ABSTRACT
Ovarian cancer is a most common lethal gynecological malignant tumor, with a gradual increasing incidence throughout the world. The mainstay treatment is cytoreductive surgery followed by platinum-based chemotherapy. However, a high percentage of patients recur, thus needing multiple treatments with a frequently poor prognosis. Apatinib is a novel and highly selective tyrosine kinase inhibitor of vascular endothelial growth factor receptor-2. Previous studies have suggested that apatinib is safe and effective in some solid tumors. We report one case with platinum-resistant advanced epithelial ovarian cancer, who had failed prior treatment with multiple chemotherapy reagents. She was negative for multiple driver genes including BRCA1/2、EGFR、KRAS/NRAS/BRAF, ALK, HER2 and cMET. Five courses of apatinib plus epirubicin were given. Due to the heavy leukothrombopenia, apatinib monotherapy, at 250 mg qd dose level, was used for maintenance therapy. The progression-free survival (PFS) time was 12.6 months. After that, the disease was slightly progressive during apatinib maintenance and then entered into a “stable” state until now. It indicated that apatinib may be a superior choice for advanced ovarian cancer patients, but further prospective studies are needed to optimize the treatment.
Introduction
Ovarian cancer (OC) has the highest mortality rate of the cancers unique to women. There were an estimated 225,000 new cases of OC and estimated 140.200 deaths worldwide each yearCitation1. Moreover, the majority (65%-75%) of women with OC are diagnosed with advanced stage disease (III and IV), and only about 30% of these women are free of disease recurrence at five years after standard therapeutic approaches include optimal surgical debulking followed by adjuvant platinum doublet chemotherapyCitation2.
In the last two decades, research has focused on the potential of target therapies to improve the survival of patients affected by OC. Angiogenesis inhibitors have been largely investigatedCitation3. Bevacizumab, a monoclonal antibody against vascular endothelial growth factor (VEGF), is one of the most studied target therapies, and it is approved for first- and second-line treatment of advanced epithelial ovarian cancer (EOC), fallopian tube, and primary peritoneal cancers in NCCN GuidelinesCitation4.Pazopanib, an oral multi-targeted TKIs that inhibits VEGFR-1, −2 and −3, platelet-derived growth factor receptor-a, and -b and c-kit, has shown encouraging results in Phase III trials in OCCitation5. Though bevacizumab and pazopanib may provide certain benefits in the treatment of OC, the former is an expensive treatment which the health insurance cannot be reimbursed in our state, and the latter in China mainland hasn’t been licensed by CFDA until 21th of Feb. 2017.
Apatinib (Hengrui Pharmaceutical Co. Ltd, Jiangsu, People’s Republic of China) is a novel tyrosine kinase inhibitor that selectively inhibits the vascular endothelial growth factor receptor-2 (VEGF-2) and inhibits endothelial cell migration and proliferation, thus blocking new blood vessel formationCitation6. Apatinib is a kind of oral anti-angiogenesis agent, ensuring the ease in use and is affordable to the patient, considering the patient’s choice of having targeted treatment at home. Apatinib was approved and launched in China in 2014 as a subsequent-line treatment for patients with advanced gastric cancer. Apatinib is currently undergoing phase II/III clinical trials for the treatment of several cancer types, such as gastric carcinomaCitation7 and hepatocellular carcinomaCitation8.
In the present study, we describe our experience with a recurrent ovarian cancer patient who was treated safely with apatinib and who achieved a partial response despite receiving eight prior chemotherapy regimens.
Case report
A 56-year-old Chinese woman, presenting with abdominal distension for two months, was referred to Cancer Center, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (Wuhan, China) in January 2015. Her serum antigen-125 (CA-125) level was 6500 U/mL. Positron Emission Tomography-Computed Tomography (PET- CT) and computed tomography (CT) scan showed a solid-cystic, heterogeneously enhancing mass lesion in the left ovary, diffuse implantinomentum with ascites, lymph node involvement of the abdominal cavity, pelvic cavity and right supraclavicular.The cytology of ascites by abdominocentesis provided a diagnosis of adenocarcinoma. The clinical diagnosis was advanced (FIGO stage IV) ovarian cancer. From February 2015, two cycles of chemotherapy with paclitaxel plus carboplatin (TC) (paclitaxel at 175 mg/m2; carboplatin [area under the curve [AUC] 6 mg/mL per min] on day 1 and every 21 days thereafter) were administered. Ascites disappear rapidly.
In April 2015, the patient has undergone hysterectomy, bilateral uterine adnexectomy, appendectomy resection, greater omentum resection and pelvic adhesionlysis. Pathological assessment showed epithelial ovarian cancer (EOC) (FIGO stage IV). Immunohistochemical analysis demonstrated the tumor were strongly positive for pancytokeratin (PCK), CA-125, Wilms tumor-1(WT-1) and the paired box-8 protein(PAX-8), partially positive for estrogen receptor (ER), while negative for P53.After surgery, four cycles of TC chemotherapy were administered, followed by radiotherapy of lymph node in supraclavicular zone. Then Etoposide and cyclophosphamide (EC) therapy was administered at 3-week intervals for 4 cycles as maintenance therapy. Following surgery and chemotherapy, the patient’s cancer CA-125 levels declined to 28.9 U/ml.
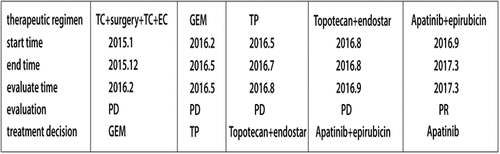
One and half months later, this patient’s CA-125 level was 120.7 U/ml on february 2016. A CT scan showed a new mass in the Vaginal stump (). Then four cycles of gemcitabine (GEM) monotherapy (1,000 mg/m2 on days 1, 8and every 21 daysthereafter) were administered, but there was no clinical response ().Laboratory analyses revealed a CA-125 level of 395.5U/ml. Thus, starting in May 2016, she received three cycles of paclitaxel plus nedaplatin (TP) (paclitaxel at 175 mg/m2 on day 1; nedaplatin 75 mg/m2 on day 1 and every 21 days thereafter).In June 2015, the patient had a complaint of stool coming from vagin. The MRI scan indicated a sigmoid colon vaginal fistula, with adjacent peritoneum and pelvic floor fascia thickening. The tumor invasion cannot be ruled out. The fistula was closed by Sigmoid colostomy in July 2016. The patient’s CA-125 level was 1423.7 U/ml on Augst 20, 2016(). CT scan showed the mass increased significantly(). The therapeutic evaluation was PD. Following this, the patient was treated with topotecan (1.5 mg/m2 for 5 consecutive days) plus endostar (rh-endostatin) (7.5 mg/m2 on days 1–14), which was repeated every 3 weeks. After one cycle, her serum CA125 level rose to 2288 U/mL in September2016.CT scan proved that the disease was PD().
Figure 1. CT scan before apatinib treatment (the lesions are indicated by arrows). A. CT scans before Apatinib therapy revealed disease progress in the Vaginal stump; B. after four cycles of gemcitabine, the metastatic mass became bigger; C. after three cycles of paclitaxel plus nedaplatin, the metastatic mass was still bigger; D. after oneone cycle of topotecan plus endostar, the metastatic mass continue to be bigger.
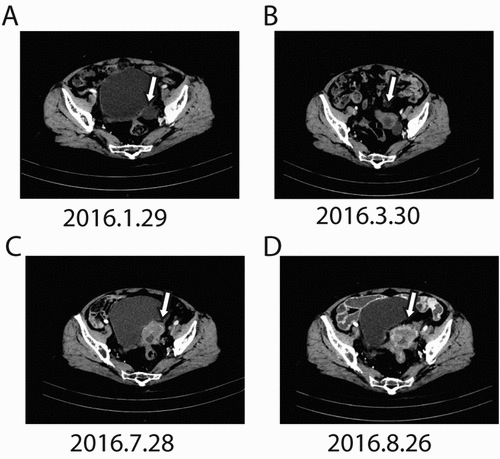
Figure 2. Changes in CA-125 levels before and after apatinib therapy. The CA-125 levels continuously increased from 28U/mL (Dec. 2015) to 2288 U/mL (Sep. 2016) before apatinib treatment. Apatinib plus epirubicin (solid arrows) began in Sep. 2016. Following initiation of this therapy, CA-125 levels dramatically decreased to 269 U/mL (Mar. 2017). Then, Apatinib monotherapy was used as maintenance therapy (open arrows), CA-125 levels come into a stable level.
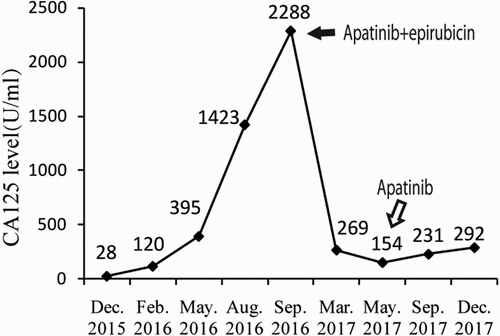
To monitor the patient’s gene mutation status, we decided to carry out next-generation sequencing (NGS) detection. Due to the long time of surgical specimens, circulating tumor DNA (ctDNA) from peripheral blood was extracted for gene analysis. A panel of 150 tumor-related diver genes was subjected to NGS. The detected mutations () may be related to the occurrence of ovarian cancer, but there is no “actionable” abnormalities for FDA approved drugs. It is suggested that in addition to antiangiogenic drugs and immunotherapy drugs, it is not recommended to try olaparibCitation9 or niraparibCitation10 that have been approved by FDA for ovarian cancertargeted therapy. Upon the NGS results, we recommended her to try epirubicin (75mg/m2 on day 1) combined with apatinib (500 mg/daily and 4 weeks as a cycle). In consideration of the off-label use of apatinib, we communicated fully with our patient before she signed the consent form of the off-label use of the medicine. But she cannot tolerate the side effect of HFS after one cycle of therapy, so apatinib at a dose of 250 mg daily was given. After five courses of combined treatment, the CT scan indicated that the pelvic mass was observably decreased (),which achieved partial response (PR) according to the RECIST 1.1. Her serum CA-125 level decreased to 269.1 U/mLdramatically in March 2017 (). Due to the severe neutropenia and thrombopenia (Grade IV) caused by combined treatment, the patient refused further chemothearpy. Subsequently, apatinib alone was used for maintenance therapy. Her CA-125 levels were decreased to 154.6 U/mL in May 2017. Response evaluations after every two months showed stable disease (SD) (). In september 2017, CT scan discovered that the mass in the vaginal stump was stable but the lymph node in front of the left external iliac artery increased () and the CA-125 increased to 231 U/ml ().The results showed a slingtly PD. In view ofa PFS time of 12.6 months since apatinb added into the treatment, we advised the patient to continue using aptinib as maintenance therapy. During maintenance, her ECOG performance status was 1. The observed nonmetabolic/laboratory events was grade 1 with hand-foot syndrome (HFS)and intermittent vaginal bleeding. No hypertension or proteinuria occurred. The last response evaluation in Dec. 2017 indicated SD ().
Figure 3. CT scan before apatinib treatment (the lesions are indicated by arrows). A. after five cycle of apatinib combined with epirubicin, CT scans showed that the metastatic mass became smaller significantly; B after more than four months of Apatinib monotherapy, CT scans showed that the metastatic mass in the vaginal stump was stable. C. after another about four months of Apatinib monotherapy, the mass in the vaginal stump was stable but the lymph node in front of the left external iliac artery increased. D. after another three months, bothe the lesion in the vaginal stump and front of the left external iliac artery were both stable.
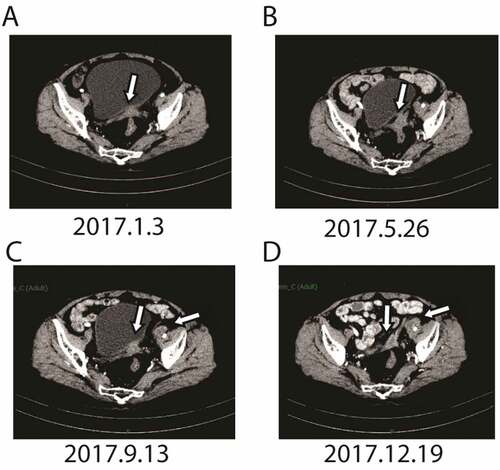
Table 1. The mutations detected by next-generation sequencing (NGS)
Discussion
The EOC is one of the most common gynecological cancers, accounting for about 60% of ovarian cancersCitation11. Most patients with EOC present with advanced stage disease, themajority of them will inevitably progress after first-line ormaintenance treatment. The standard treatment of recurrent EOC after three lines of chemotherapy is misty since no chemotherapy agent has ever demonstrated superior activity over anotherCitation12.
In the present case, the patient with advanced EOC received paclitaxel, carboplatin, etoposide, cyclophosphamide, gemcitabine, paclitaxel, topotecan and endostar (); however, these treatments were not efficacious for long time. NGS of tumors or circulating ctDNA has been heralded as a hopeful method to recognize ‘actionable’ abnormalities effective to target therapies to the mutated genesCitation13. However, there were no common mutations for FDA approved drugs targeting OC according to the NGS results in this case. The antiangiogenic therapy agents targeting theVEGF pathway have been approved to have value in the treatment of relapsed malignancy. Recently, it has been reported that apatinib is a promising drug for recurrent carcinoma, and is able to reverse multidrug resistance by inhibiting the efflux function of multiple ATP-binding cassette transportersCitation14. This patient successively entered into targeted therapy of apatinib. The treatment of apatinib plus epirubicin lasted for 6 months, that the therapeutic evaluation was PR. Duo to severe myelosuppression, apatinib monotherapy was used for maintenance treatment until now. While treatment is ongoing, the imageological evaluation indicated slightly PD in September 2017, and then entered into a “SD”state until now. The patient’s PFS was about 12.6 months since apatinib added into the treatment.
AS we known, the goals of treatment for advanced ovarian cancer, especislly for platinum-refractory OC, should be to improve the patient’s quality of life (qol) by extending PFS, prolonging life and increasing the symptom-free interval. Expected treatmentfor this group is lower in this group. Many treatment options have shown feeble response rates. The cancer stem cells are chemoresistant to conventional treatment and are able to self-renewaland propagation. Moreover, “cancer stem cells”theory partly explains why mutiple cytotoxicdrugs failed to prolong disease-free survival effectively, thus highlights the need to exploitnew agents to target alternative molecular pathways.About 15% of EOC present BRCA1/2 mutations leading to a deficiency of homologous recombinationrepair.Olaparib is a potent oral inhibitor of polyadp–ribose polymerase (PARP). In December 2014, olaparib was approved for the treatment of patients with known BRCA1 or BRCA2 mutations, which represents the first personalized therapy for ovarian cancerCitation15. Angiogenesis is also a key mechanism of ovarian cancer development. Bevacizumab is the first antiangiogenic agent that can improve patients’ outcomewhen intravenously administered as maintenance therapy for advanced EOC. Moreover, Kumar D et al reported that targeting Notch1 signaling network may regulates cancer stem cell-mediated angiogenesis and tumor progressionCitation16.
To our knowledge, this study is the first to report a patient with advanced EOC who was platinum-resistant and negative for multiple driver genes including BRCA1/2、EGFR、KRAS/NRAS/BRAF、ALK、HER2, et al, and who obtained a durable response to apatinib for 12.6 months of PFSdurations. In previous caseCitation17, the reportedOC patient was platinum-sensitive and the gene state was unknown, and apatinib was used as fourth-line chemotherapy at a daily dose of 500 mg with 11.3 months of PFS. Along with this case study, it proves that apatinib was safe and effective to patients with EOC.
Although obtained some experience in this case, there still has many issues to be resolved in further studies. For example, is apatinib also useful for other type of OC, such as clear cell carcinoma, mucinous carcinoma, et al. As for combination approaches, such as apatinib combined with other chemotherapy drugs, or combined with PARP inhibitor(olaparib) in people with BRCA1 or BRCA2 mutations or combined with immunotherapy, may be an attractive potential therapy paradigms. Besides, to find biological markers to predict drug efficacy and discriminate patients who are most likely to benefit from apatinib is also one of the challenges with apatinib. It was reported that hypertension was independent predictive factors for apatinib treatment in advanced breast cancerCitation1Citation8. The biomarkers and roles of apatinib in patients with ovarian cancer needs more explorations. Further large-scale prospective studies are required to prove the effect of apatinib in OC.
Conclusion
In this case report, apatinib may provide an additional option for the treatment ofplatinum-resistant advanced OC, especially for patients without driver genes mutation for targeted therapy and those with poor performance status.Whether it can be used in the firstline, or whether it can be used in the othet type of OC aside from EOC, or whether it can be used together with other chemotherapeutic agents, requires a large number of randomized clinical trials to confirm.
Disclosure of potential conflicts of interest
All authors have declared no potential conflicts of interest.
Additional information
Funding
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi:10.3322/caac.20107.
- Siporin C. The evolution of fluorinated quinolones: pharmacology, microbiological activity, clinical uses, and toxicities. Annu Rev Microbiol. 1989;43:601–627. doi:10.1146/annurev.mi.43.100189.003125.
- Jackson AL, Eisenhauer EL, Herzog TJ. Emerging therapies: angiogenesis inhibitors for ovarian cancer. Expert Opin Emerg Drugs. 2015;20:331–346. doi:10.1517/14728214.2015.1036739.
- Matulonis UA. Bevacizumab and its use in epithelial ovarian cancer. Future Oncol. 2011;7:365–379. doi:10.2217/fon.10.167.
- Du Bois A, Floquet A, Jw K, Rau J, Del Campo JM, Friedlander M, Pignata S, Fujiwara K, Vergote I, Colombo N, et al. Incorporation of pazopanib in maintenance therapy of ovarian cancer. J Clin Oncol. 2014;32:3374–3382. doi:10.1200/JCO.2014.55.7348.
- Zhang H. Apatinib for molecular targeted therapy in tumor. Drug Des Devel Ther. 2015;9:6075–6081. doi:10.2147/DDDT.S97235.
- Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y, Liu W, Tong J, Liu Y, Xu R, et al. Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol. 2016;34:1448–1454. doi:10.1200/JCO.2015.63.5995.
- Lu W, Jin XL, Yang C, Du P, Jiang F-Q, Ma J-P, Yang J, Xie P, Zhang Z. Comparison of efficacy between TACE combined with apatinib and TACE alone in the treatment of intermediate and advanced hepatocellular carcinoma: A single-center randomized controlled trial. Cancer Biol Ther. 2017;18:433–438. doi:10.1080/15384047.2017.1323589.
- Munroe M, Kolesar J. Olaparib for the treatment of BRCA-mutated advanced ovarian cancer. Am J Health Syst Pharm. 2016;73:1037–1041. doi:10.2146/ajhp150550.
- Caruso D, Papa A, Tomao S, Vici P, Panici PB, Tomao F. Niraparib in ovarian cancer: results to date and clinical potential. Ther Adv Med Oncol. 2017;9:579–588. doi:10.1177/1758834017718775.
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi:10.3322/caac.20073.
- Corrado G, Salutari V, Palluzzi E, Distefano MG, Scambia G, Ferrandina G. Optimizing treatment in recurrent epithelial ovarian cancer. Expert Rev Anticancer Ther. 2017;17:1147–1158. doi:10.1080/14737140.2017.1398088.
- Lim MC, Randall LM. Role and clinical application of next-generation sequencing (NGS) for ovarian cancer. J Gynecol Oncol. 2017;28:e51. doi:10.3802/jgo.2017.28.e51.
- Mi YJ, Liang YJ, Huang HB, Zhao H-Y, Wu C-P, Wang F, Tao L-Y, Zhang C-Z, Dai C-L, Tiwari AK, et al. Apatinib (YN968D1) reverses multidrug resistance by inhibiting the efflux function of multiple ATP-binding cassette transporters. Cancer Res. 2010;70:7981–7991. doi:10.1158/0008-5472.CAN-10-0111.
- Walsh CS. Two decades beyond BRCA1/2: homologous recombination, hereditary cancer risk and a target for ovarian cancer therapy. Gynecol Oncol. 2015;137:343–350. doi:10.1016/j.ygyno.2015.02.017.
- Kumar D, Kumar S, Gorain M, Tomar D, Patil HS, Radharani NNV, Kumar TVS, Patil TV, Thulasiram HV, Kundu GC. Notch1-MAPK signaling axis regulates CD133(+) cancer stem cell-mediated melanoma growth and angiogenesis. J Invest Dermatol. 2016;136:2462–2474. doi:10.1016/j.jid.2016.07.024.
- Deng L, Wang Y, Lu W, Liu Q, Wu J, Jin J. Apatinib treatment combined with chemotherapy for advanced epithelial ovarian cancer: a case report. Onco Targets Ther. 2017;10:1521–1525. doi:10.2147/OTT.S126471.