ABSTRACT
Background: Endometrial cancer (EC) occurs most commonly after menopause. A proportion of patients present with advanced age and comorbidities, and become ineligible for surgery. The optimal treatment strategy of these patients remains a clinical challenge. Aromatase inhibitor (AI) combined with Gonadotropin-releasing hormone agonist (GnRH-a) possesses profound effect in suppressing the estrogen level, has become a valid treatment in the breast cancer. However, the combined use of an AI and a GnRH-a in EC has rarely been studied.
Case presentation: Herein, we report the combination of an AI and a GnRH-a in the treatment of three patients with advanced age or comorbidities who were ineligible for surgery. The disease remained stable for two years in patients who received the combination treatment as an initial approach without any adverse effects. Moreover, an AI combined with a GnRH-a also effective as salvage treatment of recurrent patients. Further, we provide a brief review of the literature.
Conclusion: The combination of an AI and a GnRH-a presents satisfactory therapeutic effect and provides an optimal option for inoperable EC patients.
Background
Endometrial cancer (EC) is the most common female reproductive system tumour and the fourth most frequent cancer in women. Almost 70% of patients have localized disease with a 95% 5-year survival rate. Good prognosis is a result of presentation with early-stage and low-grade disease at diagnosis and the application of surgery. However, patients with advanced age or with additional medical disorders who are ineligible for surgery occur in a small percentage (3-9%).Citation1 As the elderly population is expected to grow and the incidence of both chronic disease and cancer increases with age, the number of cancer patients with advanced age and comorbidities ineligible for surgery will increase substantially. Oncologists are faced with the challenge of determining optimal treatment strategy.
Radiotherapy is currently accepted first-line treatment for patients who are ineligible for primary surgery, and may provide some measure of pelvic control and long-term progression free survival (PFS). However, complications related to vaginal, urinary, gastrointestinal (GI) systems have also been well documented. Chemotherapy is less common in elderly patients, especially when comorbidities are co-exit, which may limit treatment options due to the increasing toxicity of specific chemotherapy agents and reduced in life-expectancy.Citation2 Hormonal therapy is another effective alternative treatment for inoperable patients. Aromatase inhibitors (AIs) can reduce the oestrogen level of both pre- and postmenopausal EC patients. Gonadotropin-releasing hormone agonists (GnRH-as) are mainly used to suppress ovarian function in premenopausal women. In addition to decreasing oestrogen level, recent studies had proven its direct antitumor effects, and may also effective for postmenopausal EC patients. An AI in combined with a GnRH-a can inhibit oestrogen more deeply, and has become a valid treatment for breast cancer. However, it has been rarely studied in EC patients. Herein, we report three cases who are ineligible for surgery due to advanced age or comorbidities treated with the combination of an AI and a GnRH-a. All the three patients and their relatives were given fully explanation that this is not traditional treatment, and provide their informed consents.
Case reports
Case 1: A 72-year-old woman with a history of colon cancer surgery was referred to our hospital with the chief complaint of an intrauterine echogenic mass found 2 months prior and vaginal bleeding over the following 5 months. Bimanual examination revealed slight tenderness on the left side of the umbilicus. Ultrasound showed a 28 × 24 × 15 mm intrauterine heterogeneous mass, protruding to the uterine angle. Magnetic resonance imaging (MRI) showed that EC, which had invaded into the superficial muscle layer of the posterior wall. Dilation and curettage was performed, pathological analysis of uterus scarping demonstrated endometrial adenocarcinoma. Given the patient's advanced age and several episodes of myocardial infarction, and insufficient pulmonary function, she was considered at high-risk for surgical morbidity. Moreover, both the patient and family members refused radiotherapy and chemotherapy. Hormonal treatment consisted of letrozole (2.5 mg/day, per os) and leuprolide acetate depot (3.75 mg/28 days subcutaneously) was administered for 6 cycles. After one month of treatment, vaginal bleeding stopped, and ultrasound demonstrated obvious shrinking of the echogenic mass (-Aa and, -Ab). Disease was stable over a 2-year follow-up.
Figure 1. (Aa) 2013-5-7: ultrasound of Case 1 before treatment, tumour was 22·1 × 19·2 × 25·9 mm. (Ab) 2013-6-4: Ultrasound of Case 1 after one month of treatment, tumour was 17·1 × 13·3 × 14·4 mm. (Ba) 2016-5-3: Ultrasound of Case 2 after relapse, tumour was 16 × 14 × 12 mm. (Bb) 2016-8-2: Ultrasound of Case 2 after 2 cycles treatment for relapse, tumour was 13·8 × 13 × 7·5 mm. (Ca) 2016-7-15: Magnetic resonance imaging of Case 3 before combination therapy for relapse. (Cb) 2016-12-23: Magnetic resonance imaging of Case 3 after 6 cycles combination therapy for relapse.
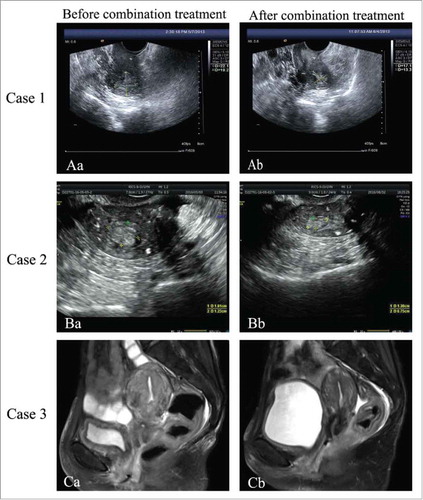
Case 2: An 89-year-old woman with a 40-year history of asthma, who had menopausal transition 37 years prior, presented to our hospital with uterine bleeding lasting one month. The ultrasound performed at another institution revealed a 25 × 29 × 17 mm intrauterine mass. She underwent hysteroscopy for uterine polyp removal and fraction curettage, the pathology indicated endometrial hyperplasia with polyp formation, local atypical hyperplasia, and focal malignant transformation. Because of her advanced age, history of asthma, hypertension, and aortic valve insufficiency, she refused surgery and radiotherapy. Letrozole (2.5 mg/day per os) and leuprolide acetate depot (3.75 mg/28 days subcutaneously) was administered over 6 cycles, vaginal bleeding was absent after one month. The disease was stable for two years after the last hormonal therapy. However, vaginal bleeding reoccurred in April 2016. Ultrasound examination demonstrated tumour was 16 × 14 × 12mm in size (-Ba). Letrozole (2.5 mg/day per os) concomitant with the use of leuprolide acetate depot (3.75 mg/28 days subcutaneously) was given for 2 cycles, vaginal bleeding stopped and the tumor reduced in size (-Bb). This patient was alive until now.
Case 3: A 49-year-old postmenopausal female admitted to our hospital due to irregular vaginal bleeding in August 2015. Ultrasound revealed a 77 × 56 × 44 mm intrauterine mass, the thickness of anterior uterus muscle wall was 0.3 cm, and both the uterine isthmus and cervix were enlarged. MRI showed that the intrauterine mass invaded the cervix and myometrium which was considered stage II. Endometrium biopsy indicated malignant tumor, considering adenocarcinoma with mucus change, and necrosis. This patient had a body mass index (BMI) of 13.72 kg/m2 and comorbidities include arrhythmia, paroxysmal atrial fibrillation, cachexia, moderate anemia. She received radiotherapy as 60Gy/30 fractions till April 2016. However, vaginal bleeding recurred in July 2016. Given her poor performance status, letrozole (2.5 mg/day per os) combined with goserelin acetate depot (3.75 mg/28 days subcutaneously) was administered as salvage treatment, vaginal bleeding was controlled after 6 cycles, changes on MRI were remarkable (-Ca and, -Cb). The patient remains with stable to date.
Discussion
Hormonal therapy is indicated for patients with advanced or recurrent EC and is more effective in hormone receptor-positive grade I or II tumours.Citation3 Furthermore, hormonal treatment is less expensive and quite convenient with fewer side effects, and is more applicable for patients with poor performance status and/or multiple comorbidities. High-dose oral progesterone or progesterone-containing intrauterine systems as conservative treatment have been frequently administered in early-stage EC and has become an alternative to hysterectomy for such women. However, many EC patients who are with old age and ineligible extremely poor candidates for surgery will not tolerate high dose oral progestin therapy due to associated adverse effects, include thromboembolism, weight gain, and edema.Citation4 The levonorgestrel-releasing intrauterine device (LNG-IUD) is usually used in young women whose lesions limited in endometrium and superior to high dose oral progestin. lists hormonal therapy as inoperable treatment for EC patients with comorbidities. LNG-IUD is frequently applied in early stage patients with the follow up ranging from 3 to 118 months. William et al.Citation5 analyze the effect of LNG-IUD in 41 inoperable atypical endometrial hyperplasia or EC patients who were younger than 45 years, which is the largest case series. Eighteen of 36 patients complete response (50%), four of the 18 complete response patients later experienced relapse of hyperplasia or cancer. Furthermore, the patients responding to treatment had significantly down-regulation expression of progesterone receptor (PR) on post-treatment biopsies than before. Additionally, decline or loss of PR isoform expression results in progesterone resistance, treatment failure and disease progression. Apart from LNG-IUD, some studies have also reported the application of AIs and GnRH-as for these patients and have demonstrated considerable results.
Table 1. Hormonal treatment as inoperable treatment for endometrial cancer patients with comorbidity.
The mechanism of action and clinical use of aromatase inhibitors
AIs block the conversion of androstenedione and testosterone to oestrone and oestradiol by inhibiting aromatase which ubiquitously expressed in different human tissues including the testis, adipose tissue, bone, skin, muscle, breast and brain, with the exception for placenta and ovaries.Citation6 There are mainly two sources of oestrogen in the postmenopausal EC patients whose ovarian production of oestrogen has ceased. In peripheral tissues, androgens can be converted into oestrogen by aromatase to increase circulating oestrogen levels. Furthermore, higher levels of aromatase activity and oestrogen synthesis have been detected in the malignant endometrium compared to normal endometrial tissue.Citation7 Thus, the increase in local and circulating concentrations of oestrogens contributes to the progression of EC.Citation7
The successful application of AIs in patients ineligible for surgery has been reported. Bogliolo et al.Citation8 reported a 79-year-old woman with advanced EC (FIGO stage IV) who was unfit for surgery due age and several comorbidities. After administration of anastrozole (1 mg/day), following chemotherapy, lung lesions disappeared, the endometrial thickness reduced and abnormal uterine bleeding stopped. Barker et al.Citation9 reported the results of a retrospective study on the effect of AIs (anastrozole or letrozole) in 16 patients with endometrial hyperplasia or endometrial adenocarcinoma ineligible for surgical treatment. They found that AIs reduced endometrial thickness in some patients with localized endometrial adenocarcinoma, but was not effective for metastatic patients in preventing disease progression. Altman et al.Citation10 conducted a study in which AIs as first-line or second-line treatment used in women with endometrial adenocarcinoma who were ineligible for surgery, clinical response rate was 70%. Mean overall survival (OS) was 151 weeks and mean treatment time was 68 weeks.
The mechanism of action and clinical use of GnRH-as
GnRH or luteinising hormone-releasing hormone (LHRH) agonists lead to an initial increase in gonadotropins secretion, with a subsequent decline of gonadal sex hormones to castrate levels.Citation11 In addition to decreasing oestrogen levels to inhibit the progression of EC, some reports have suggested that high levels of LH and FSH stimulate EC cell growth in vitro, and GnRH-as may inhibit pathogenesis by suppressing gonadotrophin levels.Citation12 Furthermore, the autocrine LHRH regulatory system is likely present in some human malignant tumours, such as breast, ovary, endometrium, and prostate cancer.Citation11
Evidences have been proven that GnRH-as have direct antitumour effects on endometrial cancer. Scientists discovered the proliferation of human endometrial cancer lines can be inhibited by GnRH-as in vitro. The most important downstream mechanisms of GnRH signaling in tumours include interference with the mitogenic pathway that results in anti-proliferative activity and protection of cells from apoptosis via activation of the NFkB pathway.Citation13 Another study has revealed that treatment with GnRH-as leading to apoptosis of human EC cells is mediated by GADD45α activation through the GnRH-I receptor, ERK1/2, and p38 MAPK signalling pathways.Citation14 Furthermore, Wu et al demonstrated that a GnRH-a promoted cell motility of EC cells through the GnRH-I receptor via the phosphorylation of ERK1/2 and JNK.Citation15 Zhao et al also found that GnRH-as promote increased apoptosis rate of EC cells and inhibit cell proliferation in a dose-dependent manner, likely caused by inhibition of ERK1/2 and Akt activity, which is in turn related to the status of PTEN status and could be offset by 17beta-oestradiol.Citation16
Some studies report effective usage of GnRH-as as inoperable treatment in EC. A Gynaecologic Oncology Group (GOG) study estimated the effects of GnRH-as in patients with advanced and recurrent EC, and showed the overall response rate was 11% (2 complete and 3 partial among 42 patients), with a 1.9-month median PFS and 7.3-month median OS. No severe or life-threatening toxicities occurred.Citation17 Noci et al.Citation18 reported one case in which leuprolide was administered primarily due to the patient's compromised conditions. Therapy was continued for over six years with no disease progression. The author deduced that the chronic treatment with LHRH analogues might have a direct or indirect effect on E-cadherin expression in endometrial tissue, in which expression is inversely correlated with both depth of myometrial invasion and para-aortic lymph node metastasis in EC.
Combination use of aromatase inhibitors and GnRH-as
GnRH-as can decrease oestrogen synthesis from gonads, while AIs can inhibit the synthesis of oestrogens from local and peripheral tissue, so that the combined administration of AI and GnRH-a can block oestrogen more deeply in premenopausal women. For postmenopausal women whose ovary function has ceased, both circulating and locally oestrogens have a negative feedback on the hypothalamic/pituitary axis. Inhibition of aromatase activity reduce oestrogen levels, and remove the negative feedback on gonadotropin, which ultimately increase synthesis and release of gonadotropin and testosterone that in turn stimulate oestrogens and aromatase.Citation19 Combination therapy with a GnRH-a effectively reduces the excess substrate and maximizes the effect of aromatase inhibition.Citation20 Moreover, the direct antitumor effects of GnRH-a and the suppression of local and peripheral oestrogen with an AI may also provide satisfactory therapeutic effect. However, the mechanisms of the combination therapy in postmenopausal EC patients should be more studied.
The efficacy of combined of GnRH-a and AI has been confirmed in early-stage, advanced, and metastatic breast cancer. Studies have shown that following treatment with AI plus GnRH-a, the oestrogen repression may exceed that of standard therapy with tamoxifen plus GnRH-a in breast cancer.Citation19 The combination of GnRH-a and AI in EC has only been reported in fertility-preserving treatment of premenopausal patients. Zhou et al.Citation21 performed a retrospective analysis evaluating GnRH-a treatment combined with a LNG-IUD or letrozole as fertility-preserving treatment in 29 young women with well-differentiated early-stage EC or complex atypical hyperplasia. Twenty-seven patients had a complete response. The authors concluded that GnRH-a plus letrozole is ideally suited to young overweight women.
Herein, we have presented three postmenopausal patients with comorbidities or advanced age with contraindications to surgery. In our cases, the treatment period was six months with disease remaining stable for two years in patients who received the combination treatment as an initial approach. As salvage treatment, an AI combined with a GnRH-a was also effective for recurrent patients. All the three patients did not report side effects such as fever, nausea, headache, bone pain, muscle and joint aches. Survival was similar to that expected from hormonal treatment, but differed from that of radiotherapy, which is currently accepted first-line treatment. However, as older women or those with poor performance status due to higher comorbidity burden are at higher risk of developing both GI and bladder toxicities after radiation, neither are also not suitable for high dose oral progesterone treatment because of the increased risk of thrombosis. Compared with radiotherapy and high dose oral progesterone, this combination treatment in EC patients with advanced age or comorbidities achieved satisfactory results and may be considered a safer and more manageable treatment strategy.
Conclusion
Given the significantly improved understanding of the mechanisms of action of AIs and GnRH-as respectively, and the mechanisms of combination treatment, the association of an AI plus a GnRH-a presents considerable therapeutic effect for EC patients. The good tolerability and favourable risk benefit profile of this combination suggests that it is a favourable treatment strategy for patients with additional comorbidities or those with advanced age who are ineligible for surgery. However, data regarding the combination of an AI plus a GnRH-a for EC are currently somewhat limited. Further studies are warranted to better define the clinical benefits of this combination treatment in EC.
Abbreviations
| EC | = | Endometrial cancer |
| PFS | = | progression free survival |
| GI | = | gastrointestinal |
| MRI | = | Magnetic resonance imaging |
| AI | = | Aromatase inhibitor |
| GnRH-a | = | Gonadotropin-releasing hormone agonist |
| LNG-IUD | = | levonorgestrel-releasing intrauterine device |
| BMI | = | body mass index |
| LHRH | = | luteinising hormone-releasing hormone |
| PR | = | progesterone receptor |
| OS | = | overall survival |
Declarations
Ethics approval and consent to participate
Ethics approval for the study was gained from the ethical review committee of Tianjin Medical University General Hospital. Patients and their relatives provide their consents to the treatment.
Consent for publication
Informed consents were obtained from patients and their relatives.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Author contributions
Manuscript outline and content: MD WT. Literature search: SJ YY CG. Figures: MD YS JG. Writing and review of the manuscript writing: MD SJ. Final manuscript approval: MD SJ WT YY CG JG YS YW FX.
Acknowledgments
This study was supported by the Natural Science Foundation of China under Grant No. 81572568 to Y. Wang and No. 81602293 to W. Tian.
Additional information
Funding
References
- van der Steen-Banasik E, Christiaens M, Shash E, Coens C, Casado A, Herrera FG, Ottevanger PB, European Organisation for Research and Treatment of Cancer, Gynaecological Cancer Group (EORTC-GCG). Systemic review: Radiation therapy alone in medical non-operable endometrial carcinoma. Eur J Cancer (Oxford, England: 1990). 2016;65:172–81. doi:10.1016/j.ejca.2016.07.005.
- Hsieh MC, Thompson T, Wu XC, Styles T, O'Flarity MB, Morris CR, Chen VW. The effect of comorbidity on the use of adjuvant chemotherapy and type of regimen for curatively resected stage III colon cancer patients. Cancer Med. 2016;5(5):871–80. doi:10.1002/cam4.632. PMID:26773804
- Colombo N, Creutzberg C, Amant F, Bosse T, Gonzalez-Martin A, Ledermann J, Marth C, Nout R, Querleu D, Mirza MR, et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: diagnosis, treatment and follow-up. Ann Oncol: official journal of the European Society for Medical Oncology. 2016;27(1):16–41. doi:10.1093/annonc/mdv484. PMID:26634381
- Lee WL, Yen MS, Chao KC, Yuan CC, Ng HT, Chao HT, Lee FK, Wang PH. Hormone therapy for patients with advanced or recurrent endometrial cancer. J Chin Med Assoc: JCMA. 2014;77(5):221–6. doi:10.1016/j.jcma.2014.02.007. PMID:24694672
- Baker WD, Pierce SR, Mills AM, Gehrig PA, Duska LR. Nonoperative management of atypical endometrial hyperplasia and grade 1 endometrial cancer with the levonorgestrel intrauterine device in medically ill post-menopausal women. Gynecol Oncol. 2017;146(1):34–8. doi:10.1016/j.ygyno.2017.04.006. PMID:28427775.
- Gao C, Wang Y, Tian W, Zhu Y, Xue F. The therapeutic significance of aromatase inhibitors in endometrial carcinoma. Gynecol Oncol. 2014;134(1):190–5. doi:10.1016/j.ygyno.2014.04.060. PMID:24811574
- Thangavelu A, Hewitt MJ, Quinton ND, Duffy SR. Neoadjuvant treatment of endometrial cancer using anastrozole: a randomised pilot study. Gynecologic Oncol. 2013;131(3):613–8. doi:10.1016/j.ygyno.2013.09.023. PMID:24076063
- Bogliolo S, Gardella B, Dominoni M, Musacchi V, Cassani C, Zanellini F, De Silvestri A, Gaggero CR, Babilonti L, Spinillo A. Effectiveness of aromatase inhibitors in the treatment of advanced endometrial adenocarcinoma. Arch Gynecol Obstet. 2016;293(4):701–8. doi:10.1007/s00404-015-3974-9. PMID:26671487
- Barker LC, Brand IR, Crawford SM. Sustained effect of the aromatase inhibitors anastrozole and letrozole on endometrial thickness in patients with endometrial hyperplasia and endometrial carcinoma. Curr Med Res Opin. 2009;25(5):1105–9. doi:10.1185/03007990902860549. PMID:19301987
- Altman AD, Thompson J, Nelson G, Chu P, Nation J, Ghatage P. Use of aromatase inhibitors as first- and second-line medical therapy in patients with endometrial adenocarcinoma: a retrospective study. J Obstet Gynaecol Canada: JOGC = Journal d'obstetrique et gynecologie du Canada: JOGC. 2012;34(7):664–72. PMID:22742486
- Teutonico D, Montanari S, Ponchel G. Leuprolide acetate: pharmaceutical use and delivery potentials. Expert Opin Drug Delivery. 2012;9(3):343–54. doi:10.1517/17425247.2012.662484. PMID:22335366
- Wan J, Gao Y, Zeng K, Yin Y, Zhao M, Wei J, Chen Q. The levels of the sex hormones are not different between type 1 and type 2 endometrial cancer. Sci Rep. 2016;6:39744. doi:10.1038/srep39744. PMID:28000774
- Grundker C, Gunthert AR, Westphalen S, Emons G. Biology of the gonadotropin-releasing hormone system in gynecological cancers. Eur J Endocrinol. 2002;146(1):1–14. doi:10.1530/eje.0.1460001. PMID:11751060
- Wu HM, Cheng JC, Wang HS, Huang HY, MacCalman CD, Leung PC. Gonadotropin-releasing hormone type II induces apoptosis of human endometrial cancer cells by activating GADD45alpha. Cancer Res. 2009;69(10):4202–8. doi:10.1158/0008-5472.CAN-08-4591. PMID:19366794
- Wu HM, Wang HS, Huang HY, Lai CH, Lee CL, Soong YK, Leung PC. Gonadotropin-releasing hormone type II (GnRH-II) agonist regulates the invasiveness of endometrial cancer cells through the GnRH-I receptor and mitogen-activated protein kinase (MAPK)-dependent activation of matrix metalloproteinase (MMP)-2. BMC Cancer. 2013;13:300. doi:10.1186/1471-2407-13-300. PMID:23786715
- Zhao LJ, Wei LH, Li XP, Wang JL. [Effect of gonadotropin-releasing hormone-I agonist and gonadotropin-releasing hormone-II on endometrial carcinoma cell lines with different states of PTEN]. Zhonghua fu chan ke za zhi. 2009;44(1):45–9. PMID:19563062
- Asbury RF, Brunetto VL, Lee RB, Reid G, Rocereto TF. Goserelin acetate as treatment for recurrent endometrial carcinoma: a Gynecologic Oncology Group study. Am J Clin Oncol. 2002;25(6):557–60. doi:10.1097/00000421-200212000-00004. PMID:12477997
- Noci I, Borri P, Bonfirraro G, Chieffi O, Arcangeli A, Cherubini A, Dabizzi S, Buccoliero AM, Paglierani M, Taddei GL. Longstanding survival without cancer progression in a patient affected by endometrial carcinoma treated primarily with leuprolide. Br J Cancer. 2001;85(3):333–6. doi:10.1054/bjoc.2001.1900. PMID:11487260
- Torrisi R, Rota S, Losurdo A, Zuradelli M, Masci G, Santoro A. Aromatase inhibitors in premenopause: Great expectations fulfilled? Crit Rev oncology/hematol. 2016;107:82–9. doi:10.1016/j.critrevonc.2016.08.009. PMID:27823654
- White J, Kearins O, Dodwell D, Horgan K, Hanby AM, Speirs V. Male breast carcinoma: increased awareness needed. Br Cancer Res: BCR. 2011;13(5):219. doi:10.1186/bcr2930. PMID:22017761
- Zhou H, Cao D, Yang J, Shen K, Lang J. Gonadotropin-Releasing Hormone Agonist Combined With a Levonorgestrel-Releasing Intrauterine System or Letrozole for Fertility-Preserving Treatment of Endometrial Carcinoma and Complex Atypical Hyperplasia in Young Women. Int J Gynecol Cancer: official journal of the International Gynecological Cancer Society. 2017;27(6):1178–82. doi:10.1097/IGC.0000000000001008. PMID:28562472
