ABSTRACT
In non-small cell lung cancer (NSCLC), driver gene alterations, such as EGFR, ALK, MET, and ROS1, are usually mutually exclusive. Few clinical cases with co-existing ROS1 fusion and de-novo MET amplification have been reported. In addition, the efficacy of crizotinib in Chinese patients with driver co-existing alterations is uncertain. A 65-year-old female was diagnosed with lung adenocarcinoma metastatic to the brain. She had sufficient tumor tissue for detection of the target gene; however, common driver gene mutations, such as EGFR-wild and ALK-negative, were not initially detected. The patient was ultimately shown to have both ZCCHC8-ROS1 and de-novo MET gene amplification through next-generation sequencing with sensitivity to the targeted therapy of crizotinib. Unfortunately, the progression-free survival was only 6 months in length. We report here the first patient with co-existing ROS1 fusion and de-novo MET amplification to receive crizotinib in China. Treatment of our patient was effective with targeted therapy based on a precise diagnosis. Advanced or metastatic NSCLC patients with co-existing ROS1 fusion and de-novo MET amplification are sensitive to crizotinib. These uncommon driver gene mutations may be missed using the current first-generation detection assay. We must be aware of the incidence of concomitant ROS1 fusion and de-novo MET amplification because NSCLC patients could benefit from targeted therapy.
Introduction
Non-small cell lung cancer (NSCLC) is the leading cause of cancer-related deaths worldwide.Citation1,Citation2 Lung adenocarcinoma is the most common sub-type of NSCLC.Citation3 An increasing number of driver gene alterations have been identified in NSCLC, most notably associated with epidermal growth factor receptor (EGFR), chromosomal rearrangements involving the anaplastic lymphoma kinase gene (ALK), and the c-ros oncogene 1 (ROS1). ROS1 encodes for an orphan receptor tyrosine kinase from the insulin receptor family that is related to ALK.Citation4 Between 1% and 2% of patients with NSCLC harbor a ROS1 rearrangement and the incidence is slightly higher in the East Asian population (2%-3%).Citation5,Citation6 Currently, a total of 16 ROS1 fusion partner genes have been reported in NSCLC, including CD74, SLC34A2, GOPC, CCDC6, SDC4, TPM3, EZR, LRIG3, KDELR2, LIMA1, MSN, CLTC, TPD52L1, FIG, TMEM106B, FAM135B, SLC6A17, and CEP72.Citation7–Citation10 In addition, MET is also a potential therapeutic target in NSCLC and MET amplification has a frequency of 5%-20%.Citation11 Both ROS1 gene rearrangements and de novo MET amplification have been described as rare oncogenic events in EGFR gene-negative NSCLC patients.
Crizotinib is a first-generation, small-molecule tyrosine kinase inhibitor (TKI) originally designed to target MET that has been shown to be effective against lung cancers harboring ALK or ROS1 alterations.Citation12,Citation13 Based on a phase I trial, crizotinib was shown to have an objective response rate (ORR) of 72% and a median progression-free survival (PFS) of 19.2 months in advanced ROS1-rearranged NSCLC.Citation14 While, some cases have shown a good response to crizotinib,Citation15 the efficacy of crizotinib for NSCLC patients with de-novo MET amplifications NSCLC has not been determined in a large clinical study.
Therefore, an accurate diagnosis is important with respect to the treatment and prognosis of NSCLC patients. Developing a multi-molecular test is a key step in identifying genetic alterations. The significance of the next generation sequencing (NGS) assay for molecular diagnoses in NSCLC patients is of increasing importance and has become common in clinical practice.Citation2
No study has reported NSCLC with a co-existing ZCCHC8-ROS1 rearrangement and MET gene amplification and the efficacy of treatment with crizotinib.
Clinical case report
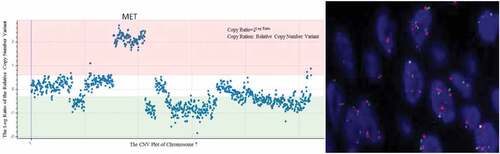
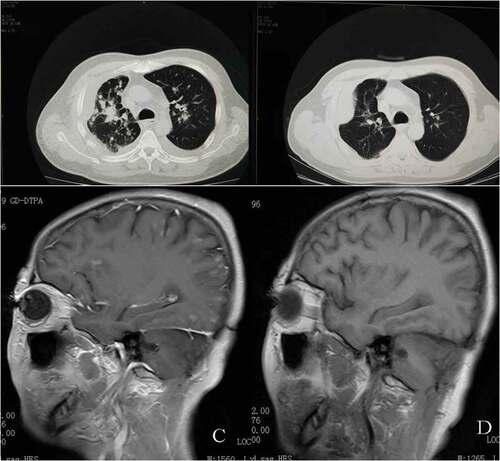
In May 2016, a 65-year-old Chinese female, a non-smoker, presented to our hospital for a physical examination that revealed pulmonary nodules. A computed tomography (CT) scan showed a mass in the right middle lung. A brain-enhanced MRI and an abdominal CT were not revealing. Then, she underwent surgery and a post-operative pathologic examination showed a peripherally invasive right lung adenocarcinoma (1.5 cm) and pleural nodule metastases. Immunohistochemical staining was positive for TTF-1 and Napsin A, and negative for CK5/6 and P40. According to the 7th edition of TNM staging, the patient was classified as stage IVA (T1N0M1a). Examination of the tumor tissue revealed wild-type epidermal growth factor receptor (EGFR) variants by ARMS (AmoyDx, Xiamen, China), and ALK protein expression was detected with the Ventana ALK IHC assay (Ventana Medical Systems, Roche, Inc., Tucson, AZ, USA). She consented to post-operative chemotherapy with two cycles of chemotherapy (docetaxel, 75 mg/m2 day 1 and cisplatin, 25 mg/m2 days 1–3 [DP]). Because of a venous thrombosis, chemotherapy was delayed and the disease had progressed in September 2016. There were multiple brain metastases, thus she received whole brain radiotherapy (WBRT; 30 Gy in 10 fractions). At the same time, the specimen was subjected to NGS and both ZCCHC8-ROS1 fusion () and de-novo MET gene amplification () were detected. De-novo MET amplification was confirmed using a MET/CEN7q Dual Color FISH Probe (Vysis; Abbott Molecular, Des Plaines, IL, USA) (). The results showed MET amplification. Because of progression of lung lesions, the patient began crizotinib therapy in November 2016 (, C). After 1 month, assessment with computed tomography and brain MRI scans revealed a partial response, according to the Response Evaluation Criteria 1.1 in Solid Tumors (, D). During crizotinib therapy, she had first-degree liver dysfunction and gastrointestinal side effects. There were no other treatment-related adverse events, including rashes, renal function, and cordis damage. Unfortunately, after 6 months, the disease progressed. Then, she received nedaplatin and apatinib and the overall survival was 22 months.
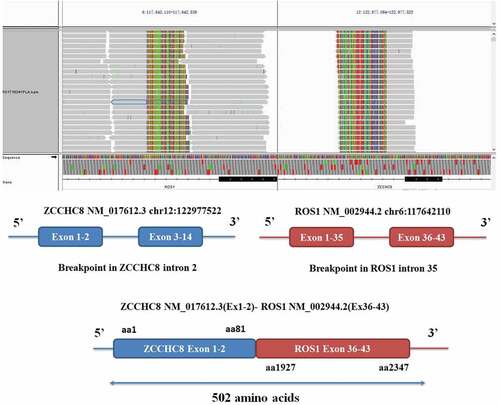
Figure 1. ZCCHC8-ROS1 fusion is clinically actionable. A, the integrative genomics viewer snapshot of ZCCHC8-ROS1. soft-clipped bases can match each other in reverse complementarity. B, schematic representation of the ZCCHC8-ROS1 fusion protein domain structure. Blue, ZCCHC8; red, ROS1; ZCCHC8-ROS1. Fusion protein is 502 amino acids in length.
Discussion
This is the first report of a co-existing ROS1 fusion and de-novo MET amplification in a patient with NSCLC. In addition, a new fusion type of ROS1 (ZCCHC8) was identified and the effect of EGFR-TKI in an NSCLC patient is reported. Considering this rare type of EGFR mutation and having a good response to TKI therapy, we recommend that clinicians should realize the existence of co-existing gene mutations in NSCLC patients.
Initially, driver gene mutations in NSCLC, such as EGFR, KRAS, HER2, BRAF, ALK, RET, ROS1, and MET, were regarded as independent, mutually exclusive events.Citation16 In recent years, more and more cases of lung cancer with co-existing alterations of EGFR, ALK, ROS1, and KRAS have been reported.Citation17–Citation19 Co-existing EGFR or KRAS mutations and ROS1 fusion were the most common associations. Warth et al.Citation20 reported ROS1 translocations occurring in conjunction with other driver mutations (EGFR, KRAS, and BRAF). In addition, Wiesweg et al.Citation21 showed that ROS1-IHC-positive patients presented with co-existing oncogenic driver mutations, such as EGFR (six cases), KRAS (two cases), PIK3CA, and BRAF. To date, only two patients harboring ROS1 and ALK concomitant rearrangements have been reported in the literature. Song et al.Citation22 and Zhu et al.Citation18 each reported one patient with a co-existing ALK/ROS1 rearrangement. Indeed, very few co-existing genes have been reported with MET gene amplification. Wang et al.Citation23 reported a patient with a KLC1-ALK fusion and MET gene amplification-positive NSCLC. Therefore, a concomitant ROS1 gene rearrangement and MET amplification is rare. Tang and colleaguesCitation19 explored co-existing genetic alterations involving ALK, RET, ROS1, or MET in 15 patients with lung adenocarcinomas, and found a case of ROS1 combined with MET; however, Tang et al.Citation19 did not show a specific fusion type and had no crizotinib treatment.
In addition, ZCCHC8-ROS1 fusion was first discovered in NSCLC. ZCCHC8, as a fusion partner of ROS1, was first demonstrated in a case of congenital glioblastoma multiforme with a t (6; 12) (q21;q24.3).Citation24 Therefore, the efficacy of inhibitors, such as crizotinib, for this type of ROS1 fusion was unknown.
Crizotinib, as an ALK/ROS1/MET inhibitor, is associated with an ORR of 72% and a median PFS of 19.2 months in advanced ROS1-rearranged NSCLC.Citation14 Recently, a phase II study involving crizotinib in East Asian patients with ROS1-positive advanced NSCLC (127 patients) resulted in an ORR of 71.7% and a median PFS of 15.9 months.Citation25 In contrast, NSCLC patients with a de novo MET amplification represents an uncommon sub-type of gene alteration in lung cancer. Although preliminary data from case reports has demonstrated promising efficacy of crizotinib treatment in NSCLC patients with de novo MET amplifications,Citation26,Citation27 studies involving a large number of patients with NSCLC and de novo MET amplifications are lacking. Therefore, crizotinib provides meaningful clinical benefit in patients with ROS1-positive or MET amplification advanced NSCLC. Wang et al.Citation23 showed a rapid response to crizotinib in a patient with brain metastases and a KLC1-ALK fusion and de-novo MET gene amplification; however, the patient did not continue crizotinib and did not achieve a PFS. Although, our patients also showed a good response to crizotinib therapy, the PFS was only 6 months. We conclude that NSCLC patients with co-existing driver gene alterations have a good response to crizotinib, but disease control time may be different from single gene mutation. Additional clinical data to verify the efficacy of crizotinib treatment in patients with advanced NSCLC and ROS1 fusion and de-novo MET amplification is warranted.
We should be aware of the phenomenon of concomitant ROS1 and de-novo MET amplification. There is currently no standard therapy for patients with co-existing driver genes. If concurrent driver fusions are identified, targeted therapy with the guidance of precise diagnosis should be offered; however, co-existing driver fusions may have potential clinical implications as biomarkers for a relatively poor prognosis.
Acknowledgments
This study was supported in part by grants from the Science and Technology Planning Project of Zhejiang Province (2015C33194) and the National Clinical Key Specialty Construction Program (2013).
Additional information
Funding
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics JA, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi:10.3322/caac.21387.
- Hiley CT, Le Quesne J, Santis G, Sharpe R, de Castro DG, Middleton G, Swanton C. 2016. Challenges in molecular testing in non-smallcell lung cancer patients with advanced disease. Lancet. 388:1002–1011. doi:10.1016/S0140-6736(16)31340-X.
- Stinchcombe TE, Socinski MA. 2009. Current treatments for advanced stage non-small cell lung cancer. Proc Am Thorac Soc. 6:233–241. doi:10.1513/pats.200809-110LC.
- Acquaviva J, Wong R, Charest A. 2009. The multifaceted roles of the receptor tyrosine kinase ROS in development and cancer. Biochim Biophys Acta. 1795:37–52. doi:10.1016/j.bbcan.2008.07.006.
- Davies KD, Le AT, Theodoro MF, Skokan M C, Aisner D L, Berge E M, Terracciano L M, Cappuzzo F, Incarbone M, Roncalli M, Alloisio M, Santoro A, Camidge D R, Varella-Garcia M, Doebele R C. Identifying and targeting ROS1 gene fusions in non-small cell lung cancer. Clin Cancer Res. 2012;18:4570–4579. doi:10.1158/1078-0432.CCR-12-0550.
- Kohno T, Nakaoku T, Tsuta K, Tsuchihara K, Matsumoto S, Yoh K, Goto K. 2015. Beyond ALK, RET, ROS1 and other oncogene fusions in lung cancer. Transl Lung Cancer Res. 4:156–164. doi:10.3978/j.issn.2218-6751.2014.11.11.
- Comprehensive molecular profiling of lung adenocarcinoma. Nature. Cancer Genome Atlas Research Network. 2014. 511(7511):543–550. doi:10.1038/nature13385.
- Zhu VW, Upadhyay D, Schrock AB, Gowen K, Ali SM, Ou S-HI. 2016. TPD52L1-ROS1, a new ROS1 fusion variant in lung adenosquamous cell carcinoma identified by comprehensive genomic profiling. Lung Cancer. 97:48–50. doi:10.1016/j.lungcan.2016.04.013.
- Gounder M M, Hakimi A A, Harding J J, Zehir A, Benayed R, Shah R H, Syed A, Middha S, Kim H R, Srinivasan P, Gao J, Chakravarty D, Devlin S M, Hellmann M D, Barron D A, Schram A M, Hameed M, Dogan S, Ross D S, Hechtman J F, DeLair D F, Yao J, Mandelker D L, Cheng D T, Chandramohan R, Mohanty A S, Ptashkin R N, Jayakumaran G, Prasad M, Syed M H, Rema A B, Liu Z Y, Nafa K, Borsu L, Sadowska J, Casanova J, Bacares R, Kiecka I J, Razumova A, Son J B, Stewart L, Baldi T, Mullaney K A, Al-Ahmadie H, Vakiani E, Abeshouse A A, Penson A V, Jonsson P, Camacho N, Chang M T, Won H H, Gross B E, Kundra R, Heins Z J, Chen H W, Phillips S, Zhang H, Wang J, Ochoa A, Wills J, Eubank M, Thomas S B, Gardos S M, Reales D N, Galle J, Durany R, Cambria R, Abida W, Cercek A, Feldman D R, Gounder M M, Hakimi A A, Harding J J, Iyer G, Janjigian Y Y, Jordan E J, Kelly C M, Lowery M A, Morris LGT, Omuro A M, Raj N, Razavi P, Shoushtari A N, Shukla N, Soumerai T E, Varghese A M, Yaeger R, Coleman J, Bochner B, Riely G J, Saltz L B, Scher H I, Sabbatini P J, Robson M E, Klimstra D S, Taylor B S, Baselga J, Schultz N, Hyman D M, Arcila M E, Solit D B, Ladanyi M, Berger M F. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med. 2017;23(6):703–713. doi:10.1038/nm.4333.
- Zhu YC, Zhou YF, Wang WX, Xu C-W, Zhuang W, Du K-Q, Chen G. CEP72-ROS1: A novel ROS1 oncogenic fusion variant in lung adenocarcinoma identified by next-generation sequencing. Thorac Cancer. 2018 Mar 8; Epub ahead of print. doi:10.1111/1759-7714.12617.
- Tsuta K, Kozu Y, Mimae T, Yoshida A, Kohno T, Sekine I, Tamura T, Asamura H, Furuta K, Tsuda H. 2012. c-MET/phospho-MET protein expression and MET gene copy number in non-small cell lung carcinomas. J Thorac Oncol. 7:331–339. doi:10.1097/JTO.0b013e318241655f.
- Christensen JG, Zou HY, Arango ME, Li Q, Lee JH, McDonnell SR, Yamazaki S, Alton GR, Mroczkowski B, Los G. 2007. Cytoreductive antitumor activity of PF-2341066, a novel inhibitor of anaplastic lymphoma kinase and c-Met, in experimental models of anaplastic large-cell lymphoma. Mol Cancer Ther. 6:3314–3322. doi:10.1158/1535-7163.MCT-07-0365.
- Yasuda H, de Figueiredo-Pontes LL, Kobayashi S, Costa DB. 2012. Preclinical rationale for use of the clinically available multitargeted tyrosine kinase inhibitor crizotinib in ROS1-translocated lung cancer. J Thorac Oncol. 7:1086–1090. doi:10.1097/JTO.0b013e3182570919.
- Shaw AT, Ou S-HI, Bang Y-J, Camidge DR, Solomon BJ, Salgia R, Riely GJ, Varella-Garcia M, Shapiro GI, Costa DB, et al. 2014. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med. 371:1963–1971. doi:10.1056/NEJMoa1406766.
- Motzer RJ, Barrios CH, Kim TM, Falcon S, Cosgriff T, Harker WG, Srimuninnimit V, Pittman K, Sabbatini R, Rha SY, et al. Phase II randomized trial comparing sequential first-line everolimus and second-line sunitinib versus first-line sunitinib and second-line everolimus in patients with metastatic renal cell carcinoma. J Clin Oncol. 2014;32(25):2765–2772. doi:10.1200/JCO.2013.54.6911.
- Gainor JF, Varghese AM, Ou S-HI, Kabraji S, Awad MM, Katayama R, Pawlak A, Mino-Kenudson M, Yeap BY, Riely GJ, et al. 2013. ALK rearrangements are mutually exclusive with mutations in EGFR or KRAS: an analysis of 1,683 patients with non-small cell lung cancer. Clin Cancer Res. 19:4273–4281. doi:10.1158/1078-0432.CCR-13-0318.
- Ju L, Han M, Zhao C, Li X. 2016. EGFR, KRAS and ROS1 variants coexist in a lung adenocarcinoma patient. Lung Cancer. 95:94–97. doi:10.1016/j.lungcan.2016.03.005.
- Xu C-W, Zhu Y-C, Ye X-Q, Yin M-X, Zhang J-X, Du K-Q, Zhang Z-H, Hu J. 2016. Lung cancer with concurrent EGFR mutation and ROS1 rearrangement: a case report and review of the literature. OncoTargets and Therapy. 9:4301–4305. doi:10.2147/OTT.
- Tang Z, Zhang J, Lu X, Wang W, Chen H, Robinson MK, Cheng J, Tang G, Medeiros LJ. Coexistent genetic alterations involving ALK, RET, ROS1 or MET in 15 cases of lung adenocarcinoma. Mod Pathol. 2018;31(2):307–312. doi:10.1038/modpathol.2017.109.
- Warth A, Muley T, Dienemann H, Goeppert B, Stenzinger A, Schnabel PA, Schirmacher P, Penzel R, Weichert W. ROS1 expression and translocations in non-small-cell lung cancer: clinicopathological analysis of 1478 cases. Histopathology. 2014;65(2):187–194. doi:10.1111/his.12379.
- Wiesweg M, Eberhardt WEE, Reis H, Ting S, Savvidou N, Skiba C, Herold T, Christoph DC, Meiler J, Worm K, et al. High prevalence of concomitant oncogene mutations in prospectively identified patients with ros1-positive metastatic lung cancer. J Thorac Oncol. 2017;12(1):54–64. doi:10.1016/j.jtho.2016.08.137.
- Song ZB, Zheng YH, Zhang YP. ALK and ROS1 rearrangements, coexistence and treatment in EGFR-wild type lung adenocarcinoma - A multicenter study of 732 cases. J Thorac Oncol. 2017;12(1):s1160–s1161. doi:10.1016/j.jtho.2016.11.1633. http://www.jto.org/article/S1556-0864(16)32874-X/fulltext.
- Wang P, Xiao P, Ye Y, Liu P, Han L, Dong L, She C, Yu J. Rapid response of brain metastasis to crizotinib in a patient with KLC1-ALK fusion and MET gene amplification positive non-small cell lung cancer: a case report. Cancer Biol Med. 2017;14(2):183–186. doi:10.20892/j.issn.2095-3941.2017.0017.
- Coccé MC, Mardin BR, Bens S, Stütz AM, Lubieniecki F, Vater I, Korbel JO, Siebert R, Alonso CN, Gallego MS. Identification of ZCCHC8 as fusion partner of ROS1 in a case of congenital glioblastoma multiforme with a t(6;12)(q21;q24.3). Genes Chromosomes Cancer. 2016;55(9):677–687. doi:10.1002/gcc.22369.
- Wu YL, Yang JC, Kim DW, Lu S, Zhou J, Seto T, Yang JJ, Yamamoto N, Ahn MJ, Takahashi T, Yamanaka T, Kemner A, Roychowdhury D, Paolini J, Usari T, Wilner KD, Goto K. Phase II study of crizotinib in East Asian Patients With ROS1-positive advanced non-small-cell lung cancer. J Clin Oncol. 2018;29:JCO2017755587. Epub ahead of print. doi:10.1200/JCO.2017.75.5587.
- Ou S-HI, Kwak EL, Siwak-Tapp C, Dy J, Bergethon K, Clark JW, Camidge DR, Solomon BJ, Maki RG, Bang Y-J, et al. 2011. Activity of crizotinib (PF02341066), a dual mesenchymal-epithelial transition (MET) and anaplastic lymphoma kinase (ALK) inhibitor, in a non-small cell lung cancer patient with de novo MET amplification. J Thorac Oncol. 6:942–946. doi:10.1097/JTO.0b013e31821528d3.
- Caparica R, Yen CT, Coudry R, Ou S-HI, Varella-Garcia M, Camidge DR, De Castro G. Responses to crizotinib can occur in high-level MET-amplified non-small cell lung cancer independent of MET Exon 14 alterations. J Thorac Oncol. 2017;12(1):141–144. doi:10.1016/j.jtho.2016.09.116.