ABSTRACT
Acute promyelocytic leukemia (APL) is one of the most fatal hematological malignancies. APL during pregnancy is a rare comorbidity and can lead to adverse outcomes, such as maternal and/or fetal death, without timely and appropriate management. Medical management for APL during pregnancy remains challenging. We reported 2 patients with no regular prenatal visits who were diagnosed with APL during pregnancy. One presented with typical hematological abnormalities related to infection, while the other presented with intracranial hemorrhage, which is rare. Although supportive measures and chemotherapy were administered after APL was diagnosed, these two patients had completely different outcomes. The pregnancy outcomes of APL patients depend greatly on the timely diagnosis and appropriate management of the disease. Physicians should pay more attention to APL during pregnancy and thus may save more maternal and fetal lives. Further study of the management of APL during pregnancy is warranted.
Abbreviations: AML: acute myeloid leukemia; APL: acute promyelocytic leukemia; WBC: white blood cell; RBC: red blood cell; Hb: hemoglobin; PT: prothrombin time; TT: thrombin time; APTT: activated partial thromboplastin time; TP: total protein; ALB: albumin; AST: aspartate transaminase; ALT: alanine aminotransferase; LDH: lactate dehydrogenase; ATRA: all-trans retinoic acid; ICH: intracranial hemorrhage; DIC: disseminated intravascular coagulation
Introduction
Leukemia is a rare comorbidity during pregnancy with a low prevalence of 1/75 000 ~ 1/100 000 pregnancies, the majority of which are cases of acute myeloid leukemia (AML).Citation1 Acute promyelocytic leukemia (APL) is an invasive subtype of AML, accounting for approximately 10% of AML. APL in the early stage may present no typical clinical manifestations, only abnormal routine blood test results, which means that the diagnosis of APL is often missed. If APL patients do not receive timely and effective treatment, they will experience adverse outcomes, such as maternal and/or fetal death. Here, we report 2 cases of new-onset APL diagnosed during pregnancy in Tianjin Medical University General Hospital. The patients’ history, clinical data, therapeutic approaches and outcomes are discussed. In addition, clinical characteristics, management and therapeutic options are also discussed to raise the awareness of physicians of APL in pregnancy, promote the early diagnosis of APL and improve pregnancy outcomes. Based on previous literature, we analyzed the characteristics, clinical decision-making and treatment options of APL during pregnancy, trying to draw the attention of physicians to this rare disease, thus perhaps improving the pregnancy outcomes of APL patients by increasing timely diagnosis and appropriate management.
Case report
Case 1
A 24-year-old woman (gravida 2, para 0) with a 25 week pregnancy was admitted to our hospital having been unconscious for 4 hours. Unconsciousness occurred suddenly without obvious causes or any accompanying symptoms, such as vomiting, twitching or incontinence. Her past medical history, family history, surgical history, and OB/GYN history were not relevant. Her guardian denied smoking or alcohol consumption or prior radiation exposure history and claimed that her routine blood test was normal in the first trimester of this pregnancy.
On admission, her vital signs were as follows: temperature 36.8°C, blood pressure 126/67 mmHg, heart rate 109 bpm, respiratory rate 29 pm, and SpO2 99%. General physical examination revealed that she had mild cyanosis, asymmetrical mydriasis, scattered petechiae on the abdominal skin and mild dropsy in her lower extremities. There were no palpable superficial lymph nodes. Tachycardia and shortness of breath were present. She was unresponsive to stimulus. The Glasgow Coma Scale was 3. The height of the uterus fundus was 25 cm, and the girth was 88 cm. No uterine contractions or vaginal bleeding or abnormal discharge were observed. The fetal heart rate was 130 bpm.
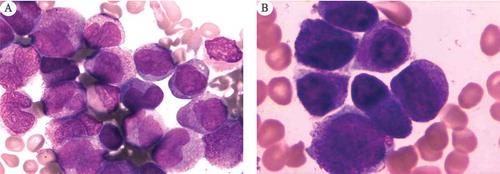
Non-contrast computerized tomography of the head was performed and revealed multiple hematomas in the bilateral cerebellar hemisphere, bilateral frontal temporal parietal lobe and right basal ganglia; subarachnoid hemorrhage; and intraventricular hemorrhage, with low density strip under the right side of the parietal bone. Obstetrical ultrasound showed a fetus at 25 weeks gestation with normal fetal anatomy. The amniotic fluid index was 12.4 cm. Abdominal ultrasound revealed no abnormalities except an enlarged spleen. Her laboratory data were as follows: white blood cell (WBC) count 162.97 × 109/L (reference value: 3.5 ~ 9.5 × 109/L), red blood cell (RBC) count 2.89 × 1012/L (3.8 ~ 5.10 × 1012/L), hemoglobin (Hb) 89 g/L (reference value: 115 ~ 150 g/L), platelet count 25 × 109/L (reference value: 125 ~ 350 × 109/L), prothrombin time (PT) 13.9 sec (reference value: 9.50–15.0 sec), thrombin time (TT) 27.2 sec (reference value: 13–25 sec), PT-INR 1.27 (reference value: 0.8–1.5), activated partial thromboplastin time (APTT) 27.2 (reference value: 20–40 sec), D-dimer 7701ng/L (reference value: 0–500 ng/L), fibrinogen 1.15g/L (reference value: 1.8–4.0 g/L), total protein (TP) 70 g/L (reference value: 63–82 g/L), albumin (ALB) 39 g/L (reference value: 35–50 g/L), aspartate transaminase (AST) 28 U/L (reference value: 15–46 U/L), alanine aminotransferase (ALT) 18 U/L (reference value: 5–69 U/L), lactate dehydrogenase (LDH) 744 U/L (reference value: 94–250 U/L), creatinine 43 μmol/L (reference value: 62–133 μmol/L), and BUN 5.1 mmol/L (reference value: 2.9–7.5 mmol/L). An initial diagnosis of multiple intracranial hemorrhage (ICH) complicated with coagulopathy was established. Ventilatory support and the monitoring of other vital signs were immediately started. Bone marrow examination revealed that 83% of the marrow cells were abnormal promyelocytic cells (), which confirmed the diagnosis of APL, AML French-American-British classification M3. A multidisciplinary consultation was held between neurosurgery, hematology and obstetrics, and advice was given. 1) The diagnosis of APL was established based on the results of routine blood and bone marrow examinations. All-trans retinoid (ATRA) treatment was recommended, if the patient’s guardian provided consent. 2) Based on the computerized tomography examination, a diagnosis of ICH with disseminated intravascular coagulation (DIC) was established. Because the patient’s general status was not stable enough to survive a high-risk operation, surgical interventions were not recommended. 3) Close monitoring of the comprehensive maternal and fetal status was recommended. After the receipt of inform consent, ATRA 45 mg/m2/day was started. Meanwhile, 1 g hydroxyurea three times a day was also administered. In addition, platelet transfusion, plasma transfusion and other supportive measures were also started immediately. Her general condition deteriorated progressively after 24 hours of treatment. Her vital signs became unstable (T 39.6°C, BP 110/60 mmHg, HR 129 bpm, RR 34 pm, SpO2 99%). Physical examination revealed that she had moderate cyanosis and bilateral mydriasis (L = 5 mm, R = 5 mm). She was still unresponsive to stimuli. The Glasgow Coma Scale score remained a 3. Laboratory investigations revealed 118.80 × 109/L WBC, 68 g/L Hb, and 21 × 1012/L platelets. She had TP 52 g/L, ALB 28 g/L, AST 106 U/L, ALT 40 U/L, LDH 831 U/L, creatinine 190 μmol/L and BUN 10.1 mmol/L, indicating the potential onset of multiple organ failure. Non-stress tests showed a basal line of 180 bpm with minimal variability and slight and irregular uterine contractions. After exhaustive explanation and communication with her guardian about the patient’s situation, they refused further treatment, giving consideration to all potential poor outcomes and the untenable finical burden. Subsequently, her guardian withdrew her medical treatment and discharged her 48 h after admission due to progressive deterioration of her general condition. Ten hours after discharge, the patient died at home.
Case 2
A 37-year-old woman (gravida 4, para 1) with a 34 week pregnancy was referred to our hospital with a history of sore throat and cough for 2 weeks that had been aggravated for 3 days. Two weeks ago, she developed a sore throat, followed by tussis and expectoration. Oral cefuroxime for 7 days was administered at the local hospital. Her symptoms resolved after the administration of antibiotics. Three days before referral, her symptoms relapsed with a maximum body temperature of 38.8°C. Routine blood tests revealed that she had pancytopenia with a WBC of 0.78 × 109/L, Hb 52 g/L, and platelet 15 × 109/L. Her past medical history, family history, surgical history, and OB/GYN history were unremarkable. She denied smoking or alcohol consumption or prior radiation exposure history. Her previous pregnancy resulted in an uneventful vaginal delivery in 2008. She claimed no abnormal routine blood work results in the first trimester. Before this referral, she had no regular antenatal examinations.
On admission, her vital signs were as follows: blood pressure 113/60 mmHg, heart rate 103 bpm, respiratory rate 21 pm, and SpO2 99%. Initial examination revealed distinctly pale skin and scattered petechiae on her right eyelid, left upper extremity and both lower extremities. Mild edema was also observed in her lower extremities. Bilateral tonsils were 1 degree swollen with purulent exudation. No obvious abnormality was found in the cardiopulmonary examination. The height of the uterus fundus was 33 cm, and the girth was 92 cm. No uterine contractions, vaginal bleeding or abnormal discharge were detected. The fetal heart rate was 136 bpm. Fetal posture was left occipital anterior and not engaged.
Obstetrical ultrasound showed a fetus at 32 weeks plus 6 days gestation with normal fetal anatomy. The amniotic fluid index was 10.2 cm. Fetal heart rate monitoring was Class I. Abdominal ultrasound showed nothing abnormal except an enlarged spleen. Her laboratory data on admission were as follows: WBC 0.81 × 109/L, Hb 45 g/L, platelets 11 × 109/L, PT 21.9 sec, PT-INR 1.99, APTT 34.4, D-dimer > 0 ng/L, fibrinogen 0.16 g/L, TP 70 g/L, ALB 30 g/L, AST 52 U/L, ALT 24 U/L, LDH 1154 U/L, creatinine 86 μmol/L and BUN 3.8 mmol/L. An initial diagnosis of pancytopenia complicated with upper respiratory tract infection was made.
Supportive care was started immediately with 2 U blood transfusion, 1 therapeutic dose of platelets, colony stimulating factor and intravenous cefotaxime. Bone marrow examination revealed that 79% of the marrow cells were abnormal promyelocytic cells (). The diagnosis of APL, AML French-American-British classification M3, was also confirmed by a molecular evaluation of the PML-RARα gene rearrangement and cytogenetic confirmation of a specific chromosomal translocation, t (15; 17). A multidisciplinary consultation was held between hematology and obstetrics, and the following advice was given: 1) the diagnosis of APL was established based on the results of routine blood tests, bone marrow examination and PML-RARα gene rearrangement. ATRA treatment should be started with patient consent. 2) During ATRA therapy, the close and comprehensive monitoring of both maternal and fetal statuses was strongly needed. After the patient provided consent, 45 mg/m2/day ATRA was administered. The fetal status in utero was monitored by ultrasound every day. As a result of treatment, her general status became stable. Changes in the hemogram of the patient are shown in . Twelve days later, she had a spontaneous and uneventful vaginal delivery in the operating room under intensive care. A 3200-g female infant was born with an Apgar score of 7/9/10 at 1, 5 and 10 min, respectively. The baby was healthy with no recognized somatic abnormalities. Post-delivery, the patient continued chemotherapy in the hematology department. The baby stayed in the neonatal intensive care unit for 3 weeks before discharge. At the time of this report, she and her baby are doing well.
Table 1. Changes to hemogram of patient 2 after treatment.
Discussion
Epidemiological background
APL is an invasive AML subtype with an age-adjusted annual prevalence of 0.23/100 000,Citation2 distinguished by balanced reciprocal translocation t (15; 17) and fusion of the PML-RARα gene. Fatal coagulopathy is another important characteristic of APL. The median age at diagnosis of APL was 44, while that of AML was 67. This may be the reason why APL occurs more commonly in childbearing and even pregnant women than AML.
Diagnosis and clinical manifestation
The diagnostic procedure of APL in pregnancy is the same as that in the nonpregnancy period. Bone marrow aspiration and trephine biopsy can be performed safely in pregnancy.Citation3 More than 30% of promyelocytic cells in a bone marrow smear is the basic evidence supporting APL diagnosis. Other means, such as molecular analysis, peripheral blood microscopy and flow cytometry, were auxiliary evidence for APL diagnosis. Both of these cases were diagnosed with APL through bone marrow aspiration. The PML-RARα of case 2 was positive, which also supported the diagnosis of APL.
APL is the most invasive type of AML. Beyond recurrent infection and fatigue, hemorrhagic risk is another characteristic of APL. Petechiae and coagulopathy are common clinical manifestations. Hemorrhagic coagulopathy is potentially fatal. Life-threatening hemorrhagic disorder, which may result from coagulopathy, is another special characteristic that leads to early death from APL.Citation4 A population-based report from Sweden revealed that hemorrhage is the main cause of early death in patients with APL.Citation5
In our two cases, case 1 initially presented as ICH with coagulopathy, which was uncommon in APL. Although timely treatment was started as soon as she was diagnosed, the outcome was still poor. Case 2 was suspected to have APL due to an abnormal blood count complicated by recurrent upper respiratory tract infection, which is a very typical manifestation of hematological disorders. Compared with case 2, case 1 presented with more severe and rare symptoms, including coagulopathy, which may be the underlying reason for her death. With supportive measures and other medications, case 2 achieved a better outcome than case 1. Neither case 1 nor case 2 had undergone regular antenatal examinations. If they had received regular antenatal examination, they might have achieved an early diagnosis and earned precious time to combat the disease. This indicated that the importance of receiving regular antenatal examinations cannot be ignored.
Pregnancy and APL
Embryo toxicity is a main limitation when drugs are used for APL during pregnancy. Because APL during pregnancy is rare, it is unclear whether the immunosuppressed state caused by hormonal and immunological alterations during pregnancy and the limitations of the available medications affect the pathogenesis and prognosis of APL in pregnancy. When APL occurs during pregnancy, the risks of abortion, perinatal mortality, intrauterine growth retardation, and preterm delivery are increased. In addition, the risks of other complications, such as infection, inflammation, and placental abruption, are also increased. A systematic study of new-onset APL during pregnancy revealed that spontaneous/therapeutic abortion/intrauterine death and neonatal complications, including newborn respiratory stress syndrome, were found in 33.3% and 25.9% of cases, respectively. The reason for the surprisingly low risk of transferred fetal leukemia may lie in the natural placental barrier or the immune elimination ability.Citation6
Management of APL during pregnancy
APL treatment for adults is divided into the following three parts: induction therapy, consolidation therapy and maintenance treatment. Induction therapy is based on ATRA, while the consolidation phase involves the use of ATRA and anthracycline drugs or cytarabine and anthracycline drugs. With this therapy, more than 80% of patients may be cured.Citation2 The clinical significance of maintenance therapy is still controversial. The discovery and application of ATRA and arsenic have made considerable improvements in APL treatment over the past several decades. Arsenic has high embryo toxicity, so it is not recommended during any trimester of pregnancy.Citation7,Citation8 As a member of the retinoid family, ATRA is considered to have high teratogenicity, potentially resulting in retinoid embryopathy and should be avoided in the first trimester.Citation9 As ATRA therapy in the second and third trimesters is also associated with fetal cardiac toxicity, the necessity of cardiac monitoring should be emphasized.Citation10,Citation11 Chemotherapy may not cause congenital abnormalities, but the risk of abortion, prematurity, low birth weight, neonatal neutropenia, and\sepsis are undoubtedly increased. Meanwhile, chemotherapy-associated toxicity, such as the potential occurrence of severe myelosuppression and secondary leukemia, cannot be ignored.Citation9,12
Comprehensive evaluation is the fundamental basis of the clinical decision-making process, including the termination of pregnancy and start of induction therapy. Gestational weeks and individual differences in maternal and fetal risk-taking are very important factors. The lack of evidence-based medical and epidemiological evidence suggests that chemotherapy agents and treatment time for APL in pregnancy remain challenging clinical dilemmas.
Termination of the pregnancy followed by ATRA therapy is often recommended because of the embryo toxicity of ATRA when used in the first trimester. Because anthracyclines had relatively less toxicity compared with ATRA, using anthracyclines alone in the first trimester and postponing ATRA until the second trimester is the only but not optimal option for APL pregnant women who insist on preserving their pregnancies. Before therapy is started, the patient should be fully informed regarding the poor efficiency and potentially increased risk of bleeding, abortion, fetal death, congenital malformations, low birth weight and other adverse outcomes.Citation9,Citation13 Anthracyclines may also affect organogenesis and fetal development. These risks mean that anthracyclines not the preferred choice, although they are the only choice if the pregnancy must be preserved. Prudent and optimally suitable therapeutic approaches should be made on the basis of gestational age, maternal and fetal status and ethical/moral considerations. Although ATRA is associated with an increase in fetal growth, low birth weight infants, abortion, and preterm birth risk in late pregnancy, ATRA had fewer teratogenic effects on the fetus than the other available drugs. Valappil et al.Citation14 reviewed 27 cases involving the administration of ATRA during pregnancy; no infant had congenital malformations, which provided evidence supporting the low risk of major fetal malformations due to ATRA administered during the 2nd and 3rd trimesters. With timely treatment, APL diagnosed in the second and third trimesters has a better prognosis than those diagnosed in the 1st trimester.Citation15 APL diagnosed in the 1st trimester mostly ends with therapeutic abortion before the start of ATRA therapy. Pregnant women with APL diagnosed in the 2nd and 3rd trimesters always have promising pregnancy outcomes.Citation15
The mode of delivery for APL patients is another challenge that requires careful assessment of patients’ general condition. If possible, a normal delivery is preferred to Cesarean section to reduce the risk of hemorrhage. Cesarean section is only the best option when a patient cannot endure the stress of a vaginal delivery; the patients should receive intensive care and preparation.Citation9,Citation10
Multidisciplinary teams are essential for individualized management of hematologic malignancies in pregnancy.Citation9,Citation16 The majority of morbidities that occur during pregnancy are associated with derangements in the coagulation system, which may culminate in DIC. In addition to ATRA and chemotherapy, supportive blood transfusion correcting DIC is of great importance.Citation17 In addition, infection is another common complication among patients with APL. Prevention and treatment of infection is very important. ICH, one of the reasons for the high levels of morbidity and mortality among patients with AML, is associated with hyperleukocytosis and prolonged PT. The incidence of ICH is higher in patients with APL than other subtypes of AML.Citation18,Citation19 Because the incidence of ICH is higher in patients with APL than other AML subtypes, APL should be suspected when physicians observe ICH in pregnant women. As maternal prognosis after ICH has been demonstrated to be poor, more prevention efforts should be made.Citation20 Timely detection of risk factors for ICH, including hypertension, vessel abnormalities, low platelet count or platelet dysfunction, coagulation factor deficiency, DIC and hyperleukocytosis, should be highlighted.
In the two cases we reported here, case 1 initially presented as multiple ICH at 25 weeks of gestation. She did not respond to ATRA therapy, and her general condition progressively deteriorated. Ultimately, adverse maternal and fetal outcomes occurred. Case 2 presented as recurrent infections and an abnormal routine blood count. ATRA and other aggressive management strategies were started in a timely manner. Fortunately, the obvious and typical symptoms and signs provided her with the opportunity for an early diagnosis, gaining treatment time for both mother and baby and making an uneventful vaginal delivery possible. Individual factors resulted in substantial differences with regard to the first symptoms presented, complications and outcomes. Timely diagnosis and aggressive management were the key factors in improving pregnancy outcomes.
In conclusion, the increasing incidence of pregnancy-associated myeloproliferative neoplasms is predominantly due to improved diagnostic techniques. The discovery and application of ATRA and arsenic, improved diagnostic tools, molecular monitoring, supportive therapy and reduced effective doses of chemotherapy resulted in marked improvements in APL treatment since the 1980s. However, when APL occurs during pregnancy, it poses a challenging therapeutic dilemma. The patient, hematologist, obstetrician, and neonatologist should all participate in the decision-making process to obtain a positive outcome for both mother and fetus. The aim of presenting these two cases is to draw the attention of physicians to the possibility of APL during pregnancy and to provide insight into the management of rare APL diagnosed during pregnancy. Timely diagnosis and intervention with ATRA can be life-saving in cases of APL.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Ethical statements
The enrolled patient provided written informed consent. The study was reviewed and approved by the ethical committee of Tianjin Medical University General Hospital.
Acknowledgments
We would like to acknowledge and thank the patients and their family members.
Additional information
Funding
References
- Brenner B, Avivi I, Lishner M. Haematological cancers in pregnancy[J]. Lancet. 2012;379(9815):580–587. doi:10.1016/S0140-6736(11)61348-2.
- NCCN Guidelines Version 1.2015 Panel Members Acute Myeloid Leukemia[EB/OL]. National Comprehensive Cancer Network; 2015. https://www.nccn.org/professionals/physician_gls/pdf/aml.pdf.
- Weisz B, Meirow D, Schiff E, Lishner M. Impact and treatment of cancer during pregnancy[J]. Expert Rev Anticancer Ther. 2004;4(5):889–902. doi:10.1586/14737140.4.5.889.
- Park JH, Qiao B, Panageas KS, Schymura MJ, Jurcic JG, Rosenblat TL, Altman JK, Douer D, Rowe JM, Tallman MS. Early death rate in acute promyelocytic leukemia remains high despite all-trans retinoic acid[J]. Blood. 2011;118(5):1248–1254. doi:10.1182/blood-2011-04-346437.
- Lehmann S, Ravn A, Carlsson L, Antunovic P, Deneberg S, Mollgard L, Derolf AR, Stockelberg D, Tidefelt U, Wahlin A, et al. Continuing high early death rate in acute promyelocytic leukemia: a population-based report from the Swedish Adult Acute Leukemia Registry[J]. Leukemia. 2011;25(7):1128–1134. doi:10.1038/leu.2011.78.
- Verma V, Giri S, Manandhar S, Pathak R, Bhatt VR. Acute promyelocytic leukemia during pregnancy: a systematic analysis of outcome[J]. Leuk Lymphoma. 2016;57(3):1–7. doi:10.3109/10428194.2015.1065977.
- Naujokas M F, Anderson B, Ahsan H, Aposhian H V, Graziano J H, Thompson C, Suk WA. The broad scope of health effects from chronic arsenic exposure: update on a worldwide public health problem[J]. Environ Health Perspect. 2013;121(3):295–302. doi:10.1289/ehp.1205875.
- Avivi I, Brenner B. Management of acute myeloid leukemia during pregnancy[J]. Future Oncol. 2014;10(8):1407–1415. doi:10.2217/fon.14.64.
- Sanz MA, Grimwade D, Tallman MS, Lowenberg B, Fenaux P, Estey EH, Naoe T, Lengfelder E, Buchner T, Dohner H, et al. Management of acute promyelocytic leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet[J]. Blood. 2009;113(9):1875–1891. doi:10.1182/blood-2008-04-150250.
- Milojkovic D, Apperley JF. How I treat leukemia during pregnancy[J]. Blood. 2014;123(7):974–984. doi:10.1182/blood-2013-08-283580.
- Terada Y, Shindo T, Endoh A, Watanabe M, Fukaya T, Yajima A. Fetal arrhythmia during treatment of pregnancy-associated acute promyelocytic leukemia with all-trans retinoic acid and favorable outcome[J]. Leukemia. 1997;11(3):454–455.
- Abedin S, Altman JK. Acute promyelocytic leukemia: preventing early complications and late toxicities[J]. Hematology Am Soc Hematol Educ Program. 2016;2016(1):10–15. doi:10.1182/asheducation-2016.1.10.
- Ali S, Jones GL, Culligan DJ, Marsden PJ, Russell N, Embleton ND, Craddock C, British Committee for Standards in Haematology. Guidelines for the diagnosis and management of acute myeloid leukaemia in pregnancy[J]. Br J Haematol. 2015;170(4):487–495. doi:10.1111/bjh.13554.
- Valappil S, Kurkar M, Howell R. Outcome of pregnancy in women treated with all-trans retinoic acid; a case report and review of literature[J]. Hematology. 2007;12(5):415–418. doi:10.1080/10245330701448594.
- Sanz MA, Montesinos P, Casale MF, Diaz-Mediavilla J, Jimenez S, Fernandez I, González-Campos, J., González, JD, Herrera, P, De Lisa, E. Maternal and fetal outcomes in pregnant women with acute promyelocytic leukemia[J]. Ann Hematol. 2015;94(8):1357–1361. doi:10.1007/s00277-015-2372-5.
- Milligan DW, Grimwade D, Cullis JO, Bond L, Swirsky D, Craddock C, Kell J, Homewood J, Campbell K, McGinley S. Guidelines on the management of acute myeloid leukaemia in adults[J]. Br J Haematol. 2006;135(4):450–474. doi:10.1111/j.1365-2141.2006.06314.x.
- Yang D, Hladnik L. Treatment of acute promyelocytic leukemia during pregnancy[J]. Pharmacotherapy. 2009;29(6):709–724. doi:10.1592/phco.29.6.709.
- Chen C Y, Tai C H, Cheng A, Wu H C, Tsay W, Liu J H, Chen PY, Huang SY, Yao M, Tang JL, Tien HF. Intracranial hemorrhage in adult patients with hematological malignancies[J]. BMC Med. 2012;10:97. doi:10.1186/1741-7015-10-97.
- Chen CY, Tai CH, Tsay W, Chen PY, Tien HF. Prediction of fatal intracranial hemorrhage in patients with acute myeloid leukemia[J]. Ann Oncol. 2009;20(6):1100–1104. doi:10.1093/annonc/mdn755.
- Takahashi JC, Iihara K, Ishii A, Watanabe E, Ikeda T, Miyamoto S. Pregnancy-associated intracranial hemorrhage: results of a survey of neurosurgical institutes across Japan[J]. J Stroke Cerebrovasc Dis. 2014;23(2):e65–e71. doi:10.1016/j.jstrokecerebrovasdis.2013.08.017.