ABSTRACT
The PD-1/PD-L1 axis is characterized as an important checkpoint of immune activation, particularly through negatively regulating T cell function. Although accumulated evidence has demonstrated the patients with some types of tumor benefit from blockade of PD-1/PD-L1 signaling pathway, the possible synergistic effect of combination of radiotherapy and anti-PD-1/PD-L1 antibody still need to be explored. Here, we report a case of patient who was diagnosed with metastatic mediastinal leiomyosarcoma and treated by combination of local radiotherapy and anti-PD-1 antibody nivolumab. Remarkable tumor regression was observed at both the irradiated focus and distant metastatic sites 2 months later after combined treatment. The patient has tumor mutational burden (8.7 somatic mutations/Mb) and positive PD-L1 expression. The increased circulating lymphocytes suggest induced activation of immune response after combination treatment. Free disease progression has reached one and a half years as far.
Background
Sarcoma is a heterogeneous group of malignancies with more than 50 distinct histological subtypes. It approximately accounts for less than 1% of all new malignancies in adults and STS comprises 7% of all children cancers.Citation1,Citation2 The average overall survival was just under 15 months in metastatic or recurrent locally advanced sarcomas.Citation3,Citation4 Currently, clinical treatment modalities to improve outcomes of sarcoma are limited by its rarity and diversity.
Immune checkpoint inhibitors targeting programmed death-1 receptor and its ligand PD-L1 have been demonstrated to contribute to dramatic improvements of clinical benefits and represent a new promising therapeutic approach in advanced tumors.Citation5,Citation6 Heine et al reported a successful treatment of refractory leiomyosarcoma with anti-PD-1 inhibitor nivolumab.Citation7 However, results of a phase II study showed that single agent of anti-PD-1 antibody nivolumab did not demonstrate benefit in a cohort of advanced patients with uterine leiomyosarcoma.Citation8 Another randomized phase II study (NCT02500797) indicated compared with patients in the anti-PD1 or anti-CTLA4 alone group, those who received combined immunotheraputic treatment achieved higher confirmed response rate and prolonged survival in patients with advanced or metastatic sarcoma.Citation9 Anti-tumor response with immune checkpoint blockade may be enhanced with combinatorial strategies. Moreover, the results of combination of RT and immunotherapy in patients with melanoma and non-small cell lung cancer (NSCLC) are especially encouragingCitation10-Citation12 However, little is known regarding the effects of anti PD-1 antibody and RT in sarcomas. Here, we firstly reported a case of tumor regression in a patient with metastatic mediastinal leiomyosarcoma treated with local radiation and nivolumab.
Case report
In February 2011, a 31-year-old female patient presented with a mediastinal nodule, for which the initial positron emission tomography/computed tomography (PET/CT) revealed increased metabolic activity (size: 2.9*2.9 cm, standard uptake value: 34.2). The patient received a wide local excision of her primary lesion following biopsy of mediastinal leiomyosarcoma in March 2011. There was no residual lesion at the primary site, and lymph nodes were not involved.
The patient remained disease-free until August 2014 when she had asthma and shortness of breath and the routine chest radiography revealed a new mediastinal mass. She then received adjuvant radiotherapy (RT) for recurrent mediastinal nodules, at the doses of 60 Gy in 30 fractions and concurrent chemotherapy with paclitaxel 45 mg/m2 body surface area (BSA) and cisplatin 25 mg/m2 BSA every week for 4 cycles. Treatments almost completely relieved moderate to severe wheeze and short breath during the recurrence period. Compared with the CT before concurrent chemoradiotherapy, subsequent chest CTs in November 2014 and January 2015 demonstrated decreased size of the irradiated nodules.
In June 2015, brain magnetic resonance imaging (MRI) indicated disease progression with a nodule in her left frontal lobe. Further ultrasound examination in July 2015 revealed mixed echogenic masses with an approximate size of 34 × 17 mm2 on her right shoulder and 25 × 62 mm2 in her waist which exhibited distinct border. One month later, the patient received stereotactic RT for her brain metastasis with total doses of 60 Gy in three fractions. Then she underwent local excision of metastases on the shoulder and waist, which was confirmed as metastatic undifferentiated leiomyosarcoma according to immunohistochemistry and SS-18 gene detection by a pathologist. Thereafter, the patient received postoperative adjuvant RT for metastatic nodules on her back at total doses of 40 Gy delivered over 10 fractions, completing in October 2015.
A follow-up CT scan revealed multiple enlarged nodules in the lungs in January 2016, demonstrating disease progression.
She then received 2 cycle of chemotherapy of doxorubicin and cisplatin. CT scan indicated progressive enlargement of the masses in the lungs and growth of new lesions in her left adrenal gland, right kidney and liver. The size of the intracranial metastases had not significantly changed under brain MRI imaging.
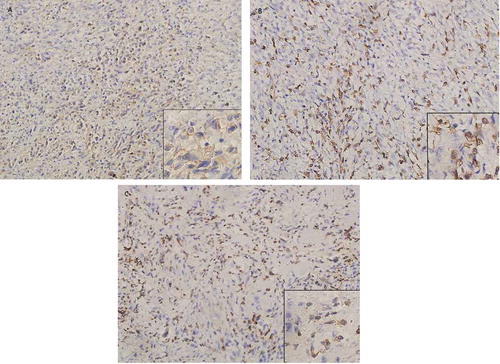
In April 2016, analysis comparing the whole exome sequencing (WES) of resected lumbar metastases and normal paired samples revealed mutational landscape (8.7 somatic mutations/Mb) and copy number variations in genome. Immunohistochemical staining showed weak positive expression of PD-L1 (20% tumor cells) () and high densities of CD3 and CD8 tumor-infiltrating lymphocytes ( and ). We discussed the results of prospective examinations extensively and supposed anti-PD-1/PD-L1 inhibitor might synergized the local and system therapeutic outcome by local radiotherapy. The patient was informed of available evidence in advanced melanoma and NSCLC. She then consented and signed the informed consent and started receiving nivolumab treatment and local RT to one of the lung metastases.
Figure 1. Sample immunohistochemical images.
Immunohistochemical staining of PD-L1, CD3 and CD8 on paraffin-embedded slides of resected lumbar metastases tissue. (a) Immunohistochemical staining for PD-L1 in tumor specimens (x200), (b) Immunohistochemical staining for CD8+ TILs (x200), (c) Immunohistochemical staining for CD3+ TILs (x200).
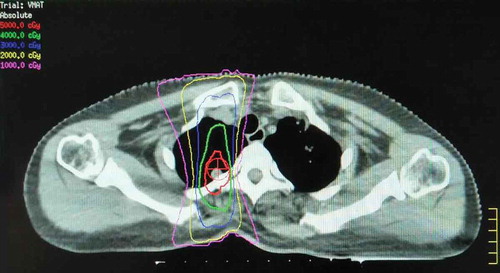
The patient received enhanced chest CT for RT treatment planning in June 2016. An isolated pulmonary nodule in the right paraspinal lesion was selected as the RT target. RT at a total dose of 50 Gy was performed in five fractions over 5 days with 6 MV photons by means of a coplanar two-field volumetric modulated arc therapy (VMAT) (). The patient was intravenously administrated with nivolumab at 2 mg per kilogram of body weight every 3 weeks from the third day after first radiation. She completed 23 doses of nivolumab and tolerated this combined treatment without any treatment-related adverse events. Maintenance of administration of nivolumab continued.
Figure 2. Registration of CT/simulation to target paraspinal lesion.
It shows the CT simulation image for radiotherapy planning. The right paraspinal lesion was selected as the irradiated target. The treatment plan was designed with 6 MV photons by means of a coplanar two-field VMAT. The isodose lines represent total doses of 50 Gy (red), 40 Gy (green), 30 Gy (blue), and 10 Gy (pink).
Results
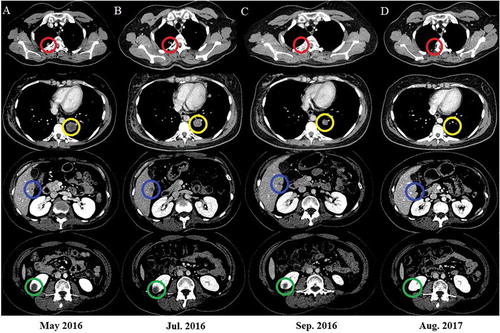
In July 2016, after one month of radiotherapy and nivolumab treatment, CT scan revealed irradiation-targeted nodule shrinkage in the right lung. Moreover, striking regressions were observed in distant metastasis (left lower lobe and left adrenal gland). However, other pulmonary metastasis and lesions in the mediastinum, right kidney, liver and left frontal lobe of brain did not response to the treatment (). Then the patient received two additional doses of nivolumab. In September 2016, remarkable clinical responses to concurrent treatment was found not only at the irradiated focus but also the distant metastatic sites outside the radiation field (pulmonary, brain, adrenal gland, renal and hepatic metastasis) (). The subsequent CT scan obtained in August 2017 (14 months after RT) showed further evidence of clinical response, with continuous presence of minimal disease ().
Figure 3. Nivolumab and radiation result in tumor regression.
Axial CT images are shown corresponding to the treatment timeline. Panel A represents the status before treatment with nivolumab and radiation. Panel B shows images 1 month later after radiotherapy. The CT scan revealed tumor shrinkage in irradiated (red circle) and pulmonary metastasis (yellow circle) and stable lesions in the liver (blue circle) and kidney (green circle). Panel C shows tumor regression in the irradiated focus and distant disease outside the radiation field. The response was durable, as shown in Panel D.
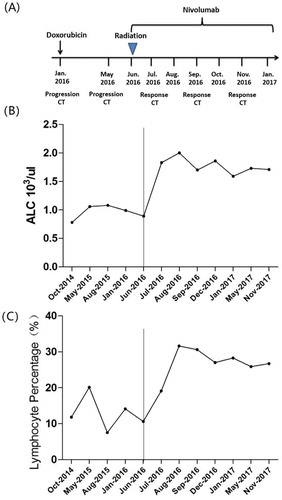
After combined treatment of RT and nivolumab, increased absolute lymphocyte counts (ALCs) in peripheral blood were found in the patient. The ALC at the bottom of 780/μl on June 2, 2016, demonstrated a dramatic rise to 2,000/μl on August 7, 2016, and thereafter was maintained at high levels. Additionally, a changing trend of lymphocyte percentage (LYM%, 7.5% of whole blood at baseline, 31.6% of whole blood at peak levels) is consistent with the change of ALC ().
Figure 4. The circulating lymphocyte counts during treatment.
A detailed clinical timeline is displayed (A). The results of ALC and LYM% in peripheral blood are displayed in accordance with the aforementioned treatment timeline. A dramatic increase in ALC and LYM% was observed after treatment with RT and nivolumab (B and C). The ALC at the bottom of 780/μl on June 2, 2016, demonstrated a dramatic rise to 2,000/μl on August 7, 2016, and thereafter was maintained at high levels (B). After combined therapy, the LYM% increased gradually, up to 31.6% and maintained at a high level throughout treatment (C).
Discussion
The association of PD-L1 expression with overall survival and clinical response treated by anti-PD-1/PD-L1 inhibitors is always inconsistent.Citation13,Citation14 In the KEYNOTE-024 phase III trial (NCT02142738), patients with advanced NSCLC and PD-L1 expression on at least 50 % of cells in tumor nests were randomly divided to receive either anti-PD-1 antibody pembrolizumab or the platinum-based chemotherapy. A promising objective respond rate (ORR) and progression free survival (PFS) were observed in the pembrolizumab group.Citation15 In contrast, Checkmate-026 reported that in the PD-L1 ≥ 5% NSCLC patients, nivolumab failed to show any benefit compared with standard chemotherapy.Citation16 These result indicated that additional factors should be considered except for PD-L1 expression when predicting the treatment efficacy of PD-1/PD-L1 checkpoint inhibitors. Moreover, the exploratory analysis of CheckMate 026 suggested that anti-PD1 antibody improved ORR and PFS when compared with standard chemotherapy in patients with high TMB. Though the optimal threshold of high TMB has not been fully established, prospective evaluation of TMB as a potential biomarker for predicting response to immunotherapy is already in progress. Immunohistochemical staining showed positive expression of PD-L1 (20% tumor cells). Moreover, WES revealed TMB (8.7 somatic mutations/Mb) and non-synonymous mutations in coding DNA. In addition, our patient had a history of RT for metastases to the chest, brain, and back. A secondary analysis of the KEYNOTE-001 trial suggests that previous treatment with RT in patients with NSCLC results in longer survival with pembrolizumab treatment than those who did not have previous RT.Citation14 Consistent with previous studies, these may be associated with the durable clinical benefit in the present case.
In recent years, combination treatment did show significantly enhanced anti-tumor response.Citation17–Citation21 In 2012, Postow MA et al. reported a patient with melanoma had a systemic response to localized RT after having had disease progression while receiving ipilimumab.Citation22 It indicated a synergistic anti-tumor effect of irradiation and immunotherapy. However, the patient in our case received concurrent anti-PD-1 therapy and local radiation after progression of multiple lesions. Therefore, tumor regression after combined treatment may be explained either by a synergistic interaction with radiation or purely by immunotherapy drugs nivolumab. Future rigorous and randomized clinical trials are needed to optimize treatment options and address unsolved puzzles in combination strategies.
In conclusion, we firstly report the delayed but burst tumor regression 2 months later after the start of nivolumab given as a concurrent regimen with fractionated RT in a patient with metastatic leiomyosarcoma. Immunologic treatment strategies in sarcoma hold substantial potential and multiple combinations may provide a novel and effective therapeutic pattern in advanced malignant tumors.
The patient in this article has given her written informed consent to publication.
Abbreviations
| PD-1 | = | programmed death-1 |
| PD-L1 | = | programmed death ligand-1 |
| NSCLC | = | non-small cell lung cancer |
| CT | = | computed tomography |
| WES | = | whole exome sequencing; |
| ALC | = | absolute lymphocyte count |
| LYM% | = | lymphocyte percentage |
| ICD | = | induce immunogenic cell death |
| TMB | = | tumor mutational burden. |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Ethics statment
All work was approved by the Medical Ethics Committee of Tianjin Medical University Cancer Institute and Hospital and complied strictly with national ethical guidelines.
Supplemental Material
Download MS Word (16.9 KB)References
- Burningham Z, Hashibe M, Spector L, Schiffman JD. The epidemiology of sarcoma. Clin Sarcoma Res. 2012;2(1):14. doi:10.1186/2045-3329-2-14.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi:10.3322/caac.21332.
- Italiano A, Mathoulin-Pelissier S, Cesne AL, Terrier P, Bonvalot S, Collin F, Michels -J-J, Blay J-Y, Coindre J-M, Bui B. Trends in survival for patients with metastatic soft-tissue sarcoma. Cancer. 2011;117(5):1049–1054. doi:10.1002/cncr.25538.
- Karavasilis V, Seddon BM, Ashley S, Al-Muderis O, Fisher C, Judson I. Significant clinical benefit of first-line palliative chemotherapy in advanced soft-tissue sarcoma: retrospective analysis and identification of prognostic factors in 488 patients. Cancer. 2008;112(7):1585–1591. doi:10.1002/cncr.23332.
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. doi:10.1056/NEJMoa1504030.
- Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi:10.1056/NEJMoa1507643.
- Heine A, Kristiansen G, Schild HH, Brossart P. Successful treatment of refractory leiomyosarcoma with the PD-1 inhibitor nivolumab. Ann Oncol. 2016;27(9):1813–1814. doi:10.1093/annonc/mdw243.
- Ben-Ami E, Barysauskas CM, Solomon S, Tahlil K, Malley R, Hohos M, Polson K, Loucks M, Severgnini M, Patel T, et al. Immunotherapy with single agent nivolumab for advanced leiomyosarcoma of the uterus: results of a phase 2 study. Cancer. 2017;123(17):3285–3290. doi:10.1002/cncr.30738.
- D’Angelo SP, Mahoney MR, Van Tine BA, Atkins J, Milhem MM, Jahagirdar BN, Antonescu CR, Horvath E, Tap WD, Schwartz GK, et al. Nivolumab with or without ipilimumab treatment for metastatic sarcoma (Alliance A091401): two open-label, non-comparative, randomised, phase 2 trials. Lancet Oncol. 2018;19(3):416–426. doi:10.1016/S1470-2045(18)30006-8.
- Stamell EF, Wolchok JD, Gnjatic S, Lee NY, Brownell I. The abscopal effect associated with a systemic anti-melanoma immune response. Int J Radiat Oncol Biol Phys. 2013;85(2):293–295. doi:10.1016/j.ijrobp.2012.03.017.
- Golden EB, Demaria S, Schiff PB, Chachoua A, Formenti SC. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol Res. 2013;1(6):365–372. doi:10.1158/2326-6066.CIR-13-0115.
- Ko EC, Raben D, Formenti SC. The integration of radiotherapy with immunotherapy for the treatment of non-small cell lung cancer. Clin Cancer Res. 2018; doi: 10.1158/1078-0432.CCR-17-3620.
- Kim C, Kim EK, Jung H, Chon HJ, Han JW, Shin K-H, Hu H, Kim KS, Choi YD, Kim S, et al. Prognostic implications of PD-L1 expression in patients with soft tissue sarcoma. BMC Cancer. 2016;16:434. doi:10.1186/s12885-016-2451-6.
- Shaverdian N, Lisberg AE, Bornazyan K, Veruttipong D, Goldman JW, Formenti SC, Garon EB, Lee P. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. 2017;18(7):895–903. doi:10.1016/S1470-2045(17)30380-7.
- Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, et al. Pembrolizumab versus chemotherapy for PD-L1-Positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. doi:10.1056/NEJMoa1606774.
- Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M, Felip E, van Den Heuvel MM, Ciuleanu T-E, Badin F, et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med. 2017;376(25):2415–2426. doi:10.1056/NEJMoa1613493.
- Demaria S, Kawashima N, Yang AM, Devitt ML, Babb JS, Allison JP, Formenti SC. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005;11(2 Pt 1):728–734.
- McDermott D, Lebbé C, Hodi FS, Maio M, Weber JS, Wolchok JD, Thompson JA, Balch CM. Durable benefit and the potential for long-term survival with immunotherapy in advanced melanoma. Cancer Treat Rev. 2014;40(9):1056–1064. doi:10.1016/j.ctrv.2014.06.012.
- Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, Fu Y-X. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124(2):687–695. doi:10.1172/JCI67313.
- Sharabi AB, Lim M, DeWeese TL, Drake CG. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol. 2015;16(13):e498–509. doi:10.1016/S1470-2045(15)00007-8.
- Vanpouille-Box C, Pilones KA, Wennerberg E, Formenti SC, Demaria S. In situ vaccination by radiotherapy to improve responses to anti-CTLA-4 treatment. Vaccine. 2015;33(51):7415–7422. doi:10.1016/j.vaccine.2015.05.105.
- Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, Mu Z, Rasalan T, Adamow M, Ritter E, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366(10):925–931. doi:10.1056/NEJMoa1112824.
