:10.1007/s00464-003-8283-x. PMID:15164277
- Al-Fozan H, Tulandi T. Safety and risks of laparoscopy in pregnancy[J]. Curr Opin Obstet Gynecol. 2002;14(4):375–9. doi:10.1097/00001703-200208000-00003. PMID:12151826
- Amant F, Halaska MJ, Fumagalli M, Dahl Steffensen K, Lok C, Van Calsteren K, Han SN, Mir O, Fruscio R, Uzan C, et al. Gynecologic cancers in pregnancy: guidelines of a second international consensus meeting[J]. Int J Gynecol Cancer. 2014;24(3):394–403. doi:10.1097/IGC.0000000000000062. PMID:24445819
- Ngu S F, Cheung VY, Pun TC. Surgical management of adnexal masses in pregnancy[J]. JSLS. 2014;18(1):71–75. doi:10.4293/108680813X13693422521007. PMID:24680147
- Deffieux X, Ballester M, Collinet P, Fauconnier A, Pierre F. Risks associated with laparoscopic entry: guidelines for clinical practice from the French College of Gynaecologists and Obstetricians[J]. Eur J Obstet Gynecol Reprod Biol. 2011;158(2):159–66. doi:10.1016/j.ejogrb.2011.04.047. PMID:21621318
- Mathevet P, Nessah K, Dargent D, Mellier G. Laparoscopic management of adnexal masses in pregnancy: a case series[J]. Eur J Obstet Gynecol Reprod Biol. 2003;108(2):217–22. doi:10.1016/S0301-2115(02)00374-3. PMID:12781415
- Balthazar U, Steiner AZ, Boggess JF, Gehrig PA. Management of a persistent adnexal mass in pregnancy: what is the ideal surgical approach?[J]. J Minim Invasive Gynecol. 2011;18(6):720–5. doi:10.1016/j.jmig.2011.07.002. PMID:21840773
- Minig L, Otano L, Cruz P, Patrono MG, Botazzi C, Zapardiel I. Laparoscopic surgery for treating adnexal masses during the first trimester of pregnancy[J]. J Minim Access Surg. 2016;12(1):22–25. doi:10.4103/0972-9941.171960. PMID:26917915
- Weiner E, Mizrachi Y, Keidar R, Kerner R, Golan A, Sagiv R. Laparoscopic surgery performed in advanced pregnancy compared to early pregnancy[J]. Arch Gynecol Obstet. 2015;292(5):1063–8. doi:10.1007/s00404-015-3744-8. PMID:25958071
- Ko ML, Lai TH, Chen SC. Laparoscopic management of complicated adnexal masses in the first trimester of pregnancy[J]. Fertil Steril. 2009;92(1):283–7. doi:10.1016/j.fertnstert.2008.04.035. PMID:18692789
- Lee JH, Lee JR, Jee BC, Suh CS, Kim SH. Safety and feasibility of a single-port laparoscopic adnexal surgery during pregnancy[J]. J Minim Invasive Gynecol. 2013;20(6):864–70. doi:10.1016/j.jmig.2013.06.002. PMID:23850362
- Fatum M, Rojansky N. Laparoscopic surgery during pregnancy[J]. Obstet Gynecol Surv. 2001;56(1):50–59. doi:10.1097/00006254-200101000-00025. PMID:11140864
- Mazze RI, Kallen B. Reproductive outcome after anesthesia and operation during pregnancy: a registry study of 5405 cases[J]. Am J Obstet Gynecol. 1989;161(5):1178–85. doi:10.1016/0002-9378(89)90659-5. PMID:2589435
- Hong JY. Adnexal mass surgery and anesthesia during pregnancy: a 10-year retrospective review[J]. Int J Obstet Anesth. 2006;15(3):212–6. doi:10.1016/j.ijoa.2006.01.004. PMID:16798446
- Visser BC, Glasgow RE, Mulvihill KK, Mulvihill SJ. Safety and timing of nonobstetric abdominal surgery in pregnancy[J]. Dig Surg. 2001;18(5):409–17. doi:10.1159/000050183. PMID:11721118
- Liu YX, Zhang Y, Huang JF, Wang L. Meta-analysis comparing the safety of laparoscopic and open surgical approaches for suspected adnexal mass during the second trimester[J]. Int J Gynaecol Obstet. 2017;136(3):272–9. doi:10.1002/ijgo.12069. PMID:28099685
- Palanivelu C, Rangarajan M, Senthilkumaran S, Parthasarathi R. Safety and efficacy of laparoscopic surgery in pregnancy: experience of a single institution[J]. J Laparoendosc Adv Surg Tech A. 2007;17(2):186–90. doi:10.1089/lap.2006.0037. PMID:17484645
- Koo YJ, Lee JE, Lim KT, Shim JU, Mok JE, Kim TJ. A 10-year experience of laparoscopic surgery for adnexal masses during pregnancy[J]. Int J Gynaecol Obstet. 2011;113(1):36–39. doi:10.1016/j.ijgo.2010.10.020. PMID:21247562
- Webb KE, Sakhel K, Chauhan SP, Abuhamad AZ. Adnexal mass during pregnancy: a review[J]. Am J Perinatol. 2015;32(11):1010–6. doi:10.1055/s-0035-1549216. PMID:26007316
- Corneille MG, Gallup TM, Bening T, Wolf SE, Brougher C, Myers JG, Dent DL, Medrano G, Xenakis E, Stewart RM. The use of laparoscopic surgery in pregnancy: evaluation of safety and efficacy[J]. Am J Surg. 2010;200(3):363–7. doi:10.1016/j.amjsurg.2009.09.022. PMID:20800715
- Cardonick E, Iacobucci A. Use of chemotherapy during human pregnancy[J]. Lancet Oncol. 2004;5(5):283–91. doi:10.1016/S1470-2045(04)01466-4. PMID:15120665
- Zemlickis D, Lishner M, Degendorfer P, Panzarella T, Sutcliffe S B, Koren G. Fetal outcome after in utero exposure to cancer chemotherapy [J]. Arch Intern Med. 1992;152(3):573–6. doi:10.1001/archinte.1992.00400150093017. PMID:1546920
- Amant F, Brepoels L, Halaska MJ, Gziri MM, Calsteren KV. Gynaecologic cancer complicating pregnancy: an overview[J]. Best Pract Res Clin Obstet Gynaecol. 2010;24(1):61–79. doi:10.1016/j.bpobgyn.2009.08.001. PMID:19740709
- Brewer M, Kueck A, Runowicz CD. Chemotherapy in pregnancy[J]. Clin Obstet Gynecol. 2011;54(4):602–18. doi:10.1097/GRF.0b013e318236e9f9. PMID:22031250
- Ebert U, Loffler H, Kirch W. Cytotoxic therapy and pregnancy[J]. Pharmacol Ther. 1997;74(2):207–20. doi:10.1016/S0163-7258(97)82004-9. PMID:9336023
- Esposito S, Tenconi R, Preti V, Groppali E, Principi N. Chemotherapy against cancer during pregnancy: A systematic review on neonatal outcomes[J]. Medicine (Baltimore). 2016;95(38):e4899. doi:10.1097/MD.0000000000004899. PMID:27661036
- Amant F, Han S N, Gziri MM, Dekrem J, Van Calsteren K. Chemotherapy during pregnancy[J]. Curr Opin Oncol. 2012;24(5):580–6. doi:10.1097/CCO.0b013e328354e754. PMID:22581358
- Van Calsteren K, Heyns L, De Smet F, Van Eycken L, Gziri MM, Van Gemert W, Halaska M, Vergote I, Ottevanger N, Amant F. Cancer during pregnancy: an analysis of 215 patients emphasizing the obstetrical and the neonatal outcomes[J]. J Clin Oncol. 2010;28(4):683–9. doi:10.1200/JCO.2009.23.2801. PMID:19841323
- Cardonick E, Usmani A, Ghaffar S. Perinatal outcomes of a pregnancy complicated by cancer, including neonatal follow-up after in utero exposure to chemotherapy: results of an international registry[J]. Am J Clin Oncol. 2010;33(3):221–8. doi:10.1097/COC.0b013e3181a44ca9 PMID:19745695
- Abdalla N, Bizon M, Piorkowski R, Stanirowski P, Cendrowski K, Sawicki W. Does Chemotherapy for Gynecological Malignancies during Pregnancy Cause Fetal Growth Restriction?[J]. Biomed Res Int. 2017;2017:7543421. doi:10.1155/2017/7543421. PMID:28626764
- Lambertini M, Kamal NS, Peccatori FA, Del Mastro L, Azim HA Jr. Exploring the safety of chemotherapy for treating breast cancer during pregnancy[J]. Expert Opin Drug Saf. 2015;14(9):1395–408. doi:10.1517/14740338.2015.1061500. PMID:26118333
- Dawood R, Instone M, Kehoe S. Neo-adjuvant chemotherapy for cervical cancer in pregnancy: a case report and literature review[J]. Eur J Obstet Gynecol Reprod Biol. 2013;171(2):205–8. doi:10.1016/j.ejogrb.2013.09.008. PMID:24139541
- Fruscio R, Villa A, Chiari S, Vergani P, Ceppi L, Dell'Orto F, Dell'Anna T, Chiappa V, Bonazzi CM, Milani R, et al. Delivery delay with neoadjuvant chemotherapy for cervical cancer patients during pregnancy: a series of nine cases and literature review[J]. Gynecol Oncol. 2012;126(2):192–7. doi:10.1016/j.ygyno.2012.04.027. PMID:22555106
- Benhaim Y, Pautier P, Bensaid C, Lhomme C, nHaie-Meder C, Morice P. Neoadjuvant chemotherapy for advanced stage cervical cancer in a pregnant patient: report of one case with rapid tumor progression[J]. Eur J Obstet Gynecol Reprod Biol. 2008;136(2):267–8. doi:10.1016/j.ejogrb.2006.10.027. PMID:17157432
- Hecking T, Abramian A, Domrose C, Engeln T, Thiesler T, Leutner C, Gembruch U, Keyver-Paik MD, Kuhn W, Kubler K. Individual management of cervical cancer in pregnancy[J]. Arch Gynecol Obstet. 2016;293(5):931–9. doi:10.1007/s00404-015-3980-y. PMID:26728388
- Ruiz RJ, Roma E, Palomar L, Poveda JL. Paclitaxel and carboplatin treatment for advanced ovarian cancer during pregnancy[J]. Chemotherapy. 2013;59(5):344–5. doi:10.1159/000360691. PMID:24820861
- Barut A, Arikan I, Barut F, Harma M, Harma MI, Payasli B. Ovarian cancer during pregnancy[J]. J Pak Med Assoc. 2011;61(9):914–6. PMID:22360037
- Doi D, Boh Y, Konishi H, Asakura H, Takeshita T. Combined chemotherapy with paclitaxel and carboplatin for mucinous cystadenocarcinoma of the ovary during pregnancy[J]. Arch Gynecol Obstet. 2009;280(4):633–6. doi:10.1007/s00404-009-0950-2. PMID:19205713
- Motegi M, Takakura S, Takano H, Tanaka T, Ochiai K. Adjuvant chemotherapy in a pregnant woman with endodermal sinus tumor of the ovary[J]. Obstet Gynecol. 2007;109(2 Pt2):537–40. doi:10.1097/01.AOG.0000245450.62758.47. PMID:17267887
- Tabata T, Nishiura K, Tanida K, Kondo E, Okugawa T, Sagawa N. Carboplatin chemotherapy in a pregnant patient with undifferentiated ovarian carcinoma: case report and review of the literature[J]. Int J Gynecol Cancer. 2008;18(1):181–4. doi:10.1111/j.1525-1438.2007.00974.x. PMID:17466045
- Buller RE, Darrow V, Manetta A, Porto M, DiSaia PJ. Conservative surgical management of dysgerminoma concomitant with pregnancy [J]. Obstet Gynecol. 1992;79(5 (Pt 2)):887–90. PMID:1565399
- Elit L, Bocking A, Kenyon C, Natale R. An endodermal sinus tumor diagnosed in pregnancy: case report and review of the literature [J]. Gynecol Oncol. 1999;72(1):123–7. doi:10.1006/gyno.1998.5190. PMID:9889045
- Karimi ZM, Behtash N, Modares GM. Good pregnancy outcome after prenatal exposure to bleomycin, etoposide and cisplatin for ovarian immature teratoma: a case report and literature review[J]. Arch Gynecol Obstet. 2008;277(1):75–78. doi:10.1007/s00404-007-0416-3. PMID:17653741
- Kluetz PG, Edelman MJ. Successful treatment of small cell lung cancer during pregnancy[J]. Lung Cancer. 2008;61(1):129–30. doi:10.1016/j.lungcan.2007.10.007. PMID:18242765
- Dark GG, Bower M, Newlands ES, Paradinas F, Rustin GJ. Surveillance policy for stage I ovarian germ cell tumors [J]. J Clin Oncol. 1997;15(2):620–4. doi:10.1200/JCO.1997.15.2.620. PMID:9053485
- Mangili G, Sigismondi C, Lorusso D, Cormio G, Scollo P, Vigano R, Gamucci T, Candiani M, Pignata S. Is surgical restaging indicated in apparent stage IA pure ovarian dysgerminoma? The MITO group retrospective experience [J]. Gynecol Oncol. 2011;121(2):280–4. doi:10.1016/j.ygyno.2011.01.006. PMID:21277010
- Zhang R, Sun YC, Zhang GY, Wu LY, Zuo J. Treatment of malignant ovarian germ cell tumors and preservation of fertility[J]. Eur J Gynaecol Oncol. 2012;33(5):489–92. PMID:23185794
- A L Husaini H, Soudy H, El Din Darwish A, Ahmed M, Eltigani A, A L Mubarak M, Sabaa AA, Edesa W, A L-Tweigeri T, Al-Badawi IA. Pure dysgerminoma of the ovary: a single institutional experience of 65 patients[J]. Med Oncol. 2012;29(4):2944–8. doi:10.1007/s12032-012-0194-z. PMID:22407668
- SCHNEIDER H, VESELL M. Dysgerminoma and pregnancy [J]. Am J Obstet Gynecol. 1947;53(4):688–91. doi:10.1016/0002-9378(47)90292-5. PMID:20291244
- WATSON SL. Dysgerminoma complicating labor [J]. Am J Obstet Gynecol. 1956;72(6):1177–9. doi:10.1016/0002-9378(56)90773-6. PMID:13372592
- MISRA S. Dysgerminoma of the ovary; report of a case complicating pregnancy [J]. J Obstet Gynaecol Br Emp. 1958;65(3):440–1. doi:10.1111/j.1471-0528.1958.tb08531.x. PMID:13564292
- PECE G. DYSGERMINOMA OF THE OVARY IN PREGNANCY; REPORT OF A CASE AND REVIEW OF THE LITERATURE [J]. Obstet Gynecol. 1964;24:768–73. PMID:14227614
- Smith AH, Ward SV. Dysgerminoma in pregnancy. Report of a case [J]. Obstet Gynecol. 1966;28(4):502–4.
- Karlen JR, Akbari A, Cook WA. Dysgerminoma associated with pregnancy [J]. Obstet Gynecol. 1979;53(3):330–5. PMID:424104
- Rabinowitz R, Granat M. Dysgerminoma of the ovary: incidental finding during cesarean section [J]. Eur J Obstet Gynecol Reprod Biol. 1985;19(2):105–8. doi:10.1016/0028-2243(85)90026-7. PMID:3987948
- Kulenthran A, Sivanesaratnam V. Dysgerminoma of the ovary associated with pregnancy [J]. Asia Oceania J Obstet Gynaecol. 1986;12(2):217–20. doi:10.1111/j.1447-0756.1986.tb00182.x. PMID:3767705
- Ueda M, Ueki M. Ovarian tumors associated with pregnancy [J]. Int J Gynaecol Obstet. 1996;55(1):59–65. doi:10.1016/0020-7292(96)02718-X. PMID:8910084
- Gauza JE, Reberti AG, Silva JC, Pope LZ, Santos JC, Quintana SM. Diagnosis of ovarian dysgerminoma during pregnancy [J]. Rev Assoc Med Bras (1992). 2010;56(5):517–9.
- Akhtar K, Ahmad SS, Kumar A, Afshan N. Dysgerminoma with pregnancy and viable baby: a case report [J]. Oman Med J. 2011;26(3):198–200. doi:10.5001/omj.2011.48. PMID:22043416
