ABSTRACT
(−)-Guaiol, a sesquiterpene alcohol with the guaiane skeleton, has been found in many Chinese medicinal plants and been reported to comprise various guaiane natural products that are well known for their antibacterial activities. Previously, we have shown its antitumor activity by inducing autophagy in NSCLC cells. However, its potential mechanism in inducing autophagy is still under our investigation. Here, data from our western blotting assays showed that, in NSCLC cells, (−)-Guaiol significantly blocked the mTORC2-AKT signaling by suppressing mTOR phosphorylation at serine 2481 (S2481) to induce autophagy, illustrated by the increasing ratio of LC3II/I. Besides, it impaired the mTORC1 signaling by inhibiting the activity of its downstream factors, such as 4E-BP1 and p70 S6K, all of which could obviously rescued by the mTOR activator MHY1485. Afterwards, results from biofunctional assays, including cell survival analysis, colony formation assays and flow cytometry assays, suggested that (−)-Guaiol triggered autophagic cell death by targeting both mTORC1 and mTORC2 signaling pathways. In summary, our studies showed that (−)-Guaiol inhibited the proliferation of NSCLC cells by specifically targeting mTOR signaling pathways, including both mTORC1 and mTORC2 signaling, providing a better therapeutic option for substituting rapamycin in treating NSCLC patients.
Introduction
(−)-Guaiol, a sesquiterpene alcohol with the guaiane skeleton, has been found in many traditional Chinese medicinal plants and been reported to compose various guaiane natural products that are acknowledged for their antibacterial activities.Citation1 In our previous studies, we have uncovered that it suppresses cell proliferation and stimulates double strand breaks (DSBs)-triggered cell apoptosis by degrading RAD51 via autophagy in non-small-cell lung cancer (NSCLC).Citation2 However, little is known about its detailed mechanisms in autophagy. Interestingly, our previous GO analysis of high throughput data revealed that it was involved in mammalian target of rapamycin (mTOR) signaling by downregulating some genes in NSCLC cells.Citation2 Therefore, in the study, we mainly investigated the mechanistic roles of (−)-Guaiol in modulating the mTOR signaling.
Lung cancer, generally regarded as an extremely aggressive malignancy, is divided into two main categories, NSCLC and small-cell lung cancer (SCLC).Citation3 NSCLC accounting for almost 80% of lung cancer cases includes large cell carcinoma, adenocarcinoma and squamous carcinoma.Citation4 It has become a prominent cause for cancer-related death in that its 5-year survival rate is merely 17%, which has barely changed in the past decades.Citation5 In spite of great improvements in current therapeutic approaches for NSCLC patients, the clinical management remains unoptimistic, due to the fact that these cells are more resistant to traditional cytotoxic therapies than SCLC cells.Citation2,Citation6 Consequently, it is imperative to develop new drugs and to clarify their underlying mechanisms to help guide a more conscious individual therapy for these patients.
mTOR, one of the phosphatidylinositol kinase-related kinase (PIKK) family, is associated with different components to form two functionally distinct complexes, including mTOR complex 1 (mTORC1), which is comprised of mTOR, mammalian lethal with SEC13 protein 8 (mLST8), the rapamycin-sensitive adapter protein of mTOR (Raptor), 40kDa Proline-rich Akt substrate (PRAS40) and DEP domain-containing mTOR-interacting protein(DEPTOR),Citation7 and mTOR complex 2(mTORC2),Citation8,Citation9 which consists of mTOR, mLST8, rapamycin-insensitive companion of mTOR (Rictor) and mammalian stress-activated protein kinase-interacting protein (mSIN1).Citation10 Previous studies have demonstrated that mTOR, as an element of mTORC1, is phosphorylated and activated at S2448 by phosphatidylinositol 3-kinase(PI3K)/Akt signaling pathway, thus promoting the translation of various pivotal proteins mediating cell cycle progression and cell survival, for instance, c-myc and Cyclin D1,Citation11 through the phosphorylation of its downstream substrates eukaryotic initiation factor 4E binding protein 1 (4E-BP1) at Thr37/46/70 and Ser65,Citation12 and p70 ribosomal S6 kinase (p70 S6K) at Thr389.Citation13 Differently, mTORC2 positively modulates cell growth through the phosphorylation of AKT at Ser473.Citation5 Accordingly, AKT functions as the upstream factor of mTORC1 whereas downstream factor of mTORC2, indicating a crucial cross-talk between the two complexes.
Macroautophagy (hereafter regarded as autophagy), a highly conserved catabolic process mediated by a large number of autophagy-related genes (ATGs), enfolds cytoplasmic components including dysfunctional cellular organelles and misfolded proteins in double-membraned vesicles, commonly known as autophagosomes, thus delivering them to lysosomes for subsequent degradation and recycling to maintain essential viability of cancer cells under stressful conditions.Citation14 Activation of mTOR, a major negative regulator of autophagy, impedes its dissociation from the complex containing ATG13 and ULK1, therefore blocking the release of ULK1 and consecutive activation of FIP200, which is required for forming autophagosomes and initiating autophagy.Citation15 However, several researchers have demonstrated that autophagy contributes to the caspase-independent cell death through the inhibition of mTOR signaling pathway in NSCLC cells,Citation5 and colorectal cancer cells.Citation13
Previous investigation has implied that aberrant activation of mTOR signaling pathways are identified in various cancers such as cervical cancer,Citation16 lung cancer and ovarian carcinoma,Citation17 promoting tumorigenesis and enhancing tumor progression. Moreover, its abnormal activation has been reported to be involved in nearly 90% of NSCLC cell lines to stimulate cell survival and assist resist to chemoradiotherapies,Citation4 implying that targeting components of the signaling has become an attractive strategy for NSCLC patients. Currently, rapamycin analogues (temsirolimus and everolimus), known as allosteric inhibitors of mTORC1, have been put into practice to treat NSCLC cancer and colorectal cancer under clinical trials.Citation18 Unfortunately, these inhibitors have been suggested to be resistant in patients, due to the feedback activation of mTORC2 substrates upon mTORC1 inhibition.Citation19 Besides, dual mTORC1/2 inhibitors AZD8055 and OSI-027, which impair activation of both mTORC1 and mTORC2, are presently used to treat glioblastoma and endometrial carcinoma in research, receiving optimistic outcomes,Citation20 suggesting that dual inhibition is of great value in cancer treatments.
In this study, we found that the tumor inhibiting drug (−)-Guaiol not only blocked mTORC2 activity by suppressing mTOR phosphorylation at S2481, leading to the inhibition of its downstream effector AKT via phosphorylation at Ser473 to induce autophagic cell death, but also impeded mTORC1 signaling by inhibiting the phosphorylation of its substrates p70 S6K at Thr389 and 4E-BP1 at Thr37/46 through mTORC2/AKT signaling pathway in NSCLC cells, further enhancing the induction of autophagy. Moreover, the following cell survival analysis, colony formation assay and flow cytometry assay further confirmed that it triggered autophagic cell death via mTOR signaling pathway, which could be reversed by using the mTOR activator MHY1485. Above all, our studies provided evidence that (−)-Guaiol was superior to rapamycin, which plays a selective role in prohibiting mTORC1 signaling, in autophagy induction related chemotherapies for NSCLC patients, because it robustly blocked both mTORC1 and mTORC2 signaling. Thus, we assumed that it could be used as a potential substitute for rapamycin in treating NSCLC in the long run.
Results
(−)-Guaiol induces autophagy by regulating mTORC2 activity in NSCLC cells
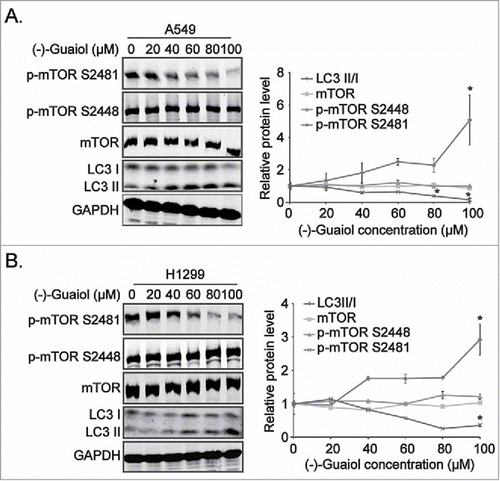
Based on the results from our previous high throughput screening,Citation2 the mTOR signaling that (−)-Guaiol was likely to participate in were selected for further investigation. To grope for its roles in the pathways, western blotting assays were applied to detect the expression of mTOR signaling effectors and autophagy associated proteins in NSCLC cells treated with different concentrations of (−)-Guaiol. Interestingly, it remarkably induced autophagy, indicated by the increasing upregulation of LC3 II/I, by specifically inhibiting the activity of mTOR, phosphorylated at Ser2481 (p-mTOR S2481) in a concentration dependent manner in both A549 and H1299 cells ( and ), without any effects on its phosphorylation at Ser2448 (p-mTOR S2448) and other pivotal autophagy associated proteins, such as PI3KC3, Cyclin D1, ATG13, pATG13 S355, BECLIN1, ULK1 andpULK1 S556, S758, S638 (Figure S1). Recent studies have shown that, upon activation, mTOR is specifically phosphorylated at Ser2448 by p70 S6K and AKT in association with mTORC1, whereas it is autophosphorylated at Ser2481 in association with mTORC2, thus these specific phosphorylation sites (Ser2448 and Ser2481) represent the activity of mTORC1 and mTORC2, respectively.Citation8,Citation9 Taking above data together, we assumed that (−)-Guaiol suppressed the activity of mTORC2 to induce autophagy in NSCLC cells.
Figure 1. (−)-Guaiol induces autophagy by inhibiting the mTORC2 activity in NSCLC cells. A-B. The total protein extracted from A549 (A) and H1299 (B) cells, treated with indicated concentrations of (−)-Guaiol, were subjected to western blotting analysis with indicated antibodies, using GAPDH as the internal control. The gray values of protein bands against GAPDH bands, automatically calculated using Image J software. Then the protein densitometry of (−)-Guaiol treated cells were normalized with that of untreated cells, which were recognized as relative protein level.
(−)-Guaiol targets the downstream effectors of mTORC2 in NSCLC cells
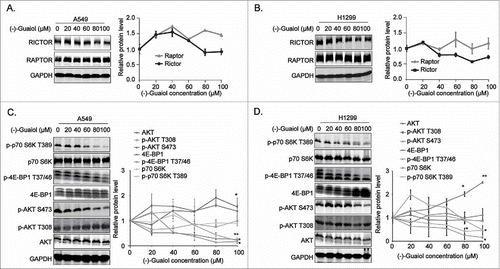
RAPTOR, a pivotal element of mTORC1, exerts the role as a scaffold to permit mTOR to bind and phosphorylate its substrates such as 4E-BP1 at Thr37/46/70 and Ser65 and p70 S6K at Thr389.Citation12,Citation13,Citation21 Whereas RICTOR acts as a critical scaffolding protein to maintain the mTORC2 integrity and regulate its downstream signals.Citation22 To gain more insights into the regulation of these complexes by (−)-Guaiol, we carried out the western blotting analysis to evaluate the expression of the pivotal components of mTORC1/2 in NSCLC cells treated with different concentrations of (−)-Guaiol. Unexpectedly, it had no obvious effects on the expression of RAPTOR and RICTOR in both A549 () and H1299 cells (). It is generally recognized that active Akt demands the phosphorylation at Ser473 by mTORC2, which leads to the enhanced phosphorylation of mTORC1 downstream substrates.Citation23 Therefore, we further explored the alternation of the downstream substrates of these two complexes. Results from western blotting assays showed that (−)-Guaiol significantly reduced the expression of p-p70 S6K T389, p-4E-BP1 T37/46 and p-AKT S473 in both A549 () and H1299 cells (), implicating that (−)-Guaiol suppressed the mTORC2 activity and its downstream factors, thus further inhibiting the mTORC1 downstream signaling factors.
Figure 2. (−)-Guaiol targets the downstream effectors of mTORC2 in NSCLC cells. A-D. The whole protein extracted from A549 (A, C) and H1299 (B, D) cells, treated with indicated concentrations of (−)-Guaiol, were applied for western blotting analysis with indicated antibodies, using GAPDH as a loading control. The relative protein levels were defined as the gray values of protein bands against GAPDH bands, which were automatically calculated using Image J software. Then the protein densitometry of (−)-Guaiol treated cells were normalized to that of untreated cells, which were recognized as relative protein level.
(−)-Guaiol impairs both mTOR signaling to induce autophagy in NSCLC cells
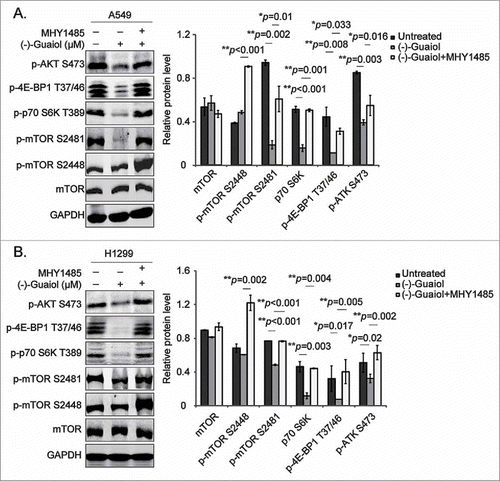
To further consolidate the role of (−)-Guaiol in regulating autophagy by targeting mTOR signaling, the mTOR activator, MHY1485,Citation24 was put into practice. Data from western blotting assays indicated that MHY1485, activating both mTORC1 and mTORC2, illustrated by p-mTOR S2488 and S2481, respectively, significantly reversed the inhibition of their downstream substrates p-p70 S6K T389, p-4E-BP1 T37/46 and p-AKT S473 in both A549 and H1299 cells ( and ). Thus, these results proved that (−)-Guaiol was engaged in promoting autophagy by targeting both mTORC1 and mTORC2 signaling, making it a better autophagy inducer than rapamycin, which was insensitive to mTORC2.Citation25
Figure 3. (−)-Guaiol induces autophagy by targeting mTOR pathways in NSCLC cells. A-B. Western blotting analysis of total protein from A549 (A) and H1299 (B) cells treated with or without (−)-Guaiol or (−)-Guaiol+MHY1485 in serum free medium for 24 h were conducted with indicated antibodies, taking GAPDH used as the internal control. The relative protein levels, which were used to statistical analysis, were calculated by normalizing the densitometry of treated cells to that of untreated cells.
(−)-Guaiol inhibits cell survival via mTOR signaling in NSCLC cells
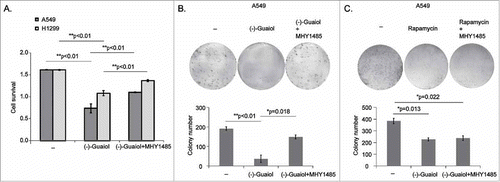
Recent studies have shown that mTORC1 promotes cell proliferation by activating p70 S6K at Thr389 and inhibiting 4E-BP1 activity, indicated by the increasing of p-4E-BP1 at Thr37/46, leading to the increasing translation of mRNAs encoding crucial proteins participating in cell proliferation, such as Cyclin D1 and c-myc.Citation16 Alternatively, active Akt has been reported to stimulate cell survival by impeding apoptosis through the inactivation of caspase-9, the BCL-2 family member Bad as well as forkhead transcription factors.Citation5 Together with our above results, we speculated that (−)-Guaiol could inhibit cell survival by targeting mTOR signaling. To confirm our assumption, we initially conducted the cell survival analysis by treating both A549 and H1299 cells with or without (−)-Guaiol or (−)-Guaiol together with MHY1485. As shown in , activation of mTOR by MHY1485 statistically reversed the inhibition of cell survival, in comparison with (−)-Guaiol treated cells. Moreover, data from colony formation assays showed that MHY1485 also significantly rescued the cell proliferation inhibited by (−)-Guaiol (). Unfortunately, the inhibition of cell survival by Rapamycin was not rescued by MHY1485 (), suggesting that (−)-Guaiol was likely to be a more specific mTOR inhibitor than Rapamycin. Therefore, we concluded that (−)-Guaiol inhibited cell survival via mTOR signaling.
Figure 4. (−)-Guaiol targets mTOR signaling to inhibit cell survival in NSCLC cells. A. Cell survival assays of A549 and H1299 cells treated with or without (−)-Guaiol or (−)-Guaiol and MHY1485 were conducted to evaluate the role of (−)-Guaiol in tumor inhibition. The ANOVA test was applied to analyze the significance. B-C. Colony formation assays were performed to verify the function of (−)-Guaiol (B) or Rapamycin (C) in suppressing cell proliferation. The Image J software was used to automatically count the colonies at a size of 80-infinity. The ANOVA test was utilized to analyze the significance.
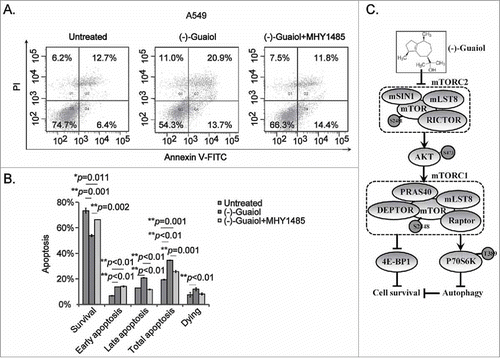
MHY1485 partially rescues the late apoptotic cells induced by (−)-Guaiol in NSCLC cells
According to data from our previous reports,Citation2 (−)-Guaiol targets RAD51 to the lysosome for degradation, triggering DSBs-induced cell apoptosis via caspase activities. Unfortunately, the pan caspase inhibitor Z-VAD-FMK could only partially rescue the apoptotic cells in (−)-Guaiol treated NSCLC cells, suggesting other signaling factors synergistically inducing apoptosis. Taking our above data into consideration, we thought that it might induce cell apoptosis via mTOR signaling. To support this hypothesis, we carried out the flow cytometry assays to test the apoptotic cells in NSCLC cells treated with or without (−)-Guaiol or (−)-Guaiol together with MHY1485. As expected, the administration of mTOR activator MHY1485 significantly reversed the percentage of late apoptotic cells in (−)-Guaiol treated A549 cells, without any significant impacts on the apoptotic cells at early stage ( and ). Consequently, our present studies provided sufficient data to support that (−)-Guaiol inhibited cell survival by targeting both mTORC1 and mTORC2 signaling in NSCLC cells, as shown in .
Figure 5. (−)-Guaiol targets mTOR signaling to promote cell apoptosis at late stage in NSCLC cells. A. Cell apoptosis was detected using flow cytometry analysis in A549 cells treated with or without (−)-Guaiol or (−)-Guaiol and MHY1485. B. The percentage of apoptotic cells (apoptosis %) from three independent tests were represented as mean ± STD and then statistically analyzed using ANOVA test. C. The schematic plot of the role of (−)-Guaiol in mTOR signaling pathway in NSCLC cells.
Discussion
Recent studies have confirmed that aberrant activation of mTOR signaling pathways are identified in a variety of cancers including ovarian carcinoma,Citation17 cervical cancer,Citation16 human primary nasopharyngeal carcinoma,Citation26 and NSCLC,Citation4 inciting cancer cells survival. Interestingly, our previous GO analysis of high throughput data revealed that (−)-Guaiol was engaged in mTOR signaling by downregulating some genes in NSCLC cells,Citation2 implicating its potential antitumor pattern. In this present investigation, our group first describe the mechanism that (−)-Guaiol blocks mTOR phosphorylation at Ser2481, without interfering with its phosphorylation at Ser2448 site and other pivotal components of autophagy associated proteins, further leading to the inhibition of mTORC2 substrates AKT activity, which in turn enhances the inhibition of the activity of mTORC1 substrates p70 S6K and 4E-BP1, to inhibit cell survival of NSCLC cells.
Results from western blotting assays demonstrated that (−)-Guaiol directly suppressed mTORC2 activity and its downstream signals, with no impact on the mTORC1 activity, demonstrated by diminished expression of p-mTOR S2481 and p-AKT S473, stable expression of p-mTOR S2448. It is commonly known that the entire AKT activation requires the prior phosphorylation at Ser473 by mTORC2 and further phosphorylation at Thr308 by 3-phophoinositide-dependent protein kinase 1 (PDK1).Citation27 From our data, (−)-Guaiol merely downregulated the expression of p-AKT S473, whereas it had no effect on p-AKT T308, implying that it wasn't involved in PDK1/AKT signaling pathway. Consequently, it is rational to consider that (−)-Guaiol has limited inhibitory effects on AKT activation. Recent studies have shown that inactive AKT inhibits mTORC1 signaling by activating its negative regulators, tuberous sclerosis complex 2 (TSC2) and PRAS40,Citation28,Citation29 thereby impairing mTORC1-mediated phosphorylation of 4E-BP1 at Thr70 and p70 S6K at Thr389.Citation23 Data from our present investigation uncovered that (−)-Guaiol significantly reduced the expression of p-p70 S6K T389 and p-4E-BP1 T37/46 in NSCLC cell lines, suggesting that it indirectly inhibited mTORC1 downstream signaling via mTORC2/AKT pathway.
It is well-known that downstream effectors of mTOR complexes including AKT,Citation10 4E-BP1 and p70 S6K,Citation16 are crucial modulators in cell survival and proliferation. Accordingly, we verified whether (−)-Guaiol could exert antitumor activities on NSCLC cells by targeting mTOR signaling through the administration of mTOR activator, MHY1485, which could impede the fusion between lysosomes and autophagosomes to inhibit autophagy.Citation24 In line with our hypothesis, results from cell survival analysis and colony formation assays demonstrated that (−)-Guaiol-triggered inhibition of cell survival and proliferation were partially rescued by MHY1485, which also activated mTORC1/2 and restored the expression of p-AKT (Ser473) and p-4E-BP1 (Thr37/46) and p-p70S6K (Thr389). It has been reported that decreased p-AKT S473 expression through mTORC2 inhibition dramatically reduces the expression of cell cycle associated proteins Cyclin D1 and CDK4, bringing about G0/G1 phase arrest and prohibited cell proliferation in hepatocellular carcinoma.Citation10 The phosphorylation of 4E-BP1 at Thr37/46 by mTORC1 stimulates its dissociation from the eukaryotic initiation factor 4E (eIF4E) and subsequent start-up of cap-dependent translation,Citation12 facilitating cervical cancer cell proliferation and cell survival by enhancing translation of some essential proteins involved in G1- to S-phase transition such as Cyclin D1 and c-myc.Citation16 Besides, activated p70 S6K modulates protein synthesis by phosphorylating ribosomal S6 protein, which promotes polypeptides extension at the ribosome, and subsequently boosting the translation of mRNA containing a 50-terminal oligopolypyrimidine, thereby stimulating glioblastoma cell growth.Citation7 Taking above mentioned into consideration, we considered (−)-Guaiol downregulated the expression of mTORC2 substrate p-AKT (Ser473), which in turn decreased the expression of mTORC1 downstream factors p-4E-BP1 (Thr37/46) and p-p70S6K (Thr389), causing slashed cell survival and proliferation, implicating that its cytotoxicity on NSCLC cells depended on mTOR signaling.
Moreover, mTOR pathways exert as pivotal regulators of autophagy initiation. Recent studies have revealed that mTORC2 negatively modulates autophagy process through activation of AKT/mTORC1 signaling pathway.Citation30 Activation of mTORC1 suppresses autophagy predominantly by regulating ULK1/Atg13/FIP200 complex activity, as indicated by phosphorylating and inhibiting ULK1 at Ser637/638/757/758 and in turn phosphorylating ATG13 at Ser318, which are involved in the initiation of autophagosome formation.Citation15 Our results demonstrated that (−)-Guaiol blocked the activation of mTORC2 and thus synergistically inhibited mTORC1/2 signaling to robustly induce autophagy. Consequently, it is rational to consider that vigorous autophagy induction by (−)-Guaiol is attributed to mTORC1-mediated suppression of autophagy initiation through mTORC2/AKT pathway in NSCLC cells. It is commonly known that autophagy exerts dichotomous function in cell survival and cell death. On the one hand, it can maintain essential viability of tumor cells under stressful conditions, such as hypoxia, nutrient deprivation, by degrading and recycling cytoplasmic components including dysfunctional cellular organelles and misfolded proteins.Citation14 On the other hand, once its level exceeds a certain threshold, it can also stimulate cell death by eliminating pivotal components required for survival, such as mitochondria.Citation3 Autophagic cell death, characterized by absent chromatin condensation, accumulation of cytoplasmic vacuolization, caspase-independent apoptosis and LC3 lipidation, has been reported to be repressed by autophagy inhibitors or knockout of ATGs.Citation31 Based on the statistics from our previous investigation,Citation2 (−)-Guaiol targeted RAD51 to the autophagy-lysosome mediated degradation, bringing about DSBs-triggered cell death, which could be partly restored by the pan caspase inhibitor Z-VAD-FMK and autophagy inhibitor 3MA in NSCLC cells, implicating that, apart from caspase-dependent apoptotic pathway, there were other ways facilitated inducing autophagic cell death. Consistently, studies from other researchers have demonstrated that Plumbagin, an antitumor drug, induces autophagic cell death in NSCLC cells through the inhibition of PI3K/Akt/mTORC1 pathway.Citation5 These results prompted us to further probe whether (−)-Guaiol functioned in a similar manner. Surprisingly, we observed that mTOR activation by MHY1485 partially restored the suppression of cell survival and cell proliferation triggered by (−)-Guaiol in NSCLC cells. Moreover, data from flow cytometry assays showed that MHY1485 could reverse the enhanced late apoptosis by (−)-Guaiol in A549 cells, accompanied with no impact on early apoptosis, implying that mTOR signaling were involved in inducing cell tapoptosis at the late stage.
Rapamycin has been commonly recognized as an allosteric inhibitor of mTORC1 for its rapid and special restraint on mTORC1, thus increasing ULK1 kinase activity and then inducing autophagy, whereas it is a relatively weaker autophagy inducer than dual mTORC1/2 inhibitors,Citation25 probably due to its restricted and inconstant influence on mTORC2.Citation32 Current studies have confirmed that mTORC1 inhibition by rapamycin decreased the protein expression of p-4E-BP1 and p-p70 S6K, leading to inhibitory cell proliferation and cell survival in cervical cancer.Citation16 Unfortunately, rapamycin administration inhibits mTORC1 activity to bring about feedback activation of mTORC2 in cancer cells, thus significantly reducing the induction of autophagy, leading to the resistance of patients to it.Citation33 Currently, our data provide a potential selection of (−)-Guaiol to substitute rapamycin, for its powerful inhibition of dual mTORC1/2 signaling pathways, indicated by reduced expression of p-p70 S6K T389, p-4E-BP1 T37/46 and p-AKT S473, thereby robustly inducing autophagic cell death in NSCLC cells. To gain more insights into the different mechanisms and antitumor effects between (−)-Guaiol and rapamycin on NSCLC cells, further studies remain to be performed.
Carcinogenic activation of mTOR signaling promotes cancer cell survival and proliferation,Citation34 giving prominence to the capacity of targeting components of the pathway as an efficient strategy against cancer. Based on our above results, (−)-Guaiol was confirmed to restrict cell growth and induce autophagic cell death in NSCLC cells by targeting mTOR signaling, making it a potent therapeutic selection for NSCLC patients. Recent studies have shown that the first generation of mTOR inhibitors rapamycin analogues (everolimus and temsirolimus), recognized as allosteric inhibitors of mTORC1, have been applied for treatment of advanced renal cell cancer,Citation35 and progressive pancreatic neuroendocrine cancer,Citation36 however, these inhibitors are comparatively well resistant in cancer patients, due to the fact that it inhibits mTORC1 to cause a feedback activation of mTORC2 downstream signals, which obviously reduces its capability in inducing autophagy.Citation19 The clinical restriction of rapamycin-based strategy accelerates the second generation of mTOR inhibitors, known as ATP-competitive inhibitors of mTOR. For instance, AZD8055 and OSI-027, which can simultaneously block mTORC1 and mTORC2 activity, are currently testing in patients with glioblastoma and endometrial carcinoma, and acquire encouraging outcomes.Citation20 Accordingly, the development of novel mTOR inhibitors, which can combinatorially target multiple members of mTOR pathway to avoid complex networks of negative feedback, is sorely required. In summary, our data provide abundant evidence that, different from mTORC1 inhibitors, (−)-Guaiol induces autophagic cell death to inhibit the proliferation and survival of NSCLC cells by powerfully suppressing both mTORC1 and mTORC2 signaling pathways, making it possibly substitute putative mTORC1 inhibitors in treating NSCLC patients with higher tumor inhibitory efficiency.
Materials and methods
Reagents and antibodies
(−)-Guaiol was commercially obtained from Sigma (448575) and diluted in methanol to prepare a stock solution at 40 mM, which was then stored at 4°C. The mTOR activator MHY1485 was purchased from Selleck (SML0810) and diluted in DMSO at a stock concentration 2 mM. Rapamycin was obtained from Sango (R706203) and diluted in DMSO at a stock concentration 5 mM. In the study, the following antibodies mTOR (MABS196), ULK1(MABC732), phospho-ULK1 Ser638 (p-ULK1 S638, MABC735) and ATG13 (ABC344), phospho-mTOR Ser2448 (p-mTOR S2448, 09–213), phospho-mTOR Ser2481(p-mTOR S2481, 09–343) were all purchased from Millipore. RICTOR (#2114), p70 S6K (#9202), phospho-p70 S6K Thr389 (p-p70 S6K T389, #9205), 4E-BP1 (#9644), phospho-4E-BP1 Thr37/46 (p-4E-BP1 T37/46, #2855), AKT (#9272), phospho-AKT Ser473/Thr308 (p-AKT S473/T308, #9271/ #9275), RAPTOR (#2280), LC3A/B (#4108), were from Cell Signaling Technology. Beclin1 (ab62557) was from Abcam, P62 (18420-1-AP) was from Proteintech, PI3KC3 (707842) was from Epitomics.
Cell culture and drug administration
The NSCLC cell lines A549 and H1299 were purchased from American Type Culture Collection (ATCC) and maintained in Dulbecco's Modified Eagle' Medium (DMEM) added with 10% FBS and 1% antibiotic at the 37°C incubator with 5% CO2. Cells at 70% density were treated with indicated concentrations of (−)-Guaiol in serum free medium for 24 h. In case of MHY1485, it was applied to treat cells at 10 μM in combination with (−)-Guaiol in serum free medium for 24 h.
Western blotting assay
After treatments, the NSCLC cells were lysed in the strong RIPA lysis supplemented with 1% phosphatase inhibitor complex and 1% protease inhibitor cocktail on ice. Then the whole protein were quantified and boiled in SDS. Afterwards, 60 μg protein were loaded onto the SDS-PAGE gels and transferred onto the nitrocellulose (NC) membrane in turn. Then the membranes were sealed with 5% BSA at room temperature for 1 h and incubated with primary antibodies at 4°C overnight. The next day, the membranes were washed with PBST for three times and incubated with appropriate secondary antibodies at 37°C for 1 h. Finally, the bands on the membranes were displayed on the Odyssey instrument. The grey values of bands, automatically calculated using Image J software, were used to statistically analyze the significance. Notably, the phosphorylated proteins were normalized with total proteins, and the total protein levels were normalized to the internal control GAPDH.
Apoptosis analysis
After treatments, the NSCLC cells were collected and washed in pre-cooled PBS. Afterwards, about 5 × 105 cells were treated with 50 μl binding buffer added with 2.5 μl Annexin-V for 10 min in a dark room, and then stained with 250 μl binding buffer added with 5 μl PI for 5 min away from light. Finally, the percentage of apoptotic cells was analyzed using the flow cytometry instrument (BD). Q1 region, dying cells, Q2 region, the late apoptotic cells, Q3 region, the survival cells, Q4 region, the early apoptotic cells. The total apoptotic cells were the amount of Q2 plus Q4.
Colony formation assay
The exponentially growing cells were trypsinized and counted using the Neubauer Counting Chamber under the microscope (Leica). About 1,000 cells were plated into 6-well plates, which were then treated with or without (−)-Guaiol or (−)-Guaiol and MHY1485 in serum free medium for 24 h the next day. Afterwards, the cells in the plates were left to grow for 10–14 days in fresh medium, which were then stained with 1% crystal violet and automatically counted using the Image J software at a size of 80-infinity.
Cell survival analysis
About 5,000 cells were seeded into the 96-well plates in triplicate and treated with or without (−)-Guaiol or (−)-Guaiol and MHY1485 in serum free medium for 24 h the next day. Afterwards, the cells were maintained in 100 μl fresh medium, which were then added with 10 μl CCK8 reagent (Beyotime) and left to incubate for another 2 h. Finally, the absorbance of 96-well plates were read at a wavelength of 450 nm using the Multiplate Reader.
Statistics
In the study, data from three independent experiments were represented as mean ± STD and statistically analyzed using the ANOVA test. One asterisk (*) means p < 0.05, two asterisks (**) mean p < 0.01.
Abbreviations
| DSBs | = | Double strand breaks |
| NSCLC | = | Non-small-cell lung cancer |
| mTOR | = | Mammalian target of rapamycin |
| SCLC | = | Small-cell lung cancer |
| PIKK | = | Phosphatidylinositol kinase-related kinase |
| mTORC1 | = | mTOR complex 1 |
| mTORC2 | = | mTOR complex 2 |
| Raptor | = | Rapamycin-sensitive adapter protein of mTOR |
| PRAS40 | = | 40kDa Proline-rich Akt substrate |
| DEPTOR | = | DEP domain-containing mTOR-interacting protein |
| Rictor | = | Rapamycin-insensitive companion of mTOR |
| mSIN1 | = | Mammalian stress-activated protein kinase-interacting protein |
| 4E-BP1 | = | Eukaryotic initiation factor 4E binding protein 1 |
| p70 S6K | = | p70 ribosomal S6 kinase |
| ATGs | = | Autophagy-related genes |
| PDK1 | = | 3-phophoinositide-dependent protein kinase 1 |
| TSC2 | = | Tuberous sclerosis complex 2 |
| eIF4E | = | Eukaryotic initiation factor 4E |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supp_mat_1451277_KCBT.zip
Download Zip (181.5 KB)Acknowledgments
This study was supported by the Natural Science Foundation of China (81502425, 81473627, 81673947, 81603590, 81371913 and 81472624).
Additional information
Funding
Reference
- Choudhary MI, Batool I, Atif M, Hussain S, Atta-Ur-Rahman. Microbial transformation of (−)-guaiol and antibacterial activity of its transformed products. J Nat Prod. 2007;70(5):849-52. doi:10.1021/np068052a.
- Yang Q, Wu J, Luo Y, Huang N, Zhen N, Zhou Y, Sun F, Li Z, Pan Q, Li Y. (−)-Guaiol regulates RAD51 stability via autophagy to induce cell apoptosis in non-small cell lung cancer. Oncotarget. 2016;7(38):62585–62597. doi:10.18632/oncotarget.11540.
- Kim N, Jeong S, Jing K, Shin S, Kim S, Heo JY, Kweon GR, Park SK, Wu T, Park JI, et al. Docosahexaenoic acid induces cell death in human non-small cell lung cancer cells by repressing mTOR via AMPK activation and PI3K/Akt inhibition. Biomed Res Int. 2015;2015:239764.
- Chen M, Du Y, Qui M, Wang M, Chen K, Huang Z, Jiang M, Xiong F, Chen J, Zhou J, et al. Ophiopogonin B-induced autophagy in non-small cell lung cancer cells via inhibition of the PI3K/Akt signaling pathway. Oncol Rep. 2013;29(2):430–6. doi:10.3892/or.2012.2131.
- Li YC, He SM, He ZX, Li M, Yang Y, Pang JX, Zhang X, Chow K, Zhou Q, Duan W, Zhou ZW, Yang T, Huang GH, et al. Plumbagin induces apoptotic and autophagic cell death through inhibition of the PI3K/Akt/mTOR pathway in human non-small cell lung cancer cells. Cancer Lett. 2014;344(2):239–59. doi:10.1016/j.canlet.2013.11.001.
- Han D, Li SJ, Zhu YT, Liu L, Li MX. LKB1/AMPK/mTOR signaling pathway in non-small-cell lung cancer. Asian Pac J Cancer Prev. 2013;14(7):4033–9. doi:10.7314/APJCP.2013.14.7.4033.
- Jhanwar-Uniyal M, Gillick JL, Neil J, Tobias M, Thwing ZE, Murali R. Distinct signaling mechanisms of mTORC1 and mTORC2 in glioblastoma multiforme: a tale of two complexes. Adv Biol Regul. 2015;57:64–74. doi:10.1016/j.jbior.2014.09.004.
- Sekulić A, Hudson CC, Homme JL, Yin P, Otterness DM, Karnitz LM, Abraham RT. A direct linkage between the phosphoinositide 3-kinase-AKT signaling pathway and the mammalian target of rapamycin in mitogen-stimulated and transformed cells. Cancer Res. 2000;60(13):3504–13.
- Copp J, Manning G, Hunter T. TORC-specific phosphorylation of mammalian target of rapamycin (mTOR): phospho-Ser2481 is a marker for intact mTOR signaling complex 2. Cancer Res. 2009;69(5):1821–7. doi:10.1158/0008-5472.CAN-08-3014.
- Chen BW, Chen W, Liang H, Liu H, Liang C, Zhi X, Hu LQ, Yu XZ, Wei T, Ma T, et al. Inhibition of mTORC2 induces cell-cycle arrest and enhances the cytotoxicity of doxorubicin by suppressing MDR1 expression in HCC cells. Mol Cancer Ther. 2015;14(8):1805–15. doi:10.1158/1535-7163.MCT-15-0029.
- Cargnello M, Tcherkezian J, Roux PP. The expanding role of mTOR in cancer cell growth and proliferation. Mutagenesis. 2015;30(2):169–76. doi:10.1093/mutage/geu045.
- Pause A, Belsham GJ, Gingras AC, Donzé O, Lin TA, Lawrence JC Jr, Sonenberg N. Insulin-dependent stimulation of protein synthesis by phosphorylation of a regulator of 5′-cap function. Nature. 1994;371(6500):762–7. doi:10.1038/371762a0.
- Huo HZ, Zhou ZY, Wang B, Qin J, Liu WY, Gu Y. Dramatic suppression of colorectal cancer cell growth by the dual mTORC1 and mTORC2 inhibitor AZD-2014. Biochem Biophys Res Commun. 2014;443(2):406–12. doi:10.1016/j.bbrc.2013.11.099.
- Degenhardt K, Mathew R, Beaudoin B, Bray K, Anderson D, Chen G, Mukherjee C, Shi Y, Gélinas C, Fan Y, et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell. 2006;10(1):p. 51. doi:10.1016/j.ccr.2006.06.001.
- Shang L, Chen S, Du F, Li S, Zhao L, Wang X. Nutrient starvation elicits an acute autophagic response mediated by Ulk1 dephosphorylation and its subsequent dissociation from AMPK. Proc Natl Acad Sci U S A. 2011;108(12):4788–93. doi:10.1073/pnas.1100844108.
- Ji J, Zheng PS. Activation of mTOR signaling pathway contributes to survival of cervical cancer cells. Gynecol Oncol. 2010;117(1):103–8. doi:10.1016/j.ygyno.2009.12.020.
- Faivre S, Kroemer G, Raymond E. Current development of mTOR inhibitors as anticancer agents. Nat Rev Drug Discov. 2006;5(8):671–88. doi:10.1038/nrd2062.
- Strimpakos AS, Karapanagiotou EM, Saif MW, Syrigos KN. The role of mTOR in the management of solid tumors: an overview. Cancer Treat Rev. 2009;35(2):148–59. doi:10.1016/j.ctrv.2008.09.006.
- Punt CJ, Boni J, Bruntsch U, Peters M, Thielert C. Phase I and pharmacokinetic study of CCI-779, a novel cytostatic cell-cycle inhibitor, in combination with 5-fluorouracil and leucovorin in patients with advanced solid tumors. Ann Oncol. 2003;14(6):931–7. doi:10.1093/annonc/mdg248.
- Zhang YJ, Duan Y, Zheng XF. Targeting the mTOR kinase domain: the second generation of mTOR inhibitors. Drug Discov Today. 2011;16(7–8):325–31. doi:10.1016/j.drudis.2011.02.008.
- Schalm SS, Blenis J. Identification of a conserved motif required for mTOR signaling. Curr Biol. 2002;12(8):632–9. doi:10.1016/S0960-9822(02)00762-5.
- Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14(14):1296–302.
- Xu Y, Liu C, Chen S, Ye Y, Guo M, Ren Q, Liu L, Zhang H, Xu C, Zhou Q, et al. Activation of AMPK and inactivation of Akt result in suppression of mTOR-mediated S6K1 and 4E-BP1 pathways leading to neuronal cell death in in vitro models of Parkinson's disease. Cell Signal. 2014;26(8):1680–9. doi:10.1016/j.cellsig.2014.04.009.
- Choi YJ, Park YJ, Park JY, Jeong HO, Kim DH, Ha YM, Kim JM, Song YM, Heo HS, Yu BP, et al. Inhibitory effect of mTOR activator MHY1485 on autophagy: suppression of lysosomal fusion. PLoS One. 2012;7(8):p. e43418. doi:10.1371/journal.pone.0043418.
- Zhou J, Tan SH, Nicolas V, Bauvy C, Yang ND, Zhang J, Xue Y, Codogno P, Shen HM. Activation of lysosomal function in the course of autophagy via mTORC1 suppression and autophagosome-lysosome fusion. Cell Res. 2013;23(4):508–23. doi:10.1038/cr.2013.11.
- Yang C, Peng J, Jiang W, Zhang Y, Chen X, Wu X, Zhu Y, Zhang H, Chen J, Wang J, Cho WC, et al. mTOR activation in immature cells of primary nasopharyngeal carcinoma and anti-tumor effect of rapamycin in vitro and in vivo. Cancer Lett. 2013;341(2):186–94. doi:10.1016/j.canlet.2013.08.004.
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307(5712):1098–101. doi:10.1126/science.1106148.
- Rajasekhar VK, Viale A, Socci ND, Wiedmann M, Hu X, Holland EC. Oncogenic Ras and Akt signaling contribute to glioblastoma formation by differential recruitment of existing mRNAs to polysomes. Mol Cell. 2003;12(4):889–901. doi:10.1016/S1097-2765(03)00395-2.
- Sancak Y, Thoreen CC, Peterson TR, Lindquist RA, Kang SA, Spooner E, Carr SA, Sabatini DM. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol Cell. 2007;25(6):903–15. doi:10.1016/j.molcel.2007.03.003.
- Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, Brown M, Fitzgerald KJ, Sabatini DM. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev Cell. 2006;11(6):859–71. doi:10.1016/j.devcel.2006.10.007.
- Liu G, Pei F, Yang F, Li L, Amin AD, Liu S, Buchan JR, Cho WC. Role of autophagy and apoptosis in non-small-cell lung cancer. Int J Mol Sci. 2017;18(2).
- Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22(2):159–68. doi:10.1016/j.molcel.2006.03.029.
- Chen Y, Wei H, Liu F, Guan JL. Hyperactivation of mammalian target of rapamycin complex 1 (mTORC1) promotes breast cancer progression through enhancing glucose starvation-induced autophagy and Akt signaling. J Biol Chem. 2014;289(2):1164–73. doi:10.1074/jbc.M113.526335.
- Polivka J Jr, Janku F. Molecular targets for cancer therapy in the PI3K/AKT/mTOR pathway. Pharmacol Ther. 2014;142(2):164–75.
- Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, Grünwald V, Thompson JA, Figlin RA, Hollaender N, et al. Efficacy of everolimus in advanced renal cell carcinoma: A double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372(9637):449–56. doi:10.1016/S0140-6736(08)61039-9.
- Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, Hobday TJ, Okusaka T, Capdevila J, de Vries EG, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):514–23. doi:10.1056/NEJMoa1009290.
