ABSTRACT
Advanced gastric cancer with a pathological complete response to neoadjuvant chemotherapy and surgery followed by early brain metastasis is rare. A 52-y-old male patient who was diagnosed with advanced gastric cancer (cT4N2M0, stage ШB). Radiological examinations after three cycles of preoperative chemotherapy with a modified FOLFOX6 (mFOLFOX6) regimen showed a partial response (PR) had been achieved. The patient underwent curative surgery consisting of proximal gastrectomy, and D2 lymph node dissection. The lack of abnormal gastric cancer cells in the primary lesion or lymph nodes confirmed a pathological complete response (pCR). Postoperative chemotherapy with oral S-1 was administrated. However, during the second cycles of postoperative chemotherapy, the patient experienced headaches, projectile vomiting and convulsion. Upon further examination, a tumor representing metastasis to the brain was recognized by cranial enhanced magnetic resonance imaging (MRI) examination and cytopathology of cerebrospinal fluid. In addition to documenting the case report, we reviewed the literature associated to features of metastatic brain malignancies that form from gastric cancer. In short, advanced gastric cancer patents achieved pCR after preoperative chemotherapy typically have good prognosis; however, great attention should be paid on detecting metastatic events.
Introduction
Gastric cancer is a major global health concern, especially in China, where nearly 679,000 newly diagnosed cases and 498,000 gastric cancer-related deaths occurred in 2015.Citation1 For the lack of a well-established screening system, approximately 90 percent of all gastric cancers were detected in advanced stages in China thereby resulting in a poor prognosis.Citation2 In recent y, clinical studies have indicated that preoperative chemotherapy of gastric cancer could reduce tumor size, downstage the primary tumor, and improve the R0 resection rates; thereby promoting a potential survival benefit.Citation3,Citation4 Cases in which advanced gastric cancer patients obtained pCR after neoadjuvant chemotherapy were less commonly seen in routine clinical practice; nonetheless, the observed cases typically showed favorable survival rates.Citation5
Clinical studies have reported that metastasis from gastric cancer usually occurs in the abdominal cavity, peritoneum, lymph nodes and liver; metastasis limited to the brain is rare.Citation6 York et al observed 3320 patients diagnosed with advanced gastric cancer at M.D. Anderson Cancer Center (Houston, TX, USA); only 24 of these patients (0.7%) developed brain metastases.Citation7 Go et al reported a similar incidence rate of brain metastasis from gastric cancer at 0.62%.Citation8
To the best of our knowledge, the incidence of the cases achieved pCR after neoadjuvant chemotherapy was rarely seen in daily clinical practice, and a better prognosis could be obtained in such patients than those haven't achieved pCR. Metastasis limited to the brain from gastric cancer is also less common. The current study presents a rare case of advanced gastric cancer with a pCR to preoperative chemotherapy and surgery followed by early brain metastasis during the course of postoperative chemotherapy.
Case report
A 52-y-old male patient presenting with meal hind full bilge for one month and was admitted to the Affiliated Cancer Hospital of Zhengzhou University (Henan, China) on February 5, 2015. Physical examination revealed slight anemia (hemoglobin [Hb], 10.4 g/dl). The serum levels of carcinoembryonic antigen (CEA) and carbohydrate antigen (CA) 19–9 were 3.79 ng/ml (normal range, <3.5 ng/ml) and 138.3 U/ml (normal range, <30 U/ml), respectively. Abdominal computed tomography (CT) showed that the gastric wall was thickened, and metastatic lymph nodes were visible of the stomach (). Gastrointestinal fiberscopy (GIF) of the upper gastrointestinal tract indicated type 3 advanced gastric carcinoma, and the biopsy specimen revealed poorly differentiated adenocarcinoma (). The patient was diagnosed with advanced gastric carcinoma (cT4N2M0, stage ШB), according to the 7th edition of the Union for International Cancer Control (UICC) TNM classification in gastric cancer. In an attempt to downstage the disease, neoadjuvant chemotherapy was performed after obtaining the patient's informed consent. Details of the mFOLFOX6 regimen were: a 2-h intravenous injection of oxaliplatin (85 mg/m2) on d 1, a 2-h intravenous injection of leucovorin (400 mg/m2) on d 1, an intravenous injection of 5-FU (400 mg/m2), and then a 46-h continuous infusion (2400 mg/m2) on d 1; two weeks one cycle. After three cycles, CT was performed to evaluate the effect of neoadjuvant chemotherapy. The toxicity of the chemotherapy was classified using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE version 4.0).Citation9 Adverse effects include grade 1 gastrointestinal disorders and grade 2 leukocytopenia; these side effects were transient and could remit spontaneously, or leukocytopenia could relieved by the using granulocyte colony-stimulating factor (G-CSF). The patient's CEA and CA19-9 levels decreased within normal limits, measuring 2.04 ng/ml and 16.83 U/ml, respectively. Abdominal CT revealed reductions in the thickness of the gastric wall and a decrease in the size of the lymph nodes along the stomach (). The clinical response was classified as a PR according to the Response Evaluation Criteria in Solid Tumors (RECIST version 1.1).Citation10
Figure 1. (A) Enhanced CT prior to preoperative chemotherapy, a mass in the gastric wall. (B) Enhanced CT following three cycles of preoperative chemotherapy revealing that the lesion was clearly decreased in size.
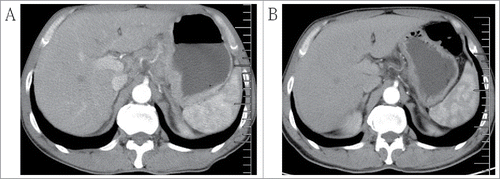
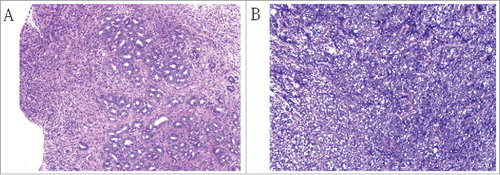
Figure 2. (A) Pre-surgical pathology of biopsy specimen revealing adenocarcinoma of the stomach, Magnification, x100. (B) Post-surgical pathology of the mass revealing a small amount of degenerated nuclear large cells and inflammatory cells infiltration, Magnification, x100.
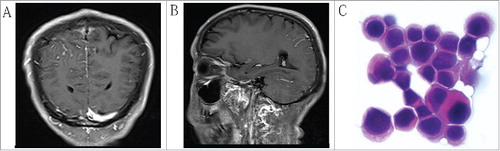
Surgery was performed on April 13, 2015. Laparotomy revealed neither ascites nor peritoneal dissemination; lymph nodes were observed in the left gastric artery, common hepatic artery and celiac artery with 1 cm × 1 cm in size, no lymph node swelling was observed in paraaortic tissues. Therefore, proximal gastrectomy and D2 lymph nodes dissection were performed. Gastric cancer cells were not detected in the primary lesion or associated lymph nodes post-surgery (). Histological change was diagnosed as significant response (Complete remission), both in the primary lesion and in the associated lymph nodes, according to NCCN clinical practice guidelines in oncology for gastric cancer.Citation11 There were no postoperative complications observed. The patient was discharged on the tenth d after surgery. On May 14, 2015, S-1 adjuvant chemotherapy (80 mg/m2 on d 1–14, followed by a 1-week drug-free interval) was delivered; the regimen was well tolerated by the patient during the first cycle. However, during the second cycles of postoperative chemotherapy, the patient began to suffer headaches, projectile vomiting, and convulsions. MRI of the brain on June 25, 2015 implied vascular image in the right parietal lobe ( and ). Scans for other sites of metastatic disease were negative. Cytopathology of cerebrospinal fluid revealed the presence of a heterocyst (). Due to financial difficulty, the patient voluntarily abandoned treatment, and died one month later.
Discussion
Clinical studies have reported that preoperative chemotherapy of gastric cancer could downstage the primary tumor, improve R0 resection rates, and is associated with favorable survival. During the course of neoadjuvant chemotherapy, the commonly used FOLFOX regimen is effective for advanced gastric cancer, with a response rate between 50–69.7%.Citation12,Citation13 A phase II study indicated the radiology response rate was 45.8% and the pCR rate was 3.0% by using the mFOLFOX6 regimen as a neoadjuvant chemotherapy strategy.Citation14 In the meantime, other studies demonstrated the pCR rates were 0–15% for this cohort of patients.Citation15-Citation17 Cho et al reviewed 22 advanced gastric cancer patients who achieved a pCR with neoadjuvant chemotherapy; although 12 patients (54.5%) did not receive postoperative chemotherapy, the OS rates at 3 and 5 y were 96% and 85%, respectively.Citation5 Zhang et al indicated 23 patients with advanced gastric cancer who achieved a pCR after neoadjuvant chemotherapy, and a favorable prognosis was achieved.Citation18 However, in our study, the patient with advanced gastric cancer with a complete response after preoperative chemotherapy and surgery developed brain metastasis and died less than three months later even with the administration of postoperative chemotherapy.
Previous studies revealed the incidence of brain metastasis from gastric cancer was less than 1% of clinical cases.Citation7,Citation8,Citation19 In one study from the USA, 714 patients received preoperative therapy for gastric or gastroesophageal junction (GEJ) adenocarcinoma from 1985 to 2009, 60 patients achieved pCR; there were no significant differences in local/regional (LR) recurrence or distant metastasis (43% LR vs 57% distant) between pCR and non-pCR groups; however, one-third of the recurrences in the pCR group were brain recurrences; first brain recurrences was the main reason of failure compared to recurrences of other sites in pCR patients (36 vs 4%, P = 0.01); furthermore, the incidence of brain recurrences in all pCR patients were 8%.Citation20 Cho et al analyzed 22 advanced gastric cancer patients who achieved a pCR after neoadjuvant chemotherapy, and only one patient (4.5%) developed brain recurrence.Citation5 Zhang et al found the incidence of brain recurrence in pCR patients were 4.3% (1/23).Citation18 The patients achieved a pCR correlated with an increased risk of brain recurrence, and the potential reasons were: firstly, those patients may have a micro-metastatic disease in systemic circulation, and are more likely to have a non- central nervous system(CNS) site of first recurrence; secondly, the blood brain barrier could potentially prevent chemotherapeutic agents from entering the CNS in the treatment of advanced gastric cancer.Citation21 He et al reported surgical trauma under general anesthesia disrupted the blood–brain barrier, which may contribute to cancer cells migrating into the CNS.Citation22 Sakurai et al found a case of early gastric cancer (T1bN0M0) developed brain metastasis eight y after curative resection.Citation23 These may indicate gastric cancer cells could lie dormant for a long time, and they could recur as a virulent resistant disease. To date, the detailed mechanism of brain metastasis is still under investigation.
Those patients with brain metastasis from gastric cancer had a poor response to pharmacological treatment and an unfavorable prognosis. Treatment options include surgical resection (SR), brain radiotherapy, steroids, chemotherapy or a combination strategy. York et al detected median survival was approximately 2 months in patients with gastric cancer with brain metastases who underwent steroids only or whole-brain radiation therapy (WBRT) combined with steroids, the median survival of patients who underwent surgery, WBRT, and steroids were slightly greater than one y; moreover, the results also indicated a younger age and less extensive systemic disease in the patients who underwent surgical resection followed by WBRT were associated with an improved survival.Citation7 Kasakura et al reported the median survival was 24.0 weeks in patients underwent surgery or surgery followed by WBRT whereas the median survival was 10.8 weeks in patients received WBRT alone, a statistically significant difference was observed between the two groups (P = 0.0177).Citation19 Matsunaga et al revealed two patients were detected combined with brain metastases from GEJ adenocarcinoma, both of which had a long-term survival by receiving surgical resection followed by stereotactic radiosurgery.Citation24 Bartelt et al indicated performance status and the number of brain metastases were independent prognostic factors for OS in patients with brain metastases from gastrointestinal tract cancer treated by WBRT.Citation25 Unfortunately, in the present case, the patient abandoned treatment because of financial hardship, and died within one month. Currently, the available treatment strategies are limited in improving survival. For patients with good performance status, aggressive treatment may contribute to a survival benefit. However, the best approach to the treatment of patients with brain metastases from gastric carcinoma remains unclear. Future studies are needed to determine the optimal treatment approach for this disease.
Conclusions
For patients of Gastric cancer, cases achieved pCR after preoperative chemotherapy have a favorable prognosis. Although the risk of brain recurrence is rare, great attention should be paid on these patients who developed CNS symptoms.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by the National Natural Science Foundation of China [grant number 81773230], the Science and Technology Innovative Research Group of Zhengzhou city [grant number 121PCXTD524], and the Joint Research Funds of Henan province and the Minister of Health of China [grant number 201201009].
Additional information
Funding
References
- Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–32. doi:10.3322/caac.21338. PMID:26808342.
- Yu M, Zheng HC, Xia P, Takahashi H, Masuda S, Takano Y, Xu HM. Comparison in pathological behaviours & prognosis of gastric cancers from general hospitals between China & Japan. Indian J Med Res. 2010;132:295–302. PMID:20847376.
- Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi:10.1056/NEJMoa055531. PMID:16822992.
- Ychou M, Boige V, Pignon JP, Conroy T, Bouché O, Lebreton G, Ducourtieux M, Bedenne L, Fabre JM, Saint-Aubert B, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29:1715–21. doi:10.1200/JCO.2010.33.0597. PMID:21444866.
- Cho H, Nakamura J, Asaumi Y, Yabusaki H, Sakon M, Takasu N, Kobayashi T, Aoki T, Shiraishi O, Kishimoto H, et al. Long-term survival outcomes of advanced gastric cancer patients who achieved a pathological complete response with neoadjuvant chemotherapy: a systematic review of the literature. Ann Surg Oncol. 2015;22:787–92. doi:10.1245/s10434-014-4084-9. PMID:25223927.
- Deng J, Liang H, Wang D, Sun D, Pan Y, Liu Y. Investigation of the recurrence patterns of gastric cancer following a curative resection. Surg Today. 2011;41:210–5. doi:10.1007/s00595-009-4251-y. PMID:21264756.
- York JE, Stringer J, Ajani JA, Wildrick DM, Gokaslan ZL. Gastric cancer and metastasis to the brain. Ann Surg Oncol. 1999;6:771–6. doi:10.1007/s10434-999-0771-3. PMID:10622506.
- Go PH, Klaassen Z, Meadows MC, Chamberlain RS. Gastrointestinal cancer and brain metastasis: a rare and ominous sign. Cancer. 2011;117:3630–40. doi:10.1002/cncr.25940. PMID:21319152.
- National Institutes of Health, National Cancer Institute, US Department of Health and Human Services. Handbook of Common Terminology Criteria for Adverse Events (CTCAE), Version 4.0. Bethesda (MD): NIH Publication. 2009.
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47. doi:10.1016/j.ejca.2008.10.026. PMID:19097774.
- Ajani JA, D'Amico TA, Almhanna K, Bentrem DJ, Chao J, Das P, Denlinger CS, Fanta P, Farjah F, Fuchs CS, et al. Gastric Cancer, Version 3.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2016;14:1286–312. doi:10.6004/jnccn.2016.0137. PMID:27697982.
- Zhang J, Chen RX, Zhang J, Cai J, Meng H, Wu GC, Zhang ZT, Wang Y, Wang KL. Efficacy and safety of neoadjuvant chemotherapy with modified FOLFOX7 regimen on the treatment of advanced gastric cancer. Chin Med J (Engl). 2012;125:2144–50. PMID:22884144.
- Li ZY, Koh CE, Bu ZD, Wu AW, Zhang LH, Wu XJ, Wu Q, Zong XL, Ren H, Tang L, et al. Neoadjuvant chemotherapy with FOLFOX: improved outcomes in Chinese patients with locally advanced gastric cancer. J Surg Oncol. 2012;105:793–9. doi:10.1002/jso.23009. PMID:22189752.
- Wang X, Zhao L, Liu H, Zhong D, Liu W, Shan G, Dong F, Gao W, Bai C, Li X. A phase II study of a modified FOLFOX6 regimen as neoadjuvant chemotherapy for locally advanced gastric cancer. Br J Cancer. 2016;114:1326–33. doi:10.1038/bjc.2016.126. PMID:27172250.
- Wang Y, Yu YY, Li W, Feng Y, Hou J, Ji Y, Sun YH, Shen KT, Shen ZB, Qin XY, et al. A phase II trial of Xeloda and oxaliplatin (XELOX) neo-adjuvant chemotherapy followed by surgery for advanced gastric cancer patients with para-aortic lymph node metastasis. Cancer Chemother Pharmacol. 2014;73:1155–61. doi:10.1007/s00280-014-2449-1. PMID:24748418.
- Jary M, Ghiringhelli F, Jacquin M, Fein F, Nguyen T, Cleau D, Nerich V, El Gani M, Mathieu P, Valmary-Degano S, et al. Phase II multicentre study of efficacy and feasibility of dose-intensified preoperative weekly cisplatin, epirubicin, and paclitaxel (PET) in resectable gastroesophageal cancer. Cancer Chemother Pharmacol. 2014;74:141–50. doi:10.1007/s00280-014-2482-0. PMID:24824852.
- Yoshikawa T, Tanabe K, Nishikawa K, Ito Y, Matsui T, Kimura Y, Hasegawa S, Aoyama T, Hayashi T, Morita S, et al. Accuracy of CT staging of locally advanced gastric cancer after neoadjuvant chemotherapy: cohort evaluation within a randomized phase II study. Ann Surg Oncol. 2014;21(Suppl 3):S385–389. doi:10.1245/s10434-014-3615-8. PMID:24595801.
- Zhang Y, Peng Z, Chen L. Survival analysis of gastric cancer cases with pathological complete response received neoadjuvant chemotherapy. Zhonghua Yi Xue Za Zhi. 2016;96:1582–4. PMID:27266687.
- Kasakura Y, Fujii M, Mochizuki F, Suzuki T, Takahashi T. Clinicopathological study of brain metastasis in gastric cancer patients. Surg Today. 2000;30:485–90. doi:10.1007/s005950070112. PMID:10883456.
- Fields RC, Strong VE, Gonen M, Goodman KA, Rizk NP, Kelsen DP, Ilson DH, Tang LH, Brennan MF, Coit DG, et al. Recurrence and survival after pathologic complete response to preoperative therapy followed by surgery for gastric or gastrooesophageal adenocarcinoma. Br J Cancer. 2011;104:1840–7. doi:10.1038/bjc.2011.175. PMID:21610705.
- Chabner BALD. Cancer chemotherapy and biotherapy: principles and practice. Philadelphia (PA): Lippincott Williams & Wilkins; 2011.
- He HJ, Wang Y, Le Y, Duan KM, Yan XB, Liao Q, Liao Y, Tong JB, Terrando N, Ouyang W. Surgery upregulates high mobility group box-1 and disrupts the blood-brain barrier causing cognitive dysfunction in aged rats. CNS Neurosci Ther. 2012;18:994–1002. doi:10.1111/cns.12018. PMID:23078219.
- Sakurai K, Muguruma K, Murata A, Toyokawa T, Amano R, Kubo N, Tanaka H, Yashiro M, Maeda K, Ohira M, et al. Early gastric cancer with suspected brain metastasis arising eight y after curative resection: a case report. BMC Res Notes. 2014;7:818. doi:10.1186/1756-0500-7-818. PMID:25411022.
- Matsunaga M, Wada S, Daa T, Harada K, Okamura K, Noguchi T. Long-term survival after resection of brain metastases from esophagogastric junction adenocarcinoma: report of two cases and review of the literature. Clin J Gastroenterol. 2014;7:213–8. doi:10.1007/s12328-014-0491-5. PMID:26183738.
- Bartelt S, Momm F, Weissenberger C, Lutterbach J. Patients with brain metastases from gastrointestinal tract cancer treated with whole brain radiation therapy: prognostic factors and survival. World J Gastroenterol. 2004;10:3345–8. doi:10.3748/wjg.v10.i22.3345. PMID:15484315.