ABSTRACT
Despite its low transfer efficiency, suicide gene therapy with HSV-TK is known for its bystander killing effect. The connexin-based gap junction is believed to mediate the bystander effect. Recently, we found that resveratrol, a polyphenol compound, increased the expression of Cx26 and Cx43, which are connexins and important constituents of gap junctions, in murine hepatoma cells. Hypothetically, the resveratrol-induced upregulation of gap junctions may improve the bystander effect that HSV-TK/GCV has on hepatoma cells. Our present investigation revealed that resveratrol could enhance intercellular communication at the gap junctions in CBRH7919 hepatoma cells and thereby enhance the bystander killing effect of GCV on CBRH7919TK cells. However, inhibition of gap junction using its long-term inhibitor alpha-glycyrrhetinic acid had a negative influence on the bystander effect of gene therapy with HSV-TK/GCV. In addition, combined resveratrol and GCV treatment in tumor-bearing mice with CBRH7919TK and CBRH7919WT cells at a ratio of 2:3 resulted in a significant decrease in the volume and weight of the tumor in comparison to GCV or only resveratrol. The present results demonstrate that resveratrol can enhance the bystander effect exerted by the HSV-TK/GCV system by enhancing connexin-mediated gap junctional communication.
Introduction
Hepatocellular carcinoma (HCC) is a malignant tumor that is one of the most fatal in the world, and the morbidity and mortality rates of this tumor are alarmingly high.Citation1 Surgery and liver transplantation are the main curative options for early-stage HCC patients, but such invasive treatment is not safe in patients with serious liver dysfunction. Transarterial chemoembolization and sorafenib treatment are other treatment options, but they are associated with a high recurrence rate and poor outcomes, respectively.Citation2 Therefore, it is essential to create alternative therapeutic methods for treating HCC.
Gene therapy with suicide genes is an attractive therapeutic strategy for HCC, and thymidine kinase (TK), which is isolated from the herpes simplex virus (HSV), is a suicide gene that has been studied in depth.Citation3 Cells that are transduced with this gene express functional HSV-TK, which induces the transformation of nontoxic ganciclovir (GCV) into a toxic GCV-triphosphated compound that inhibits DNA synthesis and thereby results in cell death.Citation4,Citation5 Toxic drug metabolites diffuse into the surrounding non-recipient cells (HSV-TK – cells) in a phenomenon known as the “bystander effect,” and this results in the extensive killing of tumor cells.Citation6 The toxic phosphorylated GCV molecules are transferred from HSV-TK–transduced cells to bystander cells via connexin (Cx)-based gap junctions.Citation7 Gap junctions (GJs) are channels present between cells that are made up of two juxtaposed transmembrane hemichannels of two adjacent cells.Citation8 Each connexon molecule contains six Cx protein subunits that surround a central pore. GCV-P, the toxic metabolite catalyzed by the suicide gene, can pass through this pore on account of its appropriate size and characteristics.Citation9 A clinical trial has already proven the feasibility and safety of HSV-TK/GCV therapy for HCC treatment.Citation10 Unfortunately, HCC cells exhibit weak cell-to-cell communication due to a lack of gap junctionsCitation11; moreover, HCC cells tend to repress the expression of Cxs.Citation12
Resveratrol, a polyphenol compound, is present in many fruits and plants, including grapes, berries peanuts and red wineCitation13, and it has received much attention for its anticancer effect because of its ability to induce apoptosis and inhibit metastasis of cancer cells.Citation14 Resveratrol has also been reported to have a protective effect against the dysregulation of gap junction intercellular communication (GJIC) that is mediated by environmental toxins.Citation15 Further, our recent study showed that resveratrol could induce the upregulation of Cx in HCC cells. Based on these findings, in the current study, we hypothesized that the killing effect of the HSV-TK suicide gene on HCC cells is enhanced by resveratrol via promotion of the bystander effect. Further, given the strong link between resveratrol and GJIC, we examined the expression of Cx’s in HCC cells after resveratrol treatment.
Results
Cytotoxicity of resveratrol in CBRH7919 cells
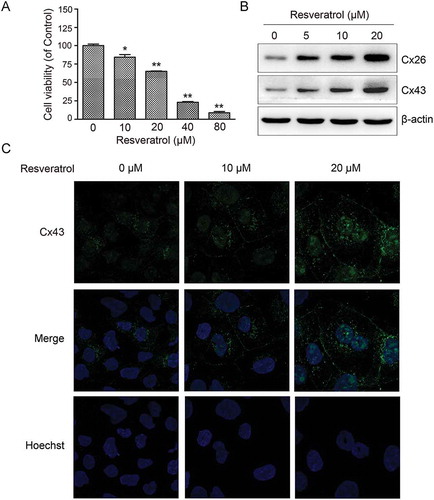
To determine the effect of resveratrol on GJIC in CBRH7919 cells, we determined the optimal concentration of resveratrol using the cell countin kit-8 (CCK8). Exposure to high concentrations of resveratrol (40 µM or 80 µM) for 48 h was highly cytotoxic to CBRH7919 cells (). In comparison, exposure to low concentrations of resveratrol (10 µM or 20 µM) for 48 h had relatively low inhibitory effect on the viability of CBRH7919 cells. Therefore, CBRH7919 cells treated with 10 μM or 20 μM resveratrol for 48 h was used for all the cellular experiments, as these concentrations showed mild toxicity to the CBRH7919 cells.
Figure 1. Resveratrol upregulated Cx expression in murine hepatoma cells. (A) Effect of resveratrol on CBRH7919 cell viability examined by CCK8. (B) Immunoblot gel images showing dose-dependent upregulation of Cx26 and Cx43 expression in CBRH7919 cells following resveratrol treatment. (C) Immunofluorescence images showing increased expression of Cx43 in resveratrol-treated CBRH7919 cells. Data are shown as mean ± standard deviation values. *P < 0.05; **P < 0.01.
Effect of resveratrol on GJIC in HCC cells
We investigated the expression of Cx proteins in CBRH7919 cells. Western blotting showed that Cx26 and Cx43 expression was upregulated dose-dependently in resveratrol-treated CBRH7919 cells (). The resveratrol-induced upregulation of Cx43 was also confirmed by immunofluorescence analysis ().
To determine whether resveratrol could promote GJIC in CBRH7919 cells, we conducted a scrape loading/dye transfer assay. When cells were cultured in the resveratrol-free medium, Lucifer yellow was mainly detected in the cells that were wounded as a result of the scrape (). In comparison, in the resveratrol-treated cells, cells adjacent to the wounded cells were also positive for the dye, and the spread of the dye was found to be dose-dependent.
Figure 2. Resveratrol promoted GJIC in CBRH7919 cells. (A) Resveratrol intensified the spread of Lucifer yellow from the wounded cells to the neighboring cells, as shown by the scrape loading/dye transfer assay. (B) Double-fluorescence assay showing the promotion of GJIC by resveratrol in CBRH7919 cells. Q1: recipient cells (calcein− DiI+), Q4: donor cells (calcein+ DiI−), Q2: DiI- and calcein-positive cell populations indicating the extent of GJIC, Q3: calcein− DiI− cells. *P < 0.05 vs. control group. Calcein, transferable green dye, passes readily through gap junctions, whereas Dil as a lipophilic red fluorescent dye does not.
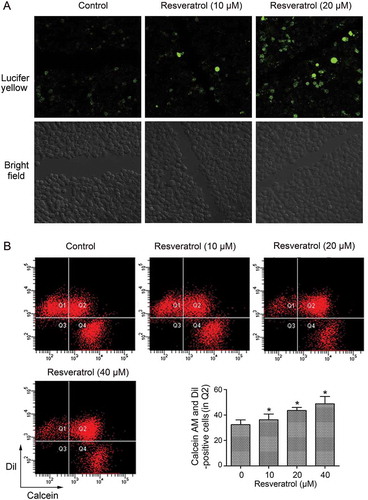
To further examine the effect of resveratrol on GJIC in HCC cells, a double-fluorescence dye transfer assay with calcein AM and Dil was also performed. Calcein AM can transfer between cells through Cx-formed gap junctions, whereas Dil can not. The percentage of double-positive cells was assessed using flow cytometry analysis, and this percentage was considered to indicate the degree of GJIC. Compared with the untreated control group (in Q2), resveratrol treatment resulted in a significant dose-dependent increase in the number of double-positive cells (). The results indicate that resveratrol enhanced GJIC in CBRH7919 cells.
Synergistic effect of resveratrol and HSV-TK/GCV therapy on inhibition of HCC cell growth
CBRH7919WT cells and CBRH7919TK cells (ratio, 9:1) were stained with propidium iodide and analyzed for apoptosis by flow cytometry after drug treatment. The apoptosis rate was low in the mixed cells treated with GCV alone (2.43 ± 0.23%); however, combined treatment with GCV and resveratrol led to an increase in the apoptotic rate (GCV + 10 µM resveratrol: 3.82 ± 0.28%; GCV + 20 µM resveratrol: 4.35 ± 0.14%; p < 0.01 vs. GCV treatment alone; ).
Figure 3. Resveratrol and HSV-TK/GCV therapy had a synergistic inhibitory effect on the growth of CBRH7919 cells. CBRH7919WT and CBRH7919tk cells (ratio, 9:1) were subjected to different treatments. (A) Cellular apoptosis was analyzed by flow cytometry with annexin V staining. (B, C) Cell viability was assessed using the MTT assay. (C) AGA treatment impaired the inhibitory effect of GCV combined with resveratrol on the mixed cells. *P < 0.05, **P < 0.01.
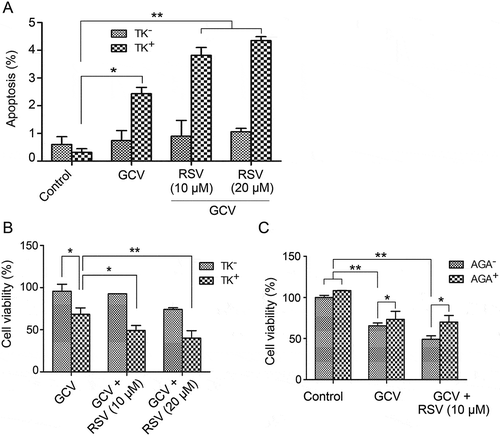
The survival fraction of the drug-treated cells was analyzed by the MTT assay. When GCV was combined with resveratrol, the cell viability (GCV + 10 µM resveratrol: 49.1 ± 6.0%; GCV + 20 µM resveratrol: 40.0 ± 8.9%) was significantly lower than that with only GCV treatment (68.3 ± 7.6%; ). The combination effect of resveratrol and GCV was statistically analyzed by the Jin’s method.Citation16 Based on the Q value, GCV combined with 10 µM (Q = 1.38) or 25 µM (Q = 1.22) resveratrol had a synergistic inhibitory influence on the growth of mixed CBRH7919 cells (Q > 1.15).
Effect of GJ inhibition on the synergistic effect of GCV and resveratrol on tk-containing mixed cells
Next, we examined whether upregulation of GJ by resveratrol mediated the bystander killing effect of the HSV-TK/GCV system in CBRH7919 cells by using AGA, which is a long-term inhibitor of GJ. The mixed cells containing 90% CBRH7919WT and 10% CBRH7919TK cultured in AGA-containing or AGA-free (control) medium were subjected to resveratrol treatment with or without GCV. Cell survival was assessed using the MTT assay. AGA or GCV treatment alone or combined AGA and GCV treatment did not have a significant effect on the growth of CBRH7919WT cells (). When the cells were mixed with 10% CBRH7919TK cells, GCV treatment alone caused a decrease in the cell viability by 65.5% ± 3.4%. Combined treatment with GCV and resveratrol, in comparison, caused a decrease in the cell viability by 49.1% ± 4.2%. The Q value (1.39) indicated that these two drugs had a synergistic effect. However, with the addition of AGA, the cell viability of the mixed group of cells treated with GCV and resveratrol was 70.1% ± 7.9% (p = 0.024 vs. GCV + resveratrol treatment). These data show that inhibition of GJ impaired the inhibitory effect of GCV combined with resveratrol on cell cultures containing 10% CBRH7919TK cells.
In vivo synergistic killing effect of resveratrol and HSV-TK/GCV therapy in CBRH7919 cells
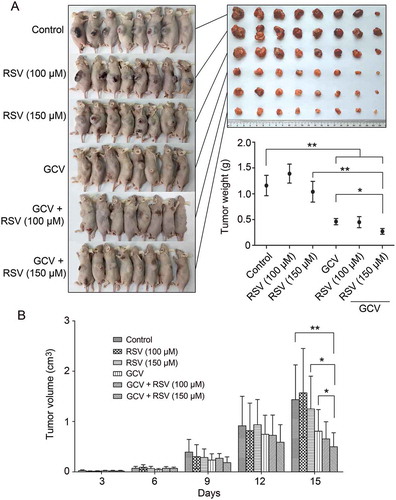
CBRH7919WT and CBRH7919tk cells (ratio, 3:2) were injected subcutaneously into nude mice that were divided into six groups, as explained in the previous section. Compared with the control group, resveratrol treatment alone did not have a significant inhibitory effect on tumor growth (p > 0.05; ). Although GCV treatment alone resulted in a 43.36% reduction in tumor volume (0.81 ± 0.43 cm3 vs. 1.43 ± 0.69 cm3) and a 60.34% decrease in tumor weight (0.46 ± 0.17 g vs. 1.16 ± 0.56 g), combined treatment with resveratrol (150 mg/kg) and GCV caused a 65.03% reduction in tumor volume (0.50 ± 0.28 cm3 vs. 1.43 ± 0.69 cm3) and a 76.72% decrease in tumor weight (0.27 ± 0.16 g vs. 1.16 ± 0.56 g). Thus, HSV-TK/GCV therapy had a more enhanced killing effect on resveratrol-treated CBRH7919 tumor cells.
Figure 4. In vivo synergistic inhibition of CBRH7919 tumor growth by resveratrol and GCV. (A) Subcutaneous tumors were induced in BALB/c mice using CBRH7919tk and CBRH7919WT cells mixed at a ratio of 2:3. When tumors reached a diameter of 0.1 mm3, the mice was divided into 6 groups and subjected to drug treatment. (A) The tumors were weighed on the 15th day after treatment. (B) Tumor volume was measured every 3 days after grouping. Data are shown as mean ± standard error values. *P < 0.05, **P < 0.01 vs. the model group; #P < 0.05 vs. GCV treatment alone.
Discussion
This study investigated the effect of resveratrol and HSV-TK/GCV therapy on cancer cells, as well as examined the underlying mechanism involving GJIC.
Eukaryotic cells express endogenous thymidine kinase. Therefore, GCV was presumed to kill cancer cells irrespective of whether the TK transgene was present. Our data, however, showed that GCV treatment barely killed WT murine hepatoma cells. This is probably because the affinity of GCV for eukaryotic thymidine kinases is 1000 times lower than that for HSV-TK. Therefore, GCV is phosphorylated solely by the viral enzyme HSV-TK.Citation17,Citation18 It is expected that the killing effect will be greater when the proportion of TK-positive cells in the mixture is higher. In this study, we mixed WT cancer cells with a small proportion of TK-positive CRBH7919 cells in order to observe the bystander effect clearly, as well as to mimic the in vivo low transfection rate.
As the half maximal inhibitory concentration (IC50) of resveratrol required for killing cancer cells is relatively high,Citation19 to efficiently kill cancer cells, in general, a high dose of resveratrol is required. The cell viability assay using CCK8 showed that resveratrol inhibits CRBH7919 cells at an IC50 of less than 40 μM. However, since HSV-TK and GCV treatment are known to result in hepatic dysfunction,Citation20 we used a low dose of resveratrol in combination with GCV treatment. On the one hand, a low dose of resveratrol reduces the burden on normal hepatocytes; on the other hand, it can effectively increase upregulation of Cxs and thereby enhance GJIC. In our study, combined treatment with resveratrol and GCV had a synergistic killing effect on CRBH7919 cells under both in vivo and in vitro conditions. Therefore, using a low dose of resveratrol combined with HSV-TK/GCV seems to be safe and also feasible for the treatment of HCC.
Cxs are capable of localizing in the cytoplasm of tumor cells; this results in the dysfunction of GJIC.Citation21 Our immunofluorescence results showed that Cx43 is mainly localized in the cell membrane of CBRH7919 cells, and that its expression remains at basal levels. Following resveratrol treatment, however, Cx43 is upregulated and predominantly localized in the cell membrane.Citation22 Cxs not only mediate GJIC, but also function as tumor suppressor proteins. In the case of cancer cells with abnormal communication, their tumorigenicity is lost or growth is downregulated when they are transfected with the appropriate Cx genes.Citation23,Citation24 Thus, Cx expression in tumor cells might have the following benefits: (a) mediation of the bystander effect and (b) tumor suppression.
The bystander effect can also be achieved in a GJ-independent manner.Citation25 In order to investigate whether a non-GJ mechanism was involved, we treated cancer cells with a long-term inhibitor of GJ (AGA) and found that the bystander effect of HSV-TK/GCV dramatically decreased. The results indicate that GJIC represents the main mechanism for the killing of neighboring cells by GCV-P. We used a GJ inhibitor and not siRNA to repress GJIC function because knock down of only one Cx in the GJ channels does not predominantly block GJIC. The data showed that inhibition of GJIC significantly decreased the bystander effect of HSV-TK/GCV therapy whether or not resveratrol treatment was also used.
To conclude, the present study shows that when administered at low doses, resveratrol worked with HSV-TK/GCV therapy in a synergistic manner to induce killing of HCC cells, and that the underlying mechanism predominantly involved GJIC.
Materials and methods
Material
GCV (≥ 99% purity, for the in vitro experiments), resveratrol (>98% purity), alpha-glycyrrhetinic acid (AGA) and Lucifer yellow (#L0259) were obtained from Sigma (St. Louis, MO, USA). Calcein AM and DiI were obtained from Invitrogen (Carlsbad, CA, USA). CCK8 was obtained from Dojindo (Tokyo, Japan). For the mouse experiments, GCV was obtained from Hubei Qianlong Pharmaceutical Co. (Qianjiang, China).
Cell lines and culture
Mouse malignant hepatoma cell line CBRH7919 (wild type, WT) was bought from the Laboratory Animal Center of Sun-yat Sun University. CBRH7919tk cells, which are CBRH7919 cells that stably express HSV-TK by lentiviral infection, were established in our lab with the lentiviral system. RPMI 1640 medium with 10% fetal bovine serum and penicillin and streptomycin (100 U/mL each) was used for cell culture.
Western blot analysis
CBRH7919 cells were cultured in 6-well plates, treated with resveratrol, and lysed in RIPA buffer (0.25 M Tris-HCl [pH 6.8], 8% SDS, 1 mM phenylmethylsulfonyl fluoride, 10 mg/ml aprotinin and 1.0 mg/ml leupeptin). The cellular extract obtained after lysing was separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred onto polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA, USA) and immunoblotted using polyclonal antibodies against Cx26 (ABclonal, Wuhan, China) and polyclonal antibodies against Cx43 (Cell Signaling Technology, Danvers, MA, USA). The internal reference used was the anti-actin antibody (Cell Signaling Technology, Danvers, MA, USA).
Immunofluorescence analysis
CBRH7919 cells that were cultured on cover slips were fixed by adding PBS with 4% paraformaldehyde for 20 min, permeabilized in 0.1% Triton X-100 for 5 min, and incubated in blocking buffer (3% horse serum in TBST) for 1 h. The cells were incubated in antibody dilution buffer (3% bovine serum albumin in TBS) containing antibodies against Cx43 for 2 h at room temperature. They were washed well in PBS and incubated for 1 h with the corresponding fluorochrome-conjugated secondary antibody. DNA was subjected to Hoechst staining. The stained cells were mounted on glass slides and observed under a confocal microscope.
Assessment of GJIC
1. Scrape loading/dye transfer assay
CBRH7919 cells were digested using EDTA-free trypsin and plated to obtain a confluent monolayer (3 × 106 cells) in 35-mm dishes with glass bottom. Following this, they were subjected to resveratrol treatment (0, 10 and 20 µM, respectively) for 48 h and washed with PBS. To prepare for scrape loading/dye transfer, the cells were exposed to a dye solution containing Lucifer yellow (2 mM) dissolved in PBS and scraped using a blade. Cells that were wounded as a result of the scrape were stained with Lucifer yellow. The cells were exposed to the dye solution for 15 min after scraping and then discarded. The Lucifer yellow is transferred from the stained cells into adjacent ones to which they are connected by functional Cx channels.Citation26 The cells were carefully rinsed and examined for dye transfer under a ZEISS LSM 800 confocal laser scanning microscope (× 630).
2. Double-fluorescence dye transfer assay
CBRH7919 cells were subjected to resveratrol treatment (0, 10 and 20 µM, respectively) in duplicate for 48 h and washed with PBS (three times). One population (donor cells) of cells in each drug-treated group was dyed with calcein AM (transferable green dye) for 30 min at 37°C, and the other population (recipient cells) was incubated with the lipophilic dye DiI (lipophilic red fluorescent dye) for 1 h at 37°C. Calcein can pass readily between functionally coupled cells in a gap junction-dependent manner, while DiI can not. After completion of incubation, the cells were washed with PBS (five times) and subjected to enzymatic dissociation with trypsin for 5 min. The donor (calcein+ DiI−) and recipient cells (calcein− DiI+) were mixed at a 1:1 ratio and left to form a confluent monolayer together in 35-mm plastic dishes. After co-culture for 3 h, the mixed cells were assayed by flow cytometry.Citation27 The percentage of double-positive cells (calcein+ DiI+) indicated the extent of GJIC.
Role of resveratrol in the bystander effect
Mixed cell populations containing 10% CBRH7919tk and 90% CBRH7919WT cells were seeded in quadruplicate in 96-well plates at 3 × 103 cells per well. When the cells had adhered on the second day, they were exposed to resveratrol (10 µM or 20 µM), GCV (15.7 µM) and DMSO (negative control) for 24 h, and GCV (15.7 μM) was added to the culture medium in one group for 24 h more. Cell viability was assessed using the MTT assay. Absorbance values were determined at 490 nm with a microplate reader (Bio-Tek ELx800; Bio-Tek Instruments Inc., Winooski, VT, USA). To determine the rate of apoptosis, the drug-treated cells were trypsinized, fixed in 70% ethanol at 4°C and stained with 40 mg/mL propidium iodide for 30 min at 37°C. Ten thousand cells from each group were analyzed with a FACStar cytofluorometer (BD Biosciences) that was equipped with an argon-ion laser (488 nm).
Experimental animals
Specific pathogen-free male BALB/c nude mice (weight, 18–22 g) were bought from the laboratory animal center of Sun Yat-Sen University. They were kept at the animal facility at Guangzhou University of Chinese Medicine. All animal studies received the approval of the Institute Research Medical Ethics Committee of Guangzhou University of Chinese Medicine.
In vivo experiments
Well-grown cultured CBRH7919WT cells and CBRH7919tk cells (ratio, 3:2) were diluted (final concentration, 2 × 106/ml) using serum-free culture medium. Then, 200 μl of the mixed cells (2 × 105) was injected into the flank of mice. When the tumor volume reached around 0.1 cm3, the mice were randomly assigned to six groups (n = 8): saline, resveratrol (100 mg/kg), resveratrol (150 mg/kg), GCV (50 mg/kg), GCV (50 mg/kg) + resveratrol (100 mg/kg), and GCV (50 mg/kg) + resveratrol (150 mg/kg). Resveratrol or saline was administered intraperitoneally once a day for 14 days. GCV treatment was initiated from day 8 and continued over a 7-day course. The volume of the xenografted tumor was measured every 3 days after grouping. On day 15, mice were killed by cervical dislocation to extract the solid tumor for measuring tumor weight.
Statistical analysis
Data were analyzed using analysis of variance (ANOVA). For differences that were significant, Fisher’s test was used to compare groups. P < 0.05 was considered to indicate statistical significance. Data were expressed as the mean ± standard deviation (SD) values with the help of the GraphPad Prism software (GraphPad Software, CA, USA).
The combination effect of the drugs was determined according to the Q value ,Citation16 which was calculated as follows: Q = EAB/[EA + EB (1-EA)]. In the eqaution, EA, EB and EAB represent the effects of drug A, drug B and the combination of both drugs, respectively. A Q value of >1.15 indicated a synergistic effect; a Q value of <0.85 indicated an antagonistic effect; Q values between 0.85 and 1.15 indicated an additive effect.
Abbreviations
| HSV | = | Herpes simplex virus |
| TK | = | Thymidine kinase |
| Cx | = | Connexin |
| GCV | = | Gancyclovir |
| WT | = | Wild type |
| HCC | = | Hepatocellular carcinoma |
| GJ | = | Gap junction |
| GJIC | = | Gap junction intercellular communication |
| AGA | = | Alpha-glycyrrhetinic acid |
| DiI | = | 1,1-dioctadecyl-3,3,3,3-tetramethylindocarbocyanine perchlorate |
| RPMI | = | Roswell Park Memorial Institute |
| SDS | = | Sodium dodecyl sulfonate |
| PVDF | = | Polyvinylidene difluoride |
| TBS | = | Tris-buffered saline |
| EDTA | = | Ethylene diamine tetraacetic acid |
| MTT | = | Methyl thiazolyl tetrazolium |
| CCK8 | = | Cell counting kit-8; ANOVA, Analysis of variance. |
Conflict of interest
The authors have no conflicts of interest to declare.
Additional information
Funding
References
- Hiraoka A, Kumada T, Kudo M, Hirooka M, Tsuji K, Itobayashi E, Kariyama K, Ishikawa T, Tajiri K, Ochi H, et al. Albumin-bilirubin (ALBI) grade as part of the evidence-based clinical practice guideline for HCC of the Japan Society of Hepatology: a comparison with the liver damage and Child-Pugh classifications. Liver Cancer. 2017;6:204–215. doi:10.1159/000452846.
- Keating GM. Sorafenib: a review in hepatocellular carcinoma. Target Oncol. 2017;12:243–253. doi:10.1007/s11523-017-0484-7.
- Vassaux G, Martin-Duque P. Use of suicide genes for cancer gene therapy: study of the different approaches. Expert Opin Biol Ther. 2004;4:519–530. doi:10.1517/14712598.4.4.519.
- Matthews T, Boehme R. Antiviral activity and mechanism of action of ganciclovir. Rev Infect Dis. 1988;10:S490–S4. PMID:2847285.
- Moolten FL. Tumor chemosensitivity conferred by inserted herpes thymidine kinase genes: paradigm for a prospective cancer control strategy. Cancer Res. 1986;46:5276–5281. PMID:3019523.
- Shao D, Li J, Pan Y, Zhang X, Zheng X, Wang Z, Zhang M, Zhang H, Chen L. Noninvasive theranostic imaging of HSV-TK/GCV suicide gene therapy in liver cancer by folate-targeted quantum dot-based liposomes. Biomater. Sci. 2015;3:833–41. doi:10.1039/C5BM00077G.
- Mesnil M, Piccoli C, Tiraby G, Willecke K, Yamasaki H. Bystander killing of cancer cells by herpes simplex virus thymidine kinase gene is mediated by connexins. Proc Natl Acad Sci. 1996;93:1831–1835, PMID:8700844. doi:10.1073/pnas.93.5.1831.
- Kumar NM, Gilula NB. The gap junction communication channel. Cell. 1996;84:381–388. PMID:8608591.
- Simpson I, Rose B, Loewenstein WR. Size limit of molecules permeating the junctional membrane channels. Science. 1977;195:294–296. PMID:831276.
- Sangro B, Mazzolini G, Ruiz M, Ruiz J, Quiroga J, Herrero I, Qian C, Benito A, Larrache J, Olagüe C, et al. A phase I clinical trial of thymidine kinase-based gene therapy in advanced hepatocellular carcinoma. Cancer Gene Ther. 2010;17:.837. doi:10.1038/cgt.2010.40.
- Fay P, Walsby A. Metabolic activities of isolated heterocysts of the blue-green alga Anabaena cylindrica. Nature. 1966;209:94. doi:10.1038/209094a0.
- Wilgenbus KK, Kirkpatrick CJ, Knuechel R, Willecke K, Traub O. Expression of Cx26, Cx32 and Cx43 gap junction proteins in normal and neoplastic human tissues. International Journal of Cancer. 1992;51:522–529. PMID:1318266.
- Du X, Zhou J, Shi J, Xu B. Supramolecular hydrogelators and hydrogels: from soft matter to molecular biomaterials. Chem Rev. 2015;115:13165–13307. doi:10.1021/acs.chemrev.5b00299.
- Varoni EM, Lo Faro AF, Sharifi-Rad J, Iriti M. Anticancer molecular mechanisms of resveratrol. Front Nutrition. 2016;3:8. doi:10.3389/fnut.2016.00008.
- Sovadinova I, Babica P, Böke H, Kumar E, Wilke A, Park J-S, Trosko JE, Upham BL, Agarwal R. Phosphatidylcholine specific PLC-induced dysregulation of gap junctions, a robust cellular response to environmental toxicants, and prevention by resveratrol in a rat liver cell model. PloS one. 2015;10:e0124454. doi:10.1371/journal.pone.0124454.
- Jin Z. About the evaluation of drug combination. Acta Pharmacol Sin. 2004;25:146–147. PMID:14769200.
- Oliver S, Bubley G, Crumpacker C. Inhibition of HSV-transformed murine cells by nucleoside analogs, 2′-NDG and 2′-nor-cGMP: mechanisms of inhibition and reversal by exogenous nucleosides. Virology. 1985;145:84–93. PMID:2990104.
- Deville-Bonne D, El Amri C, Meyer P, Chen Y, Agrofoglio LA, Janin J. Human and viral nucleoside/nucleotide kinases involved in antiviral drug activation: structural and catalytic properties. Antiviral res. 2010;86:101–20. doi:10.1016/j.antiviral.2010.02.001.
- Nivelle L, Hubert J, Courot E, Jeandet P, Aziz A, Nuzillard J-M, Renault J-H, Clément C, Martiny L, Delmas D. Anti-cancer activity of resveratrol and derivatives produced by grapevine cell suspensions in a 14 L stirred bioreactor. Molecules. 2017;22:474. doi:10.3390/molecules22030474.
- Van der Eb M, Cramer S, Vergouwe Y, Schagen F, Van Krieken J, Van der EbA, Borel Rinkes I, Van de Velde C, RC Hoeben. Severe hepatic dysfunction after adenovirus-mediated transfer of the herpes simplex virus thymidine kinase gene and ganciclovir administration. Gene therapy. 1998;5:451. doi:10.10.1038/sj.gt.3300637.
- Karjoo Z, Chen X, Hatefi A. Progress and problems with the use of suicide genes for targeted cancer therapy. Advanced drug delivery reviews. 2016;99:113–28. doi:10.1016/j.addr.2015.05.009.
- Coll J, Mesnil M, Lefebvre M, Lancon A, Favrot M. Long-term survival of immunocompetent rats with intraperitoneal colon carcinoma tumors using herpes simplex thymidine kinase/ganciclovir and IL-2 treatments. Gene Ther. 1997;4:1160. doi:10.1038/sj.gt.3300516.
- Rose B, Mehta PP, Loewenstein WR. Gap-junction protein gene suppresses tumorigenicity. Carcinogenesis. 1993;14:1073–1075. PMID:8389252.
- Hirschi KK, Xu C, Tsukamoto T, Sager R. Gap junction genes Cx26 and Cx43 individually suppress the cancer phenotype of human mammary carcinoma cells and restore differentiation potential. Cell Growth Differentiation-Publication Am Assoc Cancer Res. 1996;7:861–870. PMID:8809403.
- Kong H, Liu X, Yang L, Qi K, Zhang H, Zhang J, Huang Z, Wang H. All-trans retinoic acid enhances bystander effect of suicide gene therapy in the treatment of breast cancer. Oncology reports. 2016;35:1868–74. doi:10.3892/or.2015.4535.
- Fairweather DS, Fox M, Margison GP. The in vitro lifespan of MRC-5 cells is shortened by 5-azacytidine-induced demethylation. Exp Cell Res. 1987;168:153–159. doi:10.1016/0014-4827(87)90424-1.
- Goldberg G, Bechberger J, Naus C. A pre-loading method of evaluating gap junctional communication by fluorescent dye transfer. Biotechniques. 1995;18:490–497. PMID:7779401.
