ABSTRACT
Objective: Angiogenesis is one of the key processes in the development of malignant tumors. The vascular endothelial growth factor (VEGF) and VEGF receptor-2 (VEGFR-2) signaling pathway regulates branching angiogenesis in cancer. In this study, we analyzed the associations of VEGF/VEGFR-2 proteins and VEGFR-2 genetic variations with the prognosis of gastric cancer (GC).
Method: We collected the clinical information of patients with GC and extracted genomic DNA from paraffin-embedded tissues. Immunohistochemical methods were used to detect the expression of VEGF and VEGFR-2 in GC tissues. Four single nucleotide polymorphisms of VEGFR-2 were detected by the TaqMan assay. The Kaplan-Meier method and Cox regression model were applied to analyze the associations between clinicopathological characteristics, VEGFR-2 polymorphisms and GC prognosis.
Results: A total of 256 cases of GC were included in our study. VEGFR-2 (+) and VEGFR-2 (++/+++) protein expression levels were detected in 83 and 135 cases, respectively. High expression of the VEGFR-2 protein was associated with the poor prognosis of GC (log-rank test P = 0.026). No statistical significance was observed for the association between VEGF and the prognosis of GC. The VEGFR-2 rs1870377 A > T genetic polymorphism was discovered to be associated with the prognosis of GC (AA vs. AT, HR = 1.69, 95% CI = 1.06–2.68, P = 0.027).
Conclusion: Our study suggested that the high expression of VEGFR-2, as well as the VEGFR-2 rs1870377 A > T genetic polymorphism, may be prognostic markers for GC.
Introduction
Although the worldwide incidence of GC has declined steadily, the incidence of GC still ranks 2nd and the mortality rate of GC ranks 3rd among malignant tumors in China.Citation1 Due to the difficulty in making an early diagnosis and the atypical symptoms in the early stage, most patients are diagnosed with advanced GC, and the 5-year survival rate is only 20–30%.Citation2
With the development of molecular biology and clinical treatment with precision therapy, researchers have been exploring new prognostic markers of GC at the gene level. In malignant tumors, angiogenesis is the key to the survival of cancer cells and is closely related to the development of tumors. Rapidly growing tumor cells lack oxygen, so tumor cells secrete a variety of vascular-related growth factors to promote angiogenesis in order to maintain tumor growth.Citation3 In this process, vascular endothelial growth factor (VEGF) is considered the key driving factor. VEGF combines with its receptors, especially vascular endothelial growth factor receptor-2 (VEGFR-2), to activate its downstream signal transduction pathway and then promote angiogenesis in the malignant tumor.Citation4 VEGFR-2 is a tyrosine kinase receptor that can combine with VEGF via its extracellular domain. The dimerization and phosphorylation of VEGFR-2 lead to the activation of intracellular signaling pathways, including the mitogen-activated protein kinase (MAPK), phosphoinositide 3-kinase (PI3K) and focal adhesion kinase (FAK) pathways,Citation5 thus regulating the activity of vascular endothelial cells.
Researchers have reported that VEGFR-2 plays a key role in various malignant tumors, including breast cancer, colorectal cancer, and lung cancer.Citation6,Citation7 Previous studies indicated that the expression of VEGF/VEGFR-2 is associated with the prognosis of GC.Citation8 The expression of the VEGFR-2 protein is influenced by single nucleotide polymorphisms (SNPs) in the VEGFR-2 gene. Herein, we explored the association between VEGFR-2 and the prognosis of GC and investigated the possible mechanism underlying the association at the SNP level.
Materials and methods
Patients
A total of 271 patients who underwent gastrectomy at Xinhua Hospital, School of Medicine, Shanghai Jiao Tong University from July 2009 to July 2014 with pathologically confirmed primary GC were investigated. The exclusion criteria were as follows: (1) recurrent cases; (2) multiple foci; (3) treatment with chemotherapy or radiotherapy before gastrectomy; and (4) incomplete pathological data. The data were retrieved from medical charts and pathological records. The follow-up data were obtained from outpatient visits, phone calls and clinical databases. The median follow-up was 3.59 years. Finally, 256 GC patients were enrolled in the analysis. We excluded 15 patients who were lost to follow-up, and the dropout rate was 5.5%. We defined censoring as loss to follow-up or the nonoccurrence of the endpoint event in the summarized data. The survival time was defined as the time from the date of surgical operation to the date of the last contact or death from any cause. Written informed consent was obtained from all patients, and the study was approved by the Ethical Committee of Xinhua Hospital.
Immunohistochemical staining
Formalin-fixed, paraffin-embedded tissue specimens were cut into 7-μm-thick sections. The VEGF and VEGFR-2 proteins in the primary lesions were detected by immunohistochemistry. All primary antibodies were purchased from Abcam (Cambridge, UK). The mouse and rabbit monoclonal antibodies were purchased from Sener Biotechnology (Shanghai, China). The positive controls were obtained from known positive cases of GC, while the negative controls were subjected to the same procedure except that the primary antibody was replaced by PBS.
Two pathologists evaluated the slides independently. The immunoreactivity of VEGF was evaluated as follows: a product of the intensity and percentage of positive cells of > 6 was defined as positive. The scores for positive immunohistochemical staining were 0–20%, 1; 21%-40%, 2; 41–60%, 3; 61–80, 4; and 80–100%, 5. The color strength score was defined as follows: no coloration, 0; light brown (weak), 1; brown (medium), 2; and dark brown (strong), 3.Citation9 The immunoreactivity of VEGFR-2 was evaluated as follows: a product of intensity and the percentage of positive cells of ≤ 1 was defined as negative, 2–4 was defined as weakly positive, and ≥ 5 was defined as strongly positive. The scores for positive immunohistochemical staining were < 5%, 0; 6–25%, 1; 26–50%, 2; 51–75, 3; and 76–100%, 4. The color strength score was defined as follows: no coloration, 0; light brown (weak), 1; brown (medium), 2; dark brown (strong), 3.Citation10
DNA extraction and taqman SNP genotyping
Genomic DNA was extracted from the paraffin-embedded tissues using the TIANamp FFPE DNA kit (TIANGEN, Beijing, China). The rs1870377, rs2071559, rs2305948 and rs7667298 polymorphisms were genotyped by the TaqMan assay with an ABI7900HT Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). The genotypes were independently analyzed by two persons (Xueru Zhu and Meiling Zhu) using SDS Software 2.4 (Applied Biosystems, Foster City, CA, USA). For quality control, two random samples and two blank controls that used double distilled water instead of DNA were repeated, and the results were 100% concordant.
Statistics
The patients’ characteristics were analyzed using the chi-square test for categorical variables. The Kaplan-Meier method was used to calculate the overall survival (OS) and 3-year survival rates, and the log-rank test was used to compare the differences between the survival curves. Independent prognostic factors were analyzed by multivariate survival analysis using the Cox proportional hazards model. Factors for which P < 0.1 in univariate analysis were included in multivariate analysis. The level of significance was set at P < 0.05. Statistical analyses were conducted and graphics were generated using the SPSS 21.0 statistical package.
Results
Characteristics and clinical features of the study population
A total of 256 gastric cancer patients were included in the study; 165 were males and 91 were females. The median age was 63 years, and among them, 105 were over the age of 65 and 151 were under the age of 65. Of all patients, 31 patients smoked and 75 patients used alcohol. There were 75 patients with tumors larger than 4 cm in diameter and 181 with tumors smaller than or equal to 4 cm. According to the classification of differentiation, 88 cases were well or moderately differentiated, and 168 were poorly differentiated. Regarding the depth of tumor invasion, 123 patients were T1 and 133 were T2/T3/T4. Regarding the tumor location, the number of tumors in the cardia and in non-cardia sites was 18 and 238, respectively. There were 175 patients with stage I/II disease and 81 patients with stage III/IV disease; only 2 patients had stage IV disease. Of the patients, 39.45% (101/256) received adjuvant chemotherapy after surgery ().
Table 1. Clinicopathological characteristics of these GC patients.
Association between VEGFR-2 SNPs and the prognosis of GC patients
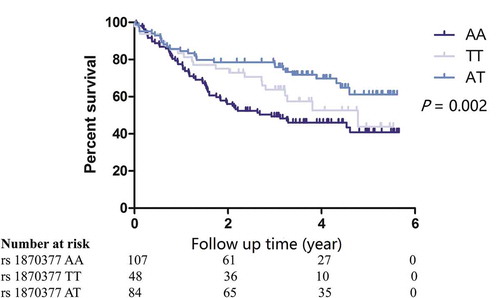
The Hardy-Weinberg equilibrium (HWE) of the four genotypes was analyzed (Supplement Table 1). The analysis of the associations between the genotypes and the prognosis of GC patients showed that the rs1870377 polymorphism was associated with the prognosis of GC patients. The median OS of patients with the AA, TT and AT genotypes was 2.93, 4.78 and 4.27 years, respectively (log-rank P = 0.002) (). Univariate analysis showed that the factors of tumor size, differentiation, depth, TNM stage and smoking were associated with the prognosis of GC (p < 0.05). No factors had a P value between 0.05 and 0.1. Further Cox regression showed that the rs1870377 genetic polymorphism was an independent prognostic factor in patients with resected GC (AA vs. AT, HR = 1.69, 95% CI = 1.06–2.68, P = 0.027), while no associations were significant for the other three SNPs ().
Table 2. Associations of genetic polymorphism of VEGFR-2 and survival of the GC patients.
Expression status of VEGF/VEGFR-2 in GC tissues and associations with VEGFR-2 SNPs
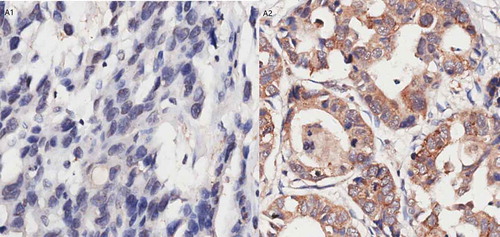
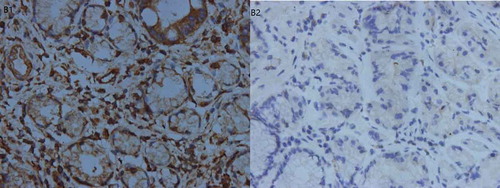
Of the 256 GC patients, 45 were VEGF (+) (17.58%) and 211 were VEGF (-) (82.42%), while 38 were VEGFR-2 (-) (14.85%), 83 were VEGFR-2 (+) (32.42%), and 135 were VEGFR-2 (++/+++) (52.73%). We did not observe a statistically significant difference between the different alleles of the four VEGFR-2 genotypes and VEGFR-2 protein expression (Supplement Table 2). The results of the immunohistochemical staining are shown in – and .
Associations between VEGF and VEGFR-2 protein expression and the clinical characteristics of GC patients
Only two patients were in stage IV, and we excluded their data and included 254 GC patients in the further survival analysis. The chi-square test showed that VEGF protein expression was high in males and patients with a tumor diameter of ≤ 4 cm; the P values were 0.014 and 0.001, respectively. However, there were associations between a tumor diameter greater than 4 cm, poor differentiation, deeper invasion (T2/T3/T4), stage III disease and the high expression of VEGFR-2; the P values were 0.001, 0.001, < 0.001 and 0.001, respectively ().
Table 3. Comparison of the clinicopathological characteristics of GC patients of VEGF/VEGFR-2 expression.
Association between VEGF, VEGFR-2 protein expression and the prognosis of GC patients
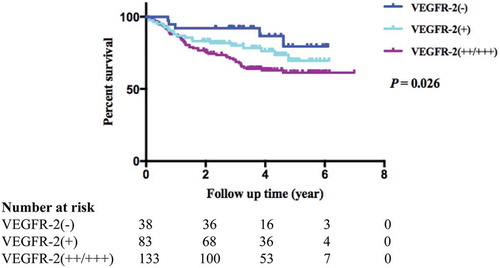
Among the 254 stage I-III GC patients, an analysis of the associations between VEGF and VEGFR-2 protein expression in GC tissue and the OS of GC patients showed that the OS of VEGFR-2 positive patients was poorer than that of patients whose VEGFR-2 expression was negative. The 3-year survival rate of VEGFR-2-negative patients was 92.1%, while the 3-year survival rates for weakly positive and strongly positive patients were 80.15 and 69.6%, respectively (log-rank P = 0.026) (). No statistical significance was observed between VEGF expression and the OS of GC patients ().
Table 4. Associations of VEGF/VEGFR-2 expression and survival of the GC patients.
Association between the clinical characteristics and the prognosis of GC patients
Analysis indicated that age, tumor diameter, differentiation, depth of invasion, LNM and TNM stage were related to the prognosis of GC. GC patients who were over 65 years of age or had a tumor diameter greater than 4 cm, poorly differentiated tumors, infiltration to additional mucosa, lymphatic metastasis or stage III disease had a poor prognosis; the P values were 0.021, < 0.001, 0.021, < 0.001, < 0.001 and < 0.001, respectively. The Cox regression analysis showed that the depth of invasion was the independent prognostic factor for GC patients.
Discussion
In this study, we analyzed the associations of VEGF and VEGFR-2 protein expression and VEGFR-2 SNPs with the prognosis of patients with resected GC. Because only two patients were stage IV, we excluded their data to better guide clinical practice. We found that the VEGFR-2 rs1870877 A > T genetic polymorphism was associated with OS in GC, and patients with the AA genotype had a poorer prognosis than those with the AT genotype. We discovered that a higher expression of VEGFR-2 conferred a poorer prognosis for GC patients. In addition, we found that the invasion depth was an independent prognostic factor for GC patients.
The overall prognosis of GC patients is poor, and the 5-year survival rate is limited. The main causes of failure in the treatment of GC are tumor invasion and metastasis, which are closely related to angiogenesis. Angiogenesis can provide nutrition and metabolites for tumor cells. In addition, it can provide advantageous conditions for hematogenous and distant metastasis.Citation11 The VEGF family includes VEGF-A, VEGF-B, VEGF-C, VEGF-D and placental growth factor (PIGF), which mainly regulate neovascularization and increase vascular permeability and lymph-angiogenesis. In terms of these family members, the VEGF/VEGFR-2 signal pathway is the key to mediating tumor angiogenesis. Currently, VEGF is generally regarded as VEGFA, which is an endothelial cell-specific mitogen that stimulates angiogenesis under physiological and pathological conditions.Citation12 In GC, the VEGF signal is regulated by tyrosine kinase, and VEGFR-2 is the main tyrosine kinase receptor.Citation6 The combination of VEGF and VEGFR-2 leads to the activation of VEGFR-2, thereby stimulating the proliferation of vascular endothelial cells and chemotaxis.Citation13 A previous study showed that inhibiting the binding of VEGF to VEGFR-2 will block neovascularization and inhibit tumor growth, invasion and distant metastasis.Citation14 The micro-vessel density is regarded as the indicator of the degree of tumor angiogenesis, and a previous study found that the micro-vessel density in VEGFR-2-positive GC tissue was higher than that in VEGFR-2-negative tissue, which indicated that VEGFR-2 is associated with angiogenesis.Citation15 Due to the key role of the VEGF/VEGFR-2 signaling pathway in angiogenesis, the expression of these proteins is closely related to the prognosis of GC. Yan et al Citation7 reported that high expression of VEGFR-2 was associated with poor differentiation and lymphatic metastasis in breast cancer, and patients with high expression of VEGFR-2 had a poorer prognosis than VEGFR-2-negative patients. Jurgensmeier et. al arrived at the same conclusion in colorectal cancer.Citation16 In the GC field, a study showed that high expression of VEGFR-2 was related to TNM stage, tumor recurrence and metastasis.Citation17 In our study, we observed the same results for the association between VEGFR-2 expression and GC prognosis but no association for VEGF expression. The reasons for this difference may be as follows: one study indicated that the effect of VEGF on the metastasis and prognosis of GC patients was weaker than that of VEGFR-2; in other words, the expression of VEGF in GC tissue may not necessarily be a risk factor for the prognosis of GC.Citation18 On the other hand, because the sample size of our study was small, we do not have enough statistical power to identify a modest effect.
he role of genetic polymorphisms in tumor development and prognosis has received much attention, and relevant studies are extensive. Scartozzi et. al observed that the VEGF rs699947 SNP was associated with the recurrence of GC and that the recurrence rate for the AC genotype was higher than that for the AA and CC genotypes, but no statistical significance was found for VEGFR-2.Citation19 A study on VEGFR-2 SNPs and micro-vessel density in colorectal cancer suggested that the VEGFR-2 polymorphism was associated with increased micro-vessel density.Citation15 Maeng et. al Citation20 found that the OS of GC patients with the VEGFR-2 rs1870377 (AA) genetic mutation was poorer than that of those in the control group. In addition, a study on the functional region genetic polymorphism of VEGF rs699947 showed that this polymorphism was associated with the prognosis of breast cancer and colorectal cancer.Citation19,Citation21 So far, only a few studies have addressed VEGFR-2 genetic polymorphisms and GC prognosis. Our analysis indicated that the VEGFR-2 rs1870877 A > T polymorphism was associated with the GC prognosis. The rs1870377 mutation is located in the coding region and is a missense mutation. This change leads to p. Gln472His, NG_012004.1: g.23789A> T, NM_002253.2: c.1416A> T, NP_002244.1: p. Gln472His.Citation22 In our analysis, the OS of patients with the AA genotype was poorer than that of patients with the TT or AT genotype. This observation may provide an important reference value for clinical practice. However, we observed that only the rs7667298 polymorphism was in HWE. We found no quality problems with the primers or paraffin-embedded samples. This disequilibrium may arise from the small sample size. To assess the clinical significance of the genetic polymorphisms, further studies with a large sample size and rigorous research are needed.
In conclusion, the results of our study are consistent with those of previous studies showing that the high expression of VEGFR-2 as well as the VEGFR-2 rs1870377 A > T genetic polymorphism may be prognostic factors for patients with resected GC. This finding has important significance for clinical practice in that it provides evidence for the diagnosis and prognosis of GC patients. However, considering that some limitations exist in our study, further studies with large samples are needed to confirm this conclusion.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Ethical Standards
This article does not contain any studies with human or animal subjects performed by any of the authors.
Supplemental Material
Download Zip (30.2 KB)Supplemental material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi:10.3322/caac.21262.
- Folli S, Dente M, Dell’Amore D, Gaudio M, Nanni O, Saragoni L, Vio A. Early gastric cancer: prognostic factors in 223 patients. Br J Surg. 1995;82(7):952–956.
- Baeriswyl V, Christofori G. The angiogenic switch in carcinogenesis. Semin Cancer Biol. 2009;19(5):329–337. doi:10.1016/j.semcancer.2009.05.003.
- Kappas NC, Zeng G, Chappell JC, Kearney JB, Hazarika S, Kallianos KG, Patterson C, Annex BH, Bautch VL. The VEGF receptor Flt-1 spatially modulates Flk-1 signaling and blood vessel branching. J Cell Biol. 2008;181(5):847–858. doi:10.1083/jcb.200709114.
- Shibuya M, Claesson-Welsh L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res. 2006;312(5):549–560. doi:10.1016/j.yexcr.2005.11.012.
- Goel HL, Mercurio AM. VEGF targets the tumour cell. Nat Rev Cancer. 2013;13(12):871–882. doi:10.1038/nrc3627.
- Yan JD, Liu Y, Zhang ZY, Liu GY, Xu JH, Liu LY, Hu YM. Expression and prognostic significance of VEGFR-2 in breast cancer. Pathol Res Pract. 2015;211(7):539–543. doi:10.1016/j.prp.2015.04.003.
- Hirashima Y, Yamada Y, Matsubara J, Takahari D, Okita N, Takashima A, Kato K, Hamaguchi T, Shirao K, Shimada Y, et al. Impact of vascular endothelial growth factor receptor 1, 2, and 3 expression on the outcome of patients with gastric cancer. Cancer Sci. 2009;100(2):310–315. doi:10.1111/j.1349-7006.2008.01020.x.
- Kowanetz M, Ferrara N. Vascular endothelial growth factor signaling pathways: therapeutic perspective. Clinl Cancer Res: off J Am Assoc Cancer Res. 2006;12(17):5018–5022. doi:10.1158/1078-0432.CCR-06-1520.
- Hsu J-T, Chen T-D, Chuang H-C, Huang S-C, Le P-H, Chen T-H, Lin C-J, Yeh T-S. Vascular endothelial growth factor expression is an independent poor prognostic factor for human epidermal growth factor receptor 2 positive gastric cancer. J Surg Res. 2017;208:40–50. doi:10.1016/j.jss.2016.09.004.
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285(21):1182–1186. doi:10.1056/NEJM197111182852108.
- Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science (New York, NY). 1989;246(4935):1306–1309. doi:10.1126/science.2479986.
- Lohela M, Bry M, Tammela T, Alitalo K. VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Curr Opin Cell Biol. 2009;21(2):154–165. doi:10.1016/j.ceb.2008.12.012.
- Su JL, Yang PC, Shih JY, Yang CY, Wei LH, Hsieh CY, Chou CH, Jeng YM, Wang MY, Chang KJ, et al. The VEGF-C/Flt-4 axis promotes invasion and metastasis of cancer cells. Cancer Cell. 2006;9(3):209–223. doi:10.1016/j.ccr.2006.02.018.
- Hansen TF, Sorensen FB, Spindler KL, Olsen DA, Andersen RF, Lindebjerg J, Brandslund I, Jakobsen A. Microvessel density and the association with single nucleotide polymorphisms of the vascular endothelial growth factor receptor 2 in patients with colorectal cancer. Virchows Archiv: int j pathol. 2010;456(3):251–260. doi:10.1007/s00428-009-0878-8.
- Jurgensmeier JM, Schmoll HJ, Robertson JD, Brooks L, Taboada M, Morgan SR, Wilson D, Hoff PM. Prognostic and predictive value of VEGF, sVEGFR-2 and CEA in mCRC studies comparing cediranib, bevacizumab and chemotherapy. Br J Cancer. 2013;108(6):1316–1323. doi:10.1038/bjc.2013.79.
- Liu X, Li Y, Wei J, Zhao Y. Role of Ang-2, Tie-2 and VEGFR-2 in angiogenesis in colorectal carcinoma and their prognostic value. Nan Fang Yi Ke Da Xue Xue Bao = Journal of Southern Medical University. 2012;32(11):1658–1662.
- Chatterjee S, Heukamp LC, Siobal M, Schottle J, Wieczorek C, Peifer M, Frasca D, Koker M, Konig K, Meder L, et al. Tumor VEGF:VEGFR2 autocrine feed-forward loop triggers angiogenesis in lung cancer. J Clin Invest. 2013;123(4):1732–1740. doi:10.1172/JCI65385.
- Scartozzi M, Loretelli C, Galizia E, Mandolesi A, Pistelli M, Bittoni A, Giampieri R, Faloppi L, Bianconi M, Del Prete M, et al. Role of vascular endothelial growth factor (VEGF) and VEGF-R genotyping in guiding the metastatic process in pT4a resected gastric cancer patients. PloS one. 2012;7(7):e38192. doi:10.1371/journal.pone.0038192.
- Maeng CH, Yi JH, Lee J, Hong JY, Choi MK, Jung HA, Park JO, Park SH, Park YS, Kang WK, et al. Effects of single nucleotide polymorphisms on treatment outcomes and toxicity in patients treated with sunitinib. Anticancer Res. 2013;33(10):4619–4626.
- Kim JG, Sohn SK, Chae YS, Cho YY, Bae HI, Yan G, Park JY, Lee MH, Chung HY, Yu W. Vascular endothelial growth factor gene polymorphisms associated with prognosis for patients with gastric cancer. Ann Oncology: Official Journal Eur Soc Med Oncol. 2007;18(6):1030–1036. doi:10.1093/annonc/mdm085.
- Allele: NM_002253.2(KDR): c.146 A > T (p.Gln472His). allele ID: 138342, variant type: single nucleotide variant. https://www.ncbi.nlm.nih.gov/clinvar/variation/134603/.