ABSTRACT
Small cell lung cancer (SCLC) is a highly aggressive disease and miRNAs may play an important role in modulating SCLC progression. We have previously screened 924 miRNAs and found that miR-886-3P was negatively associated with SCLC survival. In the current study, we further investigated the role of miR-886-3P mimic in regulating SCLC cell phenotypic alteration in vitro and xenograft tumor formation in vivo. We found that transfection of miR-886-3P mimic significantly inhibited SCLC cell proliferation, migration, and colony formation, and induced mesenchymal-epithelial transition (MET) by suppressing TGF-ß1 synthesis in vitro. Furthermore, intra-tumor injection of miR-886-3P mimic lead to necrosis and suppression of tumor invasion to the surrounding tissue in the subcutaneous xenograft tumor, and intra-vein injection of miR-886-3P mimic suppressed xenograft lung cancer growth in vivo. These findings suggested that miR-886-3P functions as a tumor suppressor in SCLC and thus, it might be a potential therapeutic molecule in the treatment of lung cancer.
Introduction
Lung cancer is one of the most aggressive malignant cancers in the world. Small cell lung cancer (SCLC) accounts for approximately 15% of all newly diagnosed lung cancer cases.Citation1 Based on whether a SCLC can be safely treated with definitive radiation fields, the American Veterans Administration Lung Study Group (VALG) defines SCLC stages as limited disease (LD) and extensive disease (ED).Citation2 Platinum-based chemotherapy in combination with radiotherapy remains as the main therapeutic strategy for SCLC.Citation3 However, despite extreme sensitivity to chemotherapy and radiotherapy, approximately 67% of newly diagnosed patients have ED, with median progression-free survival of 5.5 months and median overall survival of 6–12 months,Citation4 and 5-year survival rates of 5–10%.Citation5,Citation6
SCLC is often found early metastasis when it is diagnosed. Many factors may contribute to the aggressive phenotype of SCLC. In this content, it has been reported that miRNAs play an important role in phenotypic alteration of cancer cells through regulating epithelial-mesenchymal transition (EMT.Citation7–Citation10 EMT process involves distinct genetic and epigenetic alterations, and shifted expression of several biomarkers including E-cadherin, N-cadherin and vimenti.Citation11 The EMT process has been described in different kinds of human malignant tumors including breast cancer,Citation12 non-small cell lung cancer,Citation13 colon cancer,Citation14 and SCLC.Citation15 TGF-ß1 has been known to induce EMT in variety kinds of cells including lung cancer cells.Citation16
Due to limited therapeutic outcomes in SCLC, over the past few years, there has been a paradigm shift in cancer treatment from non-specific therapy to targeted therapies including immunotherapy and biological therapy.Citation17,Citation18 In this regard, several miRNAs have been reported to suppress or promote lung cancer cell survival or death.Citation19–Citation22 Consistent with these reports, we have previously reported that miR-886-3Pwas associated with survival of SCL.Citation23 In the current study, we further investigated role of miR-886-3P in regulating small cell lung caner cell proliferation, migration, colony formation, mesenchymal-epithelial transition (MET), and in vivo xenograft tumor formation and growth.
Materials and methods
Cell culture and transfection of exogenous mirnas
Human small cell lung cancer cell lines, NCI-H446 and NCI-H1688, were obtained from Peking Union Medical University (Beijing, China) and maintained in RPMI 1640 medium (GIBCO, USA) supplemented with 10% fetal bovine serum (FBS, GIBCO, USA) and antibiotics at 37°C and 5% CO2 atmosphere. Mimic of miR-886-3P and control miRNA were purchased from Ribobio (Guangzhou, China) and the plasmid was synthesized by Generay (Shanghai, China).
Transfection of miRNA mimic was carried out using Lipofectamine 2000 following the manufacture’s instruction (Invitrogen, USA).
Immunoblotting
Cells were harvested with lysis buffer and briefly sonicated. Total protein concentration was measured with BAC Protein Assay Kit (PPLYGEN, Beijing, China). Samples were mixed with 2 times concentrated sample loading buffer and denatured by heating at 95°C followed by cooling on ice. A total of 50µg protein was loaded into each lane and separated by SDS-PAGED (10%). After transferring to nitrocellulose membrane (GE Health), it was blocked with 5% skim milk for 2 h at room temperature. Primary antibodies of anti-E-cadherin, anti-N-cadherin, anti-Vimentin, anti-TGF-ß1 (Santa Cruz Biotechnology, Santa Cruz, USA), and anti-ß-actin (Sigma-Aldrich, St. Louise, USA) were allowed to react for overnight at 4°C. After washing and incubation with appropriate HRP-conjugated 2nd antibody for 1h at room temperature, blotting signal was analyzed using the Image Quant LAS 4000 (GE Healthcare, Life Science).
Luciferase reporter assay
The wild type and mutant 3ʹ-UTR of human TGF-ß1 was synthesized and cloned into the pGL3-luciferase reporter plasmid (Promega, Madison, USA). Cells were then co-transfected with wild type or mutated TGF-ß1 vector, and miR-886-3P mimic or control mimic, using Lipofectamine 2000. Renilla luciferase vector were co-transfected to characterize the transfection efficiency. After 48 h, cells were harvested and luciferase activity was assessed using the Dual-Luciferase reporter assay system (Promega, Madison, USA). Firefly luciferase activity was normalized to renilla luciferase activity.
Xenograft tumor formation and treatment with mirna mimic
Nude mice (3–4 weeks old) were purchased from Beijing Vital River Company (Beijing, China). Mice were randomly assigned to experimental group and control group. For the xenograft tumor formation experiment, 2 × 106 NCI-H446 cells that stably expressing miR-886-3P or NCI-H446 cells lacking of miR-886-3P were injected subcutaneously. After 4 weeks, mice were sacrificed and tumor was weighed. Tissues were fixed with formalin and sections of paraffin embedded tissues were used for histology and immunohistochemistry assessment.
For the miRNA mimic therapeutic experiment, 2 × 106 luciferase-labeled NCI-H446 cells were injected either subcutaneously or through tail veins. After formation of the xenograft tumors, equal amount of miR-886-3P mimic or control mimic were injected intra-tumor or through the tail vein. Two weeks later, mice were sacrificed, and tumors were weighed followed by fixing with formalin. Sections of paraffin embedded tumor tissue were used for histology and immunohistochemistry examination.
Immunocytochemistry
NCI-H446 cells were plated on poly-L-lysine coated coverslips in a 6-well tissue culture plate and transfected with either control miRNA mimic or miR-886-3P mimic. After fixation with 4% paraformaldehyde for 10 minutes, the cells were permeabilized with 0.1% Triton-X 100 for 15 minutes. After blocking with normal goat serum, primary antibodies including anti-E-cadherin, anti-N-cadherin, anti-TGF-ß1, and anti-vimentin (Santa Cruz Biotechnology, Santa Cruz, USA) were allowed to react overnight at 4°C. After washing, Alexa Fluor conjugated secondary antibodies (ZSGB-BIO, Beijing, China) were applied at dark for 1 hr. The coverslips were then mounted with ProLong Gold antifade with DAPI mounting medium (PPLYGEN, Beijing, China). Fluorescent images were obtained with a confocal microscope (Leica, Germany).
Histology and immunohistochemistry
All xenograft tumors were fixed with formalin for at least 24 h followed by embedding with paraffin. Serial sections of 5µm thickness from representative blocks were obtained. Sections were stained with hematoxylin and eosin (H & E) for histological examination.
For immunohistochemistry, after deparaffinization and rehydration, internal H2O2 was blocked with 3% H2O2 followed by non-specific blocking with normal goat serum. Primary antibodies were then allowed to react overnight at 4°C. After washing, appropriate HRP-conjugated secondary antibody was applied at room temperature for 1h. Expression of the target proteins was visualized using Enhance Polymer DAB Detection Kit following the manufacture’s instruction (ZSGB-BIO, Beijing, China). Images were further analyzed using Aperio Imagescope (Leica Biosystems, Germany).
Real time RT-PCR
Total RNA was extracted using TRIzol Reagent (Invitrogen Life Technologies, 15596–026). Two micrograms of total RNA were converted to complementary DNA using Superscript reverse transcriptase (Invitrogen Life Technologies, 18064–014) following the manufacturer’s instructions. RT- PCR was performed in a 20μl reaction volume according to the manufacturer’s protocol using SYBRP remix Ex Taq (Liaoning, China, RR420A).
Statistical analysis
Statistical analysis was conducted using GraphPad Prism software (GraphPad Software, La Jolla, CA, USA). Data are presented as the mean ± s.e.m. Differences in measured variables between experimental and control groups were assessed using the Student’s t-test. P values were calculated, and minimum statistical significance was accepted at P < 0.05, NS, not significant, *P < 0.05, **P < 0.01.
Results
Overexpression of mir-886-3p resulted in phenotypic alteration of SCLC cells
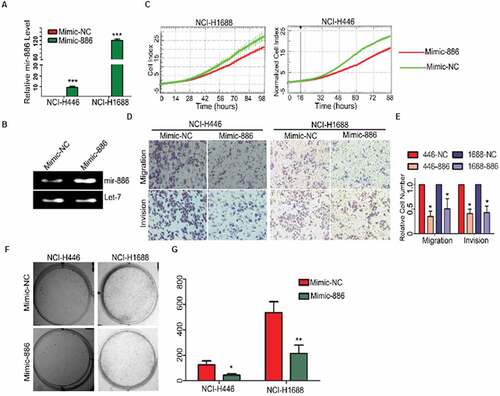
We have previously reported that miR-886-3P was down-regulated in SCLC and thus, we hypothesized that lack of miR-886-3P may be associated with aggressive phenotype of small cell lung cancer. In the current study, therefore, exogenous mimic of miR-886-3P was transfected into the two cell lines of small cell lung cancer, NCI-H446 and NCI-H1688. Phenotypes of the cells were then examined. As expected, expression of miR-886-3P significantly increased in the cells transfected with exogenous mimic of miR-886-3P both at mRNA () and protein level (). Overexpression of miR-886-3P resulted in significant suppression of cell proliferation (), cell migration and invasion into Matrigel () compared to the control cells (, migration in NCI-H446: 168 ± 25 vs 65 ± 10; Migration in NCI-H1688: 112 ± 20 vs 52 ± 22; invasion in NCI-446: 195 ± 36 vs 79 ± 15; invasion in NCI-H1688 132 ± 19 vs 65 ± 14, p < 0.05). In addition, ability of colony formation was also significantly reduced in the cells over-expressing miR-886-3P compared to the control cells ( and G, NCI-H446: 148 ± 32 vs 31 ± 5; NCI-H1688: 521 ± 79 vs 209 ± 53, p < 0.05).
Figure 1. In vitro suppression of SCLC cell proliferation, migration and invasion by exogenous miR-886-3P.
TGF-ß1 was targeted by miR-886-3P
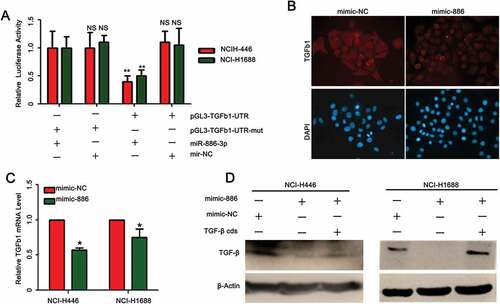
By searching databases including http://www.microrna.org/microrna/getTargets, we found that TGF-ß1 is a predicted target for miR-886-3P and thus effect of miR-886-3P mimic on TGF-ß1 expression was assessed. It was demonstrated by the luciferase reporter assay that TGF-ß1 promoter activity was significantly reduced in the cells transfected with miR-886-3P mimic compared to the cells transfected with negative control mimic (, NCI-H1688: 912 ± 149 of miR-886-3P mimic vs (2.12 ± 0.36)× 103 of control mimic; NCI-H446: (5.54 ± 0.45) × 103 of miR-886-3P mimic vs (1.42 ± 0.56)× 104 of control mimic, p < 0.01). Furthermore, TGF-ß1 protein was significantly reduced in the small cell lung cancer cells transfected with miR-886-3P mimic compared to the cells transfected with control mimic as evidenced by immunofluorescence staining ( and ) and immunoblotting ().
Figure 2. Effect of miR-886-3P mimic on TGF-ß1 expression by the SCLC cells.
Microrna-886-3p resulted in mesenchymal-epithelial transition (MET) through suppressing TGF-ß1
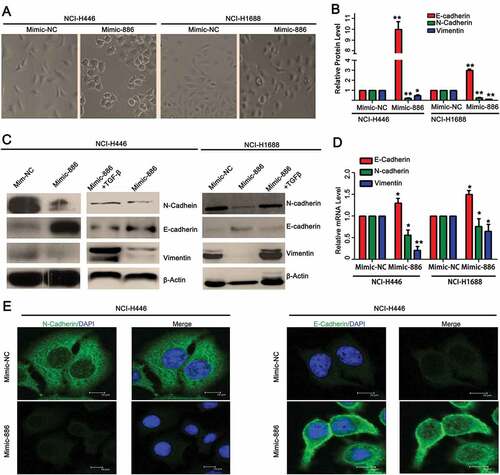
TGF-ß1 is known to induce epithelial-mesenchymal transition (EMT), one of the mechanisms by which cancer cell invades surrounding tissue. Since miR-886-3P targeting TGF-ß1, we further examined if miR-886-3p could induce mesenchymal-epithelial transition (MET) through suppressing TGF-ß1. As shown in , transfection of exogenous miR-886-3P mimic resulted in the morphological transition of the lung cancer cell lines from spindle-shape to round shape (). Next, miR-886-3P mimic-induced MET in small cell lung cancer cells was further examined by assessing the expression of biomarkers including E-cadherin, N-cadherin, and vimentin ( and ). It was found that miR-886-3P significantly increased E-cadherin (NCI-H446: 0.49 ± 0.05 of miR-886-3P mimic vs 0.05 ± 0.01 of control mimic; NCI-H1688: 0.12 ± 0.01 of miR-886-3P mimic vs 0.03 ± 0.01 of control mimic, p < 0.05, ), and in contrast, it significantly suppressed N-cadherin (NCI-H446: 0.02 ± 0.01 of miR-886-3P mimic vs 0.98 ± 0.06 of control mimic; NCI-H1688: 0.09 ± 0.01 of miR-886-3P mimic vs 0.75 ± 0.06 of control mimic, p < 0.05, ) and vimentin (NCI-H446: 0.03 ± 0.01 of miR-886-3P mimic vs 0.02 ± 0.01 of control mimic; NCI-H1688: 0.01 ± 0.01 of miR-886-3P mimic vs 0.82 ± 0.04 of control mimic, p < 0.05, ), which was partially or completely blocked in the presence of TGF-ß1 as evidenced by immunoblotting ( and ) or by confocal immunofluorescence staining (). Consistently, expression of miR-886-3P in the SCLCs resulted in significant up-regulation of E-cadherin (NCI-H446: 1.95 ± 0.21 of miR-886-3P mimic vs 1.49 ± 0.05 of control mimic; NCI-H1688: 1.04 ± 0.02 of miR-886-3P mimic vs 6.49 ± 0.87 of control mimic, p < 0.05, ), but down-regulation of N-cadherin (NCI-H446: 4.88 ± 0.92 of miR-886-3P mimic vs 9.27 ± 0.29 of control mimic; NCI-H1688: 3.65 ± 0.21 of miR-886-3P mimic vs 5.62 ± 0.40 of control mimic, p < 0.05, ) and vimentin (NCI-H446: 6.55 ± 2.55 of miR-886-3P mimic vs 3.12 ± 0.06 of control mimic; NCI-H1688: 5.50 ± 1.03 of miR-886-3P mimic vs 9.32 ± 1.25 of control mimic, p < 0.05, ).
Figure 3. Effect of miR-886-3P mimic on TGF-ß1-induced EMT and expression of biomarkers of EMT.
Effect of mir-886-3p mimic on subcutaneous tumor formation and growth
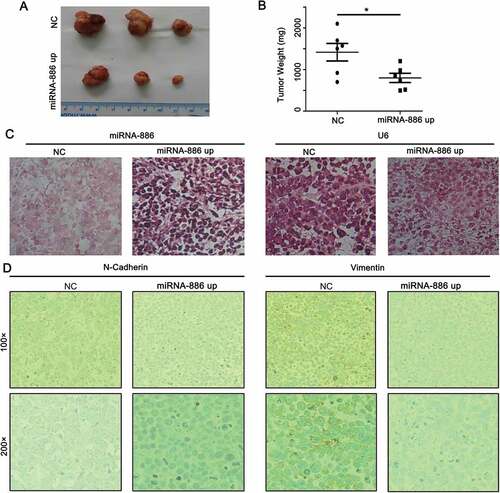
To further determine whether miR-886-3P affect tumor growth in vivo, a xenograft tumor model was established by subcutaneously injecting NCI-H446 cells into the nude mice. As expected, xenograft tumors formed by the cells transfected with miR-886-3P mimic were significantly smaller and less weight in average compared to that formed by the cells transfected with control mimic ( and , 0.755 ± 0.219 × 103 mg of miR-886-3P mimic vs 1.42 ± 0.484 × 104 mg of control mimic, p < 0.05). Stable expression of miR-886-3P in the xenograft tumor was confirmed by in situ hybridization (). Moreover, effect of miR-886-3P mimic on the expression of EMT/MET biomarkers in the xenograft tumor tissues was assessed by immunocytochemistry. As shown in , N-cadherin and vimentin were highly expressed in the tumors formed by the cells transfected with control mimic, while they were significantly suppressed in the tumors formed by the cells transfected with miR-886-3P mimic.
Figure 4. Subcutaneous tumor formation and growth by the SCLC cells transfected with miR-886-3P mimic or control mimic.
Antitumor effect of mir-886-3p mimic delivered locally or systemically
Next, in order to examine antitumor effect of miR-886-3P, following two different strategies were applied. First, NCI-H446 cells were injected subcutaneously and allowed to form xenograft tumors. The cholesterol conjugated miR-886-3P mimic or control mimic was then locally injected into the subcutaneous tumors. As shown in , intra-tumor injection of miR-886-3P mimic resulted in necrosis of the tumor tissue as well as suppression of intra-muscular invasion of the tumor. Consistent with the suppression of invasion, expressions of N-cadherin and TGF-ß1 were significantly reduced in the tumor injected with miR-886-3P mimic compared to that injected with control mimic ().
Figure 5. In vivo effect of miR-886-3P mimic on xenograft tumor formation, growth, and invasion.
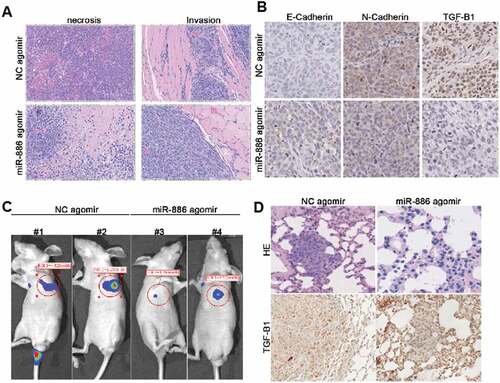
Second, luciferase-labeled NCI-H446 cells were injected into the tail veins and established a model of lung metastasis tumor. The cholesterol conjugated miR-886-3P mimic or control mimic was then systemically delivered by injecting into the tail veins. As shown in , the size of metastatic lung cancer was significantly smaller in the mice received cholesterol conjugated miR-886-3P mimic than in the mice injected with control mimic as evidenced by lung cancer luciferase imaging. Histological examination and immunohistochemistry further confirmed that systemic delivery of miR-886-3P mimic resulted in suppression of metastatic lung xenograft tumor growth although expression of TGF-ß1 was not significantly affected by the miR-886-3p mimic ().
Discussion
In the current study, we investigated the effect of miR-886-3P in suppressing proliferation, migration and formation of xenograft tumor by the small cell lung cancer (SCLC) cell lines. We found that transfection of exogenous miR-886-3P mimic resulted in alteration of SCLC cells from aggressive to non-aggressive phenotype, which includes reduced proliferation, migration, and colony formation. In addition, expression of TGF-ß1, N-cadherin, and vimentin was significantly suppressed, and in contrast, expression of E-cadherin was significantly increased in the cells overexpressing miR-886-3P. Furthermore, subcutaneous xenograft tumor formation and growth was significantly reduced in the SCLC cells containing exogenous miR-886-3P mimic compared to the cells transfected with control mimic. Intra-tumor or intra-venous injection of exogenous miR-886-3P mimic resulted in necrosis of the tumor tissue and suppression of the tumor invasion into the lung tissues. These findings suggested that lack of miR-886-3P may contribute the aggressive phenotype of small cell lung cancer.
Small cell lung cancer is a highly aggressive disease and most of the patients die of metastasis and recurrence. It has been reported that about 25% of small cell lung cancer patients have distance metastases at initial diagnosis.Citation24 While multiple factors may be associated with early metastasis of SCLC, recent studies indicated that miRNA be one of the crucial factors that contribute to the aggressive phenotype of SCLC.Citation25,Citation26 Consistently, with limited number of cases, we have also previously reported that miR-886-3P might be associated with poor prognosis of SCL.Citation23 In the current study, we further demonstrated that transfection of miR-886-3P mimic into the SCLC cell lines resulted in suppression of cell proliferation, migration and formation of subcutaneous xenograft tumor, suggesting miR-886-3P could modulate phenotype of small cell lung caner.
Epithelial-mesenchymal transition (EMT) is a crucial step for a cancer cell migration and aggressive invasion to the surrounding tissue or metastasis distance organs.Citation27,Citation28 Accumulating evidence from in vitro studies has demonstrated that TGF-ß1 plays an important role in EMT induction in variety kinds of cells including lung cancer cells.Citation11,Citation16,Citation29 Blockade of TGF-ß1 synthesis and its biological action in tumor and surrounding tissue, therefore, may result in suppression of EMT and consequent migration of the tumor cells. In support of this concept, the current study demonstrated that exogenous miR-886-3P mimic not only inhibited TGF-ß1 production by the SCLC cell lines, but also morphological alteration of SCLC cells from spindle-shape into round shape cells, a phenomenon of mesenchymal-epithelial transition (MET). Furthermore, miR-886-3P transfection in the SCLC cells leads to down-regulation of N-cadherin and vimentin, typical biomarkers of mesenchymal type and aggressive tumor cells; and in contrast, up-regulation of E-cadherin, a biomarker of epithelial cells. In addition, effect of miR-886-3P mimic on the alteration of EMT/MET biomarkers was partially blocked by the exogenous TGF-ß1. These findings indicated that TGF-ß1 play a role in the process of EMT and tumor cell migration, and that miR-886-3P suppress EMT and tumor cell migration partially through regulating TGF-ß1 synthesis and its biological function on EMT of the SCLC cells.
Subcutaneous xenograft tumor formation in nude mice is often used as an in vivo model of tumor stud.Citation30 Using this model, for the first time, we demonstrated that miR-886-3P mimic could inhibit subcutaneous xenograft tumor formation, growth, and invasion into the surrounding tissue, as well as significant suppression of N-cadherin and vimentin expression in the xenograft tumor tissue. In addition, local injection of miR-886-3P mimic into the subcutaneous xenograft tumor resulted in topical necrosis of the subcutaneous tumor and suppression of TGF-ß1 and N-cadherin expression. Furthermore, intravenous injection of miR-886-3P mimic could dramatically reduce the tumor size of metastatic xenograft lung cancer. These findings further suggested that miR-866-3P could be used as a therapeutic molecule that could be delivered either locally (intra-tumor injection) or systemically (intravenous injection).
Taken together, the current study demonstrated that exogenous miR-886-3P could lead to phenotypic alteration of small cell lung cancer cells from aggressive and mesenchymal phenotype into non-aggressive epithelial cell type as evidenced by down-regulation of N-cadherin and vimentin, and up-regulation of E-cadherin. Over-expression of miR-886-3P in the SCLC cell lines resulted in suppression of cell proliferation, migration, colony formation, and TGF-ß1 production. Injection of miR-886-3P mimic could suppress xenograft tumor formation, growth and invasion into the surrounding tissue. The findings of the current study suggested that miR-886-3P could be a biological therapeutic tool, which could be delivered either locally or systemically, in the treatment of small cell lung cancer.
Additional information
Funding
References
- Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, Dicker DJ, Chimed-Orchir O, Dandona R, Dandona L, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A systematic analysis for the global burden of disease study. JAMA Oncol. 2016;3:524.
- Micke P, Faldum A, Metz T, Beeh KM, Bittinger F, Hengstler JG, Buhl R. Staging small cell lung cancer: veterans administration lung study group versus international association for the study of lung cancer–what limits limited disease? Lung Cancer. 2002;37(3):271–276.
- Kim YH, Mishima M. Second-line chemotherapy for small-cell lung cancer (SCLC). Cancer Treat Rev. 2011; 37(2):143–150. doi:10.1016/j.ctrv.2010.05.004.
- Wolfson AH, Bae K, Komaki R, Meyers C, Movsas B, Le Pechoux C, Werner-Wasik M, Videtic GM, Garces YI, Choy H. Primary analysis of a phase II randomized trial radiation Therapy Oncology Group (RTOG) 0212: impact of different total doses and schedules of prophylactic cranial irradiation on chronic neurotoxicity and quality of life for patients with limited-disease small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2011; 81(1):77–84. doi:10.1016/j.ijrobp.2010.05.013.
- Videtic GM, Stitt LW, Dar AR, Kocha WI, Tomiak AT, Truong PT, Vincent MD, Ew Y. Continued cigarette smoking by patients receiving concurrent chemoradiotherapy for limited-stage small-cell lung cancer is associated with decreased survival. J Clin Oncol. 2003; 21(8):1544–1549. doi:10.1200/JCO.2003.10.089.
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015; 65(2):87–108. doi:10.3322/caac.21262.
- Ren J, Chen Y, Song H, Chen L, Wang R. Inhibition of ZEB1 reverses EMT and chemoresistance in docetaxel-resistant human lung adenocarcinoma cell line. J Cell Biochem. 2013; 114(6):1395–1403. doi:10.1002/jcb.24481.
- Tsai WC, Hsu PW, Lai TC, Chau GY, Lin CW, Chen CM, Lin CD, Liao YL, Wang JL, Chau YP, et al. MicroRNA-122, a tumor suppressor microRNA that regulates intrahepatic metastasis of hepatocellular carcinoma. Hepatology. 2009; 49(5):1571–1582. doi:10.1002/hep.22806.
- Li H, Zhang P, Sun X, Sun Y, Shi C, Liu H, Liu X. MicroRNA-181a regulates epithelial-mesenchymal transition by targeting PTEN in drug-resistant lung adenocarcinoma cells. Int J Oncol. 2015; 47(4):1379–1392. doi:10.3892/ijo.2015.3144.
- Kundu ST, Byers LA, Peng DH, Roybal JD, Diao L, Wang J, Tong P, Creighton CJ, Dl G. The miR-200 family and the miR-183~96~182 cluster target Foxf2 to inhibit invasion and metastasis in lung cancers. Oncogene. 2016; 35(2):173–186. doi:10.1038/onc.2015.71.
- Singh R, Shankar BS, Kb S. TGF-beta1-ROS-ATM-CREB signaling axis in macrophage mediated migration of human breast cancer MCF7 cells. Cell Signal. 2014; 26(7):1604–1615. doi:10.1016/j.cellsig.2014.03.028.
- Skalamera D, Dahmer-Heath M, Stevenson AJ, Pinto C, Shah ET, Daignault SM, Said NA, Davis M, Haass NK, Williams ED, et al. Genome-wide gain-of-function screen for genes that induce epithelial-to-mesenchymal transition in breast cancer. Oncotarget. 2016; 7(38):61000–61020. doi:10.18632/oncotarget.11314.
- Rastogi I, Rajanna S, Webb A, Chhabra G, Foster B, Webb B, Puri N. Mechanism of c-Met and EGFR tyrosine kinase inhibitor resistance through epithelial mesenchymal transition in non-small cell lung cancer. Biochem Biophys Res Commun. 2016; 477(4):937–944. doi:10.1016/j.bbrc.2016.07.003.
- Buhrmann C, Kraehe P, Lueders C, Shayan P, Goel A, Shakibaei M. Curcumin suppresses crosstalk between colon cancer stem cells and stromal fibroblasts in the tumor microenvironment: potential role of EMT. PLoS One. 2014; 9(9):e107514. doi:10.1371/journal.pone.0107514.
- Ito T, Kudoh S, Ichimura T, Fujino K, Hassan WA, Udaka N. Small cell lung cancer, an epithelial to mesenchymal transition (EMT)-like cancer: significance of inactive Notch signaling and expression of achaete-scute complex homologue 1. Hum Cell. 2017; 30(1):1–10. doi:10.1007/s13577-016-0149-3.
- Liu X. Inflammatory cytokines augments TGF-beta1-induced epithelial-mesenchymal transition in A549 cells by up-regulating TbetaR-I. Cell Motil Cytoskeleton. 2008; 65(12):935–944. doi:10.1002/cm.20315.
- Rafei H, El-Bahesh E, Finianos A, Nassereddine S, Tabbara I. Immune-based therapies for non-small cell lung cancer. Anticancer Res. 2017; 37(2):377–387. doi:10.21873/anticanres.11330.
- Osho AA, Azzoli CJ, Pai S, Mino-Kenudson M, Faquin WC, Huynh TG, Lanuti M, Mathisen DJ, Muniappan A. Successful treatment of an aggressive tracheal malignancy with immunotherapy. Ann Thorac Surg. 2017;103(2): e123-e125. doi:10.1016/j.athoracsur.2016.08.021.
- Sun CC, Li SJ, Zhang F, Zhang YD, Zuo ZY, Xi YY, Wang L, Dj L. The novel miR-9600 suppresses tumor progression and promotes paclitaxel sensitivity in non-small-cell lung cancer through altering STAT3 expression. Mol Ther Nucleic Acids. 2016; 5:e387. doi:10.1038/mtna.2016.96.
- Borzi C, Calzolari L, Centonze G, Milione M, Sozzi G, Fortunato O. mir-660-p53-mir-486 network: A new key regulatory pathway in lung tumorigenesis. Int J Mol Sci. 2017;18(1): doi:10.3390/ijms18010222.
- Wang L, Yao J, Sun H, He K, Tong D, Song T, Huang C. MicroRNA-101 suppresses progression of lung cancer through the PTEN/AKT signaling pathway by targeting DNA methyltransferase 3A. Oncol Lett. 2017; 13(1):329–338. doi:10.3892/ol.2016.5423.
- Lv Q, Hu JX, Li YJ, Xie N, Song DD, Zhao W, Yan YF, Li BS, Wang PY, Sy X. MiR-320a effectively suppresses lung adenocarcinoma cell proliferation and metastasis by regulating STAT3 signals. Cancer Biol Ther. 2017; doi: 10.1080/15384047.2017.1281497.
- Cao J, Song Y, Bi N, Shen J, Liu W, Fan J, Sun G, Tong T, He J, Shi Y, et al. DNA methylation-mediated repression of miR-886-3p predicts poor outcome of human small cell lung cancer. Cancer Res. 2013; 73(11):3326–3335. doi:10.1158/0008-5472.CAN-12-3055.
- Alvarado-Luna G, Morales-Espinosa D. Treatment for small cell lung cancer, where are we now?-a review. Transl Lung Cancer Res. 2016; 5(1):26–38. doi:10.3978/j.issn.2218-6751.2016.01.13.
- Herbst RS, Heymach JV, Sm L. Lung cancer. N Engl J Med. 2008; 359(13):1367–1380. doi:10.1056/NEJMra0802714.
- Jansson MD, Ah L. MicroRNA and cancer. Mol Oncol. 2012; 6(6):590–610. doi:10.1016/j.molonc.2012.09.006.
- Toden S, Okugawa Y, Jascur T, Wodarz D, Komarova NL, Buhrmann C, Shakibaei M, Boland CR, Goel A. Curcumin mediates chemosensitization to 5-fluorouracil through miRNA-induced suppression of epithelial-to-mesenchymal transition in chemoresistant colorectal cancer. Carcinogenesis. 2015; 36(3):355–367. doi:10.1093/carcin/bgv006.
- Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002; 2(6):442–454. doi:10.1038/nrc822.
- Puppo M, Bucci G, Rossi M, Giovarelli M, Bordo D, Moshiri A, Gorlero F, Gherzi R, Briata P. miRNA-mediated khsrp silencing rewires distinct post-transcriptional programs during tgf-beta-induced epithelial-to-mesenchymal transition. Cell Rep. 2016; 16(4):967–978. doi:10.1016/j.celrep.2016.06.055.
- Cespedes MV, Casanova I, Parreno M, Mangues R. Mouse models in oncogenesis and cancer therapy. Clin Transl Oncol. 2006;8(5):318–329.
