ABSTRACT
Genetic factors contribute to more than 40% of prostate cancer risk, and mutations in BRCA1 and BRCA2 are well-established risk factors. By using target capture-based deep sequencing to identify potential pathogenic germline mutations, followed by Sanger sequencing to determine the loci of the mutations, we identified a novel pathogenic BRCA2 mutation caused by a cytosine-to-guanine base substitution at position 4211, resulting in protein truncation (p.Ser1404Ter), which was confirmed by immunohistochemistry. Analysis of peripheral blood also identified benign polymorphisms in BRCA2 (c.7397T>C, p.Val2466Ala) and SRD5A2 (c.87G>C, p.Lys29Asn). Analysis of tumor tissues revealed seven somatic mutations in prostate tumor tissue and nine somatic mutations in esophageal squamous carcinoma tissue (single nucleotide polymorphisms, insertions, and deletions). Five-year follow-up results indicate that ADT combined with radiotherapy successfully treated the prostate cancer. To our knowledge, we are the first to report the germline BRCA2 mutation c.4211C>G (p.Ser1404Ter) in prostate cancer. Combined ADT and radiotherapy may be effective in treating other patients with prostate cancer caused by this or similar mutations.
Introduction
Prostate cancer (PCa) is one of the most common cancers affecting men, especially in developed countries. For example, in the United States it is estimated that there will be 161,360 new PCa cases in 2017, and 26,730 men will die from PCa.Citation1 Other than advanced age, family history is the strongest risk factor for PCa,Citation2 with approximately 42% of the risk for this disease attributed to genetic factors.Citation3 Compared with PCa caused by somatic mutations, hereditary PCa has earlier onset and higher rates of metastasis and mortality.Citation4 Previous studies have identified more than 70 PCa susceptible loci, which account for approximately 30% of the familial PCa risk.Citation5 Mutations in the tumor suppressor genes BRCA1 and BRCA2 are well-known genetic risk factors for this cancer.
BRCA1 and BRCA2 mutations are associated with an increased risk for many cancers including breast, ovarian, pancreatic, stomach, laryngeal, and fallopian tube cancer, as well as PCa.Citation6 The increased cancer risk in carriers of the BRCA1/BRCA2 mutations is predominantly in breast cancer and ovarian cancer for womenCitation7 and PCa for men.Citation8 Germline BRCA2 and BRCA1 mutations are present in 1.2% and 0.44% of PCa tumors, respectively.Citation9 Compared with the general population, the relative risk of PCa is 3.8 for carriers of BRCA1 mutations up to 65 years of ageCitation10 and 5 to 7 for carriers of BRCA2 mutations.Citation11,Citation12 Male BRCA mutation carriers with localized PCa are at substantially higher risk of dying from PCa than their non-mutation–carrying counterparts.Citation13 Moreover, BRCA2 contributes to early onset, with 1.2% patients younger than 65 years old carrying germline BRCA2 mutations.Citation9 BRCA1/2 mutations are also associated with higher Gleason scores,Citation14 and germline BRCA1/2 mutations confer a more aggressive phenotype with a higher probability of nodal involvement, distant metastasis, and shorter survival.Citation15
Tumors in BRCA mutation carriers that have defects in homologous recombination can be treated with radiotherapy, cisplatin, anthracyclines, or poly(ADP-ribose) polymerase inhibitors.Citation16,Citation17 In addition, radical local therapy (e.g., radical prostatectomy or radiotherapy) can be effective when performed early for PCa with BRCA2 mutations.Citation18 For metastatic castration-resistant PCa with biallelic inactivation of BRCA2, chemotherapy with platinum agents has been suggested.Citation19 In addition, Bryant et al.Citation16 reported that poly(ADP-ribose) polymerase inhibitors are efficacious in cancers with homologous recombination defects in tumors deficient in BRCA1 and BRCA2 but not in tumors with functional BRCA1 or BRCA2 proteins. However, optimal treatment strategies for specific mutations are unclear. Here we report a patient with locally advanced PCa carrying a novel germline BRCA2 mutation. The patient was treated with androgen deprivation therapy (ADT) combined with radiotherapy, and serum prostate-specific antigen (PSA) levels were within the normal range for almost 4 years, even after stopping ADT.
Results
Case presentation
In May 2011, a 46-year-old Chinese man with dysuria lasting for 6 months was referred to Daping Hospital of the Third Military Medical University. Results of digital rectal examination revealed a hard and enlarged prostate with irregularities. Laboratory test results showed elevated serum levels of total PSA (56.39 ng/ml) and free PSA (10.30 ng/ml). Pelvic magnetic resonance imaging (MRI) scans showed lesions in the peripheral zone of the prostate with low-intensity signal (3.4 × 4.6 cm) and multiple enlarged pelvic lymph nodes with high-intensity signal (largest node, approximately 1.5 × 2.2 cm) on T2-weighted imaging (). Ultrasound-guided transrectal needle biopsies obtained June 23, 2011 confirmed prostate adenocarcinoma (Gleason score 5 + 4). Immunohistochemistry results showed positive staining for Ki-67, p504S, and PSA, and negative results for p63 and 34βE2. Results of positron emission tomography (PET)-computed tomography (CT) showed a high level of asymmetrical 18F fluorodeoxyglucose (FDG) uptake in the prostate along the left side, confirming metastasis in pelvic lymph nodes. The patient had no relevant family history, and his parents had died of unknown causes.
Figure 1. MRI scans of the patient before and after treatment for prostate cancer. (A–E) T1-weighted image (T1WI) and T2-weighted image (T2WI), as well as the sagittal view and lymph node images from T2WI from June 21, 2011 to April 13, 2017. Red arrows indicate the tumor in the prostate and lymph node metastasis.
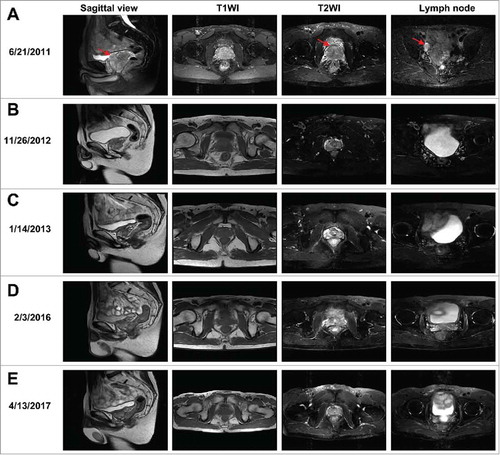
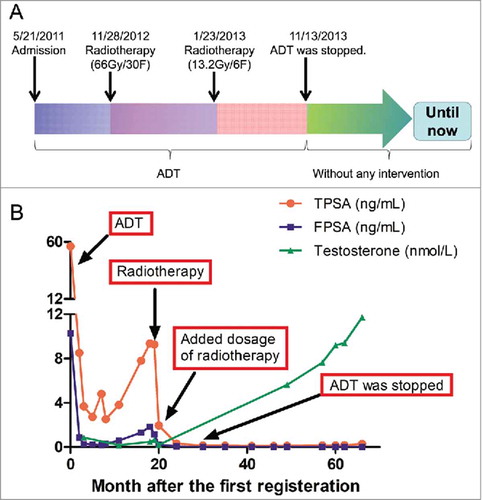
To treat this locally advanced PCa, ADT was initiated immediately with goserelin injections and oral bicalutamide, and testosterone and total/free PSA levels were monitored during and after treatment (). Serum PSA levels were maintained within the normal range for approximately 17 months, but total PSA (9.36 ng/ml) was found to be elevated on November 9, 2012. Results of MRI performed on November 26, 2012 () showed that the pelvic lymph nodes and tumor had shrunk (2.1 × 2.7 cm), presumably as a result of ADT. Local radiotherapy (66 Gy/30 F or 2.2 Gy/F) was carried out November 28, 2012. MRI results on January 4, 2013 showed the tumor had shrunk further (1.6 × 2.4 cm) (). To treat lymph nodes, pelvic radiotherapy (13.2 Gy/6 F or 2.2 Gy/F) was continued until January 23, 2013. Because total and free PSA levels were still within the normal range, ADT was discontinued November 13, 2013, and on follow-up MRI on February 3, 2016, the prostate tumor was barely detectable (). The prostate tumor appeared to be under control for 4 years without further intervention (). In addition, serum testosterone level had returned to normal, and the patient's libido and sexual activity recovered completely.
Figure 2. Treatment regimen and laboratory test results during and after treatment for prostate cancer. (A) Therapeutic schedule. (B) Serum levels of TPSA, FPSA, and testosterone during and after treatment. FPSA, free prostate-specific antigen; TPSA, total prostate-specific antigen. ADT: Androgen deprivation therapy.
In May 2015, the patient was again referred to our hospital because of dysphagia lasting for a month. Gastroscopy revealed an esophageal mass (29 × 34 cm) far from the incisors, and biopsy results suggested esophageal squamous carcinoma. PET-CT images showed a high level of asymmetrical FDG uptake in the esophagus only. On May 11, 2015, the patient underwent esophageal resection and thoracoscopic lymph node resection.
Identification of pathogenic mutation
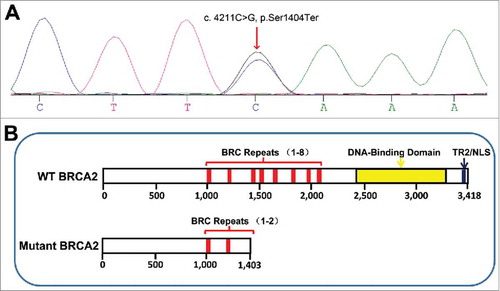
To identify potential pathogenic mutations, we screened a panel of seven genes (BRCA2, CHEK2, ELAC2, HSD17B3, HSD3B2, RNASEL, and SRD5A2) using the patient's peripheral blood and identified a novel germline BRCA2 mutation: Ser1404Ter caused by a C>G point mutation at position 4211 (). We also identified two germline mutations in BRCA2 (c.7397T>C, p.Val2466Ala) and SRD5A2 (c.87G>C, p.Lys29Asn) that resulted in benign polymorphisms (data not shown). In addition, somatic mutations were identified by whole exome sequencing. The following seven somatic single nucleotide polymorphism/indel mutations were identified in PCa tumor tissue: TP53 (c.1049T>C, p.L350P), PIK3CB (c.2527G>C, p.A843P), MLL (c.2806T>A, p.S936T), PTCH1 (c.2075>A, p.V692E), and TERT (c.-58-u5148C>A; c.-58-u3620G>A; c.-58-u1324T>C). The following nine somatic single nucleotide polymorphism/indel mutations were identified in esophageal squamous carcinoma tissue: TP53 (c.743G>A, p.R248Q; c.713G>C, p.C238S), PIK3CA (c.1636C>A, p.Q546K), PTPRD (c.5083G>A, p.E1695K), MLL3 (c.4093-2A>G; c.10249C>A, p.Q3417K), OR4C6 (c.541C>A, p.Q181K), MSH6 (c.124C>G, p.P42A), and TMPRSS2 (c.589G>A, p.V197M).
Figure 3. (A) BRCA2 mutation in patient with prostate cancer and esophageal squamous carcinoma. (B) Diagram of wild type (WT) Citation(ref. 37) and mutant BRCA2 proteins, as predicted from cDNA and genomic sequencing. NLS, nuclear localization signal; TR2, RAD51-binding domain.
Expression and subcellular location of the truncated BRCA2 protein
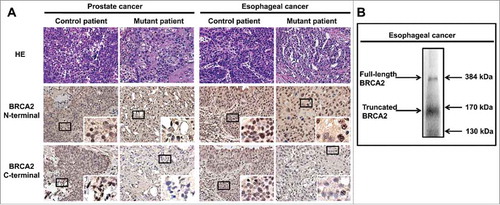
To determine whether the patient's BRCA2 mutation resulted in a truncated protein, we analyzed prostate and esophageal tumor tissues by immunohistochemistry. Using the BRCA2 C-terminal antibody, fewer BRCA2-positive cells were detected in the patient's prostate and esophagus tumor tissues compared with control tissues (). However, using the BRCA2 N-terminal antibody, the number of BRCA2-positive cells in the tumor tissues was comparable between the patient and control, confirming the presence of a truncated protein (). Levels of full-length BRCA2 in the patient's tumor tissues were considerably lower than those of control tissues, presumably due to the heterozygosity of the BRCA2 mutation expressing the truncated protein. Truncated BRCA2 protein was detected primarily in the cytoplasm, whereas full-length BRCA2 protein was detected primarily in the nucleus (. Furthermore, by using western blot, we identified the truncated BRCA2 protein at around 170 kDa ().
Figure 4. Immunohistochemistry and western blot analysis of tumor tissues. (A) The patient's prostate and esophageal tumor tissues were stained with antibodies against BRCA (C-terminus and N-terminus) and compared with the corresponding control tumor tissues. HE, hematoxylin and eosin. (B) The patient's esophageal tumor tissues kept in liquid nitrogen were collected, lysed and then analyzed with western blot assays with specific antibodies against BRCA2 N-terminus.
Discussion
To the best of our knowledge, this is the first description of PCa caused by a germline BRCA2 mutation in a Chinese patient, and the first report of the BRCA mutation c.4211C>G, which results in a truncated protein (p.Ser1404Ter). We also demonstrated that PCa associated with this mutation is sensitive to ADT combined with radiotherapy.
BRCA2 encodes a 3418-amino acid protein containing eight BRC repeats, a DNA-binding domain, and a nuclear localization signal.Citation20 As a component of the double-strand break (DSB) repair machinery, BRCA2 interacts with RAD51 through the BRC repeats and the RAD51-binding domain at its C-terminus (residues 3196–3232).Citation21,Citation22 The mutation identified in this report introduces a premature stop codon, resulting in a truncated 1403-amino acid protein (). Analysis of previously reported BRCA2 mutations suggests that truncation producing a protein smaller than 3308 amino acids severely affects protein function.Citation23 The truncated BRAC2 identified in our patient contains only two of the eight BRC repeats and lacks the essential C-terminal domain. Loss of the nuclear localization signal () may account for cytoplasmic localization of the truncated BRAC2. Based on these observations, we conclude that this mutation leads to loss of function.
Previous studies have described BRCA2 mutations associated with esophagus cancer, including the mutations c.203G>A,Citation24 c.10462A>G (p.Ile3412Val), c.8415G>T (p.Lys2729Asn),Citation25 and c.10204A>T (p.Lys3326Ter).Citation26,Citation27 The mutation identified in this case (C.4211C>G, p.Ser1404Ter) also appears increase the risk of esophageal cancer, suggesting that special attention should be paid to patients with germline mutations of BRCA2 during examination of the upper aero-digestive tract. This mutation may also increase the risk of other cancers, given the important role of BRCA2 in DNA repair.
According to the two-hit hypothesis,Citation28 multiple mutations are necessary to cause cancer. Thus, accumulation of acquired and uncorrected somatic mutations is expected in individuals with germline BRCA2 mutations. Indeed, whole exome sequencing revealed multiple somatic mutations in our patient's prostate and esophageal tumor tissues. Misrepair or inefficient repair of DSBs can lead to genetic instability and ultimately carcinogenesis.Citation29,Citation30 Indeed, BRCA2 has been identified as one of the most common mutations among the 63 pathogenic germline mutations (PPGMs) in cancers.Citation31 In our patient, haploinsufficiency of BRCA2 and inefficient DSB repair is likely to be the pathologic mechanism underlying the development of cancer. Consistent with the finding that most cancer-causing somatic mutations are associated with chromatin remodeling and DNA repair,Citation32 we found that both tumors in our patient had mutations in the tumor protein p53 (TP53), phosphatidylinositol-4,5-bisphosphate 3-kinase (PIK3C), and mixed lineage leukemia (MLL) pathways. However, we do not have direct evidence linking these somatic mutations to the BRCA2 truncation.
Most PCa patients carrying BRCA2 mutations are treated with radical local therapy with or without adjuvant ADT.Citation32 Here we assessed the effect of initial ADT and subsequent radiotherapy on PCa associated with a BRCA2 mutation. The initial ADT appeared to be effective, as evidenced by decreased PSA level and reduced tumor size, suggesting an ADT-sensitive tumor. However, the tumor become ADT-resistant, as evidenced by an increase in total PSA level (9.36 ng/ml); therefore, radiotherapy was administered.
Radiation therapy, which is used to treat many solid tumors, directly induces DSBs and indirectly induces other types of DNA damage, in part by producing reactive oxygen species. Signals from the damaged DNA trigger cell cycle arrestCitation33 and activate the DNA repair machinery. Although unrepaired DNA damage in normal cells can lead to tumorigenesis, irreparable DNA damage in cancer cells leads to apoptosis.Citation34 Because of its role in DNA repair, BRCA2 plays pivotal roles in both tumorigenesis and radiotherapy. BRCA2 haploinsufficiency increases sensitivity to DNA-damaging agents, as evidenced by multiple somatic mutations in the patient's prostate and esophagus tumors. On the other hand, cancer cells expressing truncated BRCA2 are expected to be more responsive to radiotherapy. Polymorphisms in genes such as XRCC3 and RAD51 are also associated with radiosensitivity,Citation29 but whether the SRD5A2 mutation (c.87G>C, p.Lys29Asn) identified in our patient plays a role in radiosensitivity is unclear. Nevertheless, the treatment outcome of our patient indicates that ADT combined with radiotherapy was effective for PCa caused by this BRCA2 germline mutation.
In conclusion, we believe we are the first to describe this germline mutation of BRCA2 (c.4211C>G, p.Ser1404Ter) in a patient with PCa, which was effectively treated with ADT and radiotherapy.
Materials and methods
All procedures involving human participants were carried out in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Daping Hospital of Third Military Medical University waived institutional review board approval for the study; however, written informed consent for the use of medical records and related images was obtained from the patient.
Identification of patient mutations
Germline DNA was extracted from the patient's leukocytes, and tumor DNA was extracted from PCa and esophageal squamous carcinoma tissues using the QIAamp DNA Micro Kit (Qiagen, Hilden, Germany), according to the manufacturer's instructions. In addition, total DNA purified from the patient's peripheral blood was analyzed by target capture-based deep sequencing (BGI Health, China) to identify potential mutations in the following genes: BRCA2, CHEK2, ELAC2, HSD17B3, HSD3B2, RNASEL, and SRD5A2. The potential mutations were analyzed by Sanger sequencing to determine the loci and then compared with reference sequences in GenBank (http://www.ncbi.nlm.nih.gov/genbank/) using Mutation Surveyor version 2.51 (SoftGenetics LLC). Whole exome sequencing of the tumor DNA was performed by Geneplus-Beijing Institute (Beijing, China).
Immunohistochemistry
Tissue biopsies (greatest dimension, 1.5 mm) were taken from clinical specimens of the patient and two controls lacking the identified mutation (another patient with PCa and a patient with esophagus cancer). As previously described,Citation35,Citation36 the paraffin-embedded tissues were sectioned (4 mm thick), mounted on glass slides, and baked at 60°C for 6 h. After deparaffinization with xylene, the sections were rehydrated in graded ethanol and 3% hydrogen peroxide to block endogenous peroxidase activity. Goat serum (ZSGB-BIO, China) was used to block nonspecific interactions before incubating the sections at 4°C with specific primary antibodies against the BRCA2 C-terminus (amino acids 2587–2601, Abcam ab53887, Cambridge, MA, USA) and BRCA2 N-terminus (amino acids 100–150 amino acids, Proteintech 19791-1-ap, Rosemont, IL, USA). After incubation with secondary antibody conjugated to streptavidin-biotin-horseradish peroxidase complex, the tissue sections were counterstained with hematoxylin and eosin, dehydrated, and covered with coverslips. Images of the slides were obtained with an Olympus CCD camera (Tokyo, Japan) connected to a Nikon Eclipse Ti-S inverted microscope (Tokyo, Japan) and captured with NIS-Elements F3.2 imaging software. The images were assessed by two urological pathologists.
Western blot
The esophageal squamous carcinoma tissues of the patient kept in liquid nitrogen were collected and lysed in a RIPA buffer containing a protease inhibitor cocktail tablet and phosphotase inhibitor cocktail (KeyGEN BioTECH, NanJing, China). 80 ug of tissue protein were separated by SDS-PAGE and transferred onto PVDF membrane (PALL, NY). After 1 h of incubation in blocking solution (5% BSA in PBS), primary antibody: BRCA2 N-terminus (amino acids 100–150 amino acids, Proteintech 19791-1-ap, Rosemont, IL, USA) was used to dected the truncated protein.
Table 1. The levels of TPSA, FPSA and testosterone of the patient during the treatment.
Disclosure of potential conflicts of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Acknowledgments
We thank all the patients for their participation. Medbanks (Beijing, China) Network Technology Co., Ltd was thanked for the data collection.
References
- American Society of Clinical O: The State of Cancer Care in America, 2017: A Report by the American Society of Clinical Oncology. J Oncol Pract. 2017; 13(4): p. e353–e394.
- Edwards SM, Eeles RA. Unravelling the genetics of prostate cancer. Am J Med Genet C Semin Med Genet. 2004;129C(1):65–73. doi:10.1002/ajmg.c.30027. PMID:15264274.
- Lichtenstein P, Holm NV, Verkasalo PK, Iliadou A, Kaprio J, Koskenvuo M, Pukkala E, Skytthe A, Hemminki K. Environmental and heritable factors in the causation of cancer–analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. 2000;343(2):78–85. doi:10.1056/NEJM200007133430201. PMID:10891514.
- Cremers RG, Aben KK, van Oort IM, Sedelaar JP, Vasen HF, Vermeulen SH, Kiemeney LA. The clinical phenotype of hereditary versus sporadic prostate cancer: HPC definition revisited. The Prostate. 2016;76(10):897–904. doi:10.1002/pros.23179. PMID:26989049.
- Eeles RA, Olama AA, Benlloch S, Saunders EJ, Leongamornlert DA, Tymrakiewicz M, Ghoussaini M, Luccarini C, Dennis J. Jugurnauth-Little S, et al. Identification of 23 new prostate cancer susceptibility loci using the iCOGS custom genotyping array. Nat Genet. 2013;45(4):385–391, 391e381–382. doi:10.1038/ng.2560.
- Roy R, Chun J, Powell SN. BRCA1 and BRCA2: different roles in a common pathway of genome protection. Nat Rev Cancer. 2011;12(1):68–78. doi:10.1038/nrc3181. PMID:22193408.
- King MC, Marks JH, Mandell JB. New York Breast Cancer Study G: Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302(5645):643–6. doi:10.1126/science.1088759. PMID:14576434.
- Levy-Lahad E, Friedman E. Cancer risks among BRCA1 and BRCA2 mutation carriers. Br J Cancer. 2007;96(1):11–15. doi:10.1038/sj.bjc.6603535. PMID:17213823.
- Kote-Jarai Z, Leongamornlert D, Saunders E, Tymrakiewicz M, Castro E, Mahmud N, Guy M, Edwards S, O'Brien L, Sawyer E, et al. BRCA2 is a moderate penetrance gene contributing to young-onset prostate cancer: implications for genetic testing in prostate cancer patients. Br J Cancer. 2011;105(8):1230–4. doi:10.1038/bjc.2011.383. PMID:21952622.
- Leongamornlert D, Mahmud N, Tymrakiewicz M, Saunders E, Dadaev T, Castro E, Goh C, Govindasami K, Guy M, O'Brien L, et al. Germline BRCA1 mutations increase prostate cancer risk. Br J Cancer. 2012;106(10):1697–701. doi:10.1038/bjc.2012.146. PMID:22516946.
- Thompson D, Easton D, Breast Cancer Linkage C. Variation in cancer risks, by mutation position, in BRCA2 mutation carriers. Am J Hum Genet. 2001;68(2):410–9. doi:10.1086/318181. PMID:11170890.
- Breast Cancer Linkage C. Cancer risks in BRCA2 mutation carriers. J Natl Cancer Inst. 1999;91(15):1310–6. doi:10.1093/jnci/91.15.1310. PMID:10433620.
- Castro E, Goh C, Leongamornlert D, Saunders E, Tymrakiewicz M, Dadaev T, Govindasami K, Guy M, Ellis S, Frost D, et al. Effect of BRCA Mutations on Metastatic Relapse and Cause-specific Survival After Radical Treatment for Localised Prostate Cancer. Eur Urol. 2015;68(2):186–93. doi:10.1016/j.eururo.2014.10.022. PMID:25454609.
- Mitra A, Fisher C, Foster CS, Jameson C, Barbachanno Y, Bartlett J, Bancroft E, Doherty R, Kote-Jarai Z, Peock S, et al. Prostate cancer in male BRCA1 and BRCA2 mutation carriers has a more aggressive phenotype. Br J Cancer. 2008;98(2):502–7. doi:10.1038/sj.bjc.6604132. PMID:18182994.
- Castro E, Goh C, Olmos D, Saunders E, Leongamornlert D, Tymrakiewicz M, Mahmud N, Dadaev T, Govindasami K, Guy M, et al. Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J Clin Oncol. 2013;31(14):1748–57. doi:10.1200/JCO.2012.43.1882. PMID:23569316.
- Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, Kyle S, Meuth M, Curtin NJ, Helleday T. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434(7035):913–7. doi:10.1038/nature03443. PMID:15829966.
- Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, Santarosa M, Dillon KJ, Hickson I, Knights C, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434(7035):917–21. doi:10.1038/nature03445. PMID:15829967.
- Bratt O, Loman N. Clinical Management of Prostate Cancer in Men with BRCA Mutations. Eur Urol. 2015;68(2):194–5. doi:10.1016/j.eururo.2014.11.005. PMID:25465969.
- Cheng HH, Pritchard CC, Boyd T, Nelson PS, Montgomery B. Biallelic Inactivation of BRCA2 in Platinum-sensitive Metastatic Castration-resistant Prostate Cancer. Eur Urol. 2016;69(6):992–5. doi:10.1016/j.eururo.2015.11.022. PMID:26724258.
- McAllister KA, Haugen-Strano A, Hagevik S, Brownlee HA, Collins NK, Futreal PA, Bennett LM, Wiseman RW. Characterization of the rat and mouse homologues of the BRCA2 breast cancer susceptibility gene. Cancer Res. 1997;57(15):3121–5. PMID:9242436.
- Davies OR, Pellegrini L. Interaction with the BRCA2 C terminus protects RAD51-DNA filaments from disassembly by BRC repeats. Nat Struct Mol Biol. 2007;14(6):475–83. doi:10.1038/nsmb1251.
- Esashi F, Galkin VE, Yu X, Egelman EH, West SC. Stabilization of RAD51 nucleoprotein filaments by the C-terminal region of BRCA2. Nat Struct Mol Biol. 2007;14(6):468–74. doi:10.1038/nsmb1245.
- Sugano K, Nakamura S, Ando J, Takayama S, Kamata H, Sekiguchi I, Ubukata M, Kodama T, Arai M, Kasumi F, et al. Cross-sectional analysis of germline BRCA1 and BRCA2 mutations in Japanese patients suspected to have hereditary breast/ovarian cancer. Cancer Sci. 2008;99(10):1967–76. doi:10.1111/j.1349-7006.2008.00944.x. PMID:19016756.
- Hu N, Li WJ, Su H, Wang C, Goldstein AM, Albert PS, Emmert-Buck MR, Kong LH, Roth MJ, Dawsey SM, et al. Common genetic variants of TP53 and BRCA2 in esophageal cancer patients and healthy individuals from low and high risk areas of northern China. Cancer Detect Prev. 2003;27(2):132–8. doi:10.1016/S0361-090X(03)00031-X. PMID:12670525.
- Kaushal M, Chattopadhyay I, Phukan R, Purkayastha J, Mahanta J, Kapur S, Saxena S. Contribution of germ line BRCA2 sequence alterations to risk of familial esophageal cancer in a high-risk area of India. Dis Esophagus. 2010;23(1):71–75. doi:10.1111/j.1442-2050.2009.00975.x. PMID:19473207.
- Delahaye-Sourdeix M, Anantharaman D, Timofeeva MN, Gaborieau V, Chabrier A, Vallee MP, Lagiou P, Holcatova I, Richiardi L, Kjaerheim K, et al. A rare truncating BRCA2 variant and genetic susceptibility to upper aerodigestive tract cancer. J Natl Cancer Inst. 2015;107(5): djv037. doi:10.1093/jnci/djv037.
- Akbari MR, Malekzadeh R, Nasrollahzadeh D, Amanian D, Islami F, Li S, Zandvakili I, Shakeri R, Sotoudeh M, Aghcheli K, et al. Germline BRCA2 mutations and the risk of esophageal squamous cell carcinoma. Oncogene. 2008;27(9):1290–6. doi:10.1038/sj.onc.1210739. PMID:17724471.
- Knudson AG, Jr. Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A. 1971;68(4):820–3. doi:10.1073/pnas.68.4.820. PMID:5279523.
- Vral A, Willems P, Claes K, Poppe B, Perletti G, Thierens H. Combined effect of polymorphisms in Rad51 and Xrcc3 on breast cancer risk and chromosomal radiosensitivity. Mol Med Rep. 2011;4(5):901–12. PMID:21725594.
- Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396(6712):643–9. doi:10.1038/25292. PMID:9872311.
- Robinson DR, Wu YM, Lonigro RJ, Vats P, Cobain E, Everett J, Cao X, Rabban E, Kumar-Sinha C, Raymond V, et al. Integrative clinical genomics of metastatic cancer. Nature. 2017;548(7667):297–303. doi:10.1038/nature23306. PMID:28783718.
- Borrego-Soto G, Ortiz-Lopez R, Rojas-Martinez A. Ionizing radiation-induced DNA injury and damage detection in patients with breast cancer. Genet Mol Biol. 2015;38(4):420–32. doi:10.1590/S1415-475738420150019. PMID:26692152.
- Redon CE, Nakamura AJ, Zhang YW, Ji JJ, Bonner WM, Kinders RJ, Parchment RE, Doroshow JH, Pommier Y. Histone gammaH2AX and poly(ADP-ribose) as clinical pharmacodynamic biomarkers. Clin Cancer Res. 2010;16(18):4532–42. doi:10.1158/1078-0432.CCR-10-0523. PMID:20823146.
- Deckbar D, Jeggo PA, Lobrich M. Understanding the limitations of radiation-induced cell cycle checkpoints. Crit Rev Biochem Mol Biol. 2011;46(4):271–83. doi:10.3109/10409238.2011.575764. PMID:21524151.
- Tong D, Liu Q, Liu G, Xu J, Lan W, Jiang Y, Xiao H, Zhang D, Jiang J. Metformin inhibits castration-induced EMT in prostate cancer by repressing COX2/PGE2/STAT3 axis. Cancer letters. 2016;389:23–32. doi:10.1016/j.canlet.2016.12.031. PMID:28043910.
- Liu Q, Yuan W, Tong D, Liu G, Lan W, Zhang D, Xiao H, Zhang Y, Huang Z, Yang J, et al. Metformin represses bladder cancer progression by inhibiting stem cell repopulation via COX2/PGE2/STAT3 axis. Oncotarget. 2016;7(19):28235–46. doi:10.18632/oncotarget.8595. PMID:27058422.
- Edwards SL, Brough R, Lord CJ, Natrajan R, Vatcheva R, Levine DA, Boyd J, Reis-Filho JS, Ashworth A. Resistance to therapy caused by intragenic deletion in BRCA2. Nature. 2008;451(7182):1111–5. doi:10.1038/nature06548. PMID:18264088.
