ABSTRACT
The aim of this study was to investigate the mechanism by which KLF6-SV1 promoted lung cancer metastasis through tumor-associated macrophages (TAMs). Plasmid transfection was used to construct cells that upregulated or silenced gene. Tumor-bearing mouse model was established using A549 cells. SP staining was performed to detect the CD163 and CD68. Six-well plates and Transwell chamber were used for co-culture of lung cancer A549 cells and macrophages. CCK-8 and Transwell assay were applied to detected the cell viability and migration respectively. Protein and mRNA were tested by Western blot and quantitative real-time polymerase chain reaction (qRT-PCR).KLF6-SV1 overexpression promoted the expression levels of TWIST1 and CCL2, and also induce macrophage polarization to M2 and epithelial-mesenchymal transition (EMT). In vitro experiments showed that KLF6-SV1 might regulate the migration of lung cancer cells by regulating the expression of TWIST1 and CCL-2. M2 macrophages did not affect the expression of KLF6-SV1, TWIST1 and CCL-2. The co-culture system could up-regulate the EMT of A549 cells.Overexpression of KLF6-SV1 promoted the expression of TWIST1 and CCL2, and up-regulation of TWIST1 expression might promote the infiltration of M2 macrophages, which promoted the involvement of EMT in the metastasis of lung cancer cells.
Introduction
Lung cancer is one of the world’s highest mortality malignant tumors, of which non-small cell lung cancer (NSCLC) is the most common, accounting for about 80% of the total number of lung cancer.Citation1 The 5-year predicted survival rate of NSCLC is only 15.9%.Citation2 Although many large clinical trials in the world continue to try new comprehensive treatments, the overall treatment of NSCLC is still not satisfactory, and the survival of patients has not been effectively extended, so new therapeutic targets are urgently needed.Citation3-Citation5
In recent years, studies have found that metastasis could occur in the early stage of malignant tumors, and tumor metastasis is regulated by various factors.Citation6,Citation7 The tumor microenvironment is a research hotspot in recent years. The tumor microenvironment is a complex complex of cells and structural components, including tumor cells, macrophages, fibroblasts, vascular endothelial cells, and so on, the tumor microenvironment can support the growth and metastasis of cancer.Citation8-Citation10 Macrophage is one of the resident cells of the lung, and among the various cells recruited, macrophages exist at various stages of tumor progression and have a huge number.Citation11 In many clinical and basic researches in recent years, it has been found that macrophages play a role in promoting tumor growth and metastasis which are called tumor associated macrophages (TAMs).Citation12,Citation13
Macrophages can be activated into different forms under different circumstances. Generally, macrophages can be induced by chemokines or cytokines to be polarized to be class-activated (M1) which promote pro-inflammatory or alternatively activated (M2) which support anti-inflammatory.Citation14,Citation15 Polarization of macrophages to M1 can help the virus to clear and participate in the inflammatory response.Citation16 Polarization of macrophages to M2 can promote tumor development and inhibit inflammation, participate in tumor immune escape and promoting epithelial-mesenchymal transition (EMT).Citation17,Citation18 EMT is regulated by a variety of genes and pathways, TWIST1 has been involved in the metastasis and development of tumors by many studies, but its mechanism for promoting the metastasis in lung cancer is still unclear.Citation19-Citation21
Krüppel like factor 6 (KLF6) is a member of the Krüppel-likefactors (KLF) family and plays an important role intissue development. As a tumor suppressor gene, KLF6 is inactivated or reduced in expression in many cancers.Citation22,Citation23 Krüppel like factor 6 splice variant 1 (KLF6-SV1) is a splice variant of KLF6. In contrast to KLF6, the increased expression of KLF6-SV1 is associated with tumorigenesis and metastasis, and KLF6-SV1 is associated with poor prognosis in breast cancer.Citation24,Citation25 However, there is still a lack of research on the mechanism of KLF6-SV1 leading to lung cancer metastasis.
Therefore, this study mainly exploresd the mechanism by which KLF6-SV1 gene promoted EMT in lung cancer through macrophages, analyzed the role of macrophages in lung cancer metastasis, and provided new ideas for the treatment of lung cancer.
Materials and methods
Cells culture and transfection
The human lung cancer cell line A549 and human monocyte THP-1were purchased from ATCC (USA). Cells were cultured in RPMI 1640 medium containing 10% FBS at 37 °C in 5% CO2 incubator. Culture related reagents were purchased from GIBCO invitrogen (USA).
The KLF6-SV1 coding sequences were subcloned into pcDNA3.1 (Sangon Biotech, China) to construct pcDNA expression vectors. KLF6-SV1 transfections were performed using Lipofectamine 2000 (Invitrogen,Carlsbad,CA) according to the manufacturer’s instruction. siKLF6-SV1 and siTWIST1 was purchased from GenePharma (China) and the siRNA transfections were performed by Lipofectamine TM 2000. The empty plasmid was used as control. The cells were harvested at 48 h after transfection for subsequent experiments.
Modeling and sample collection
BALB/c mice (6–8 weeks, 22 g-26 g, SFP) were purchased from Laboratory Animal Cecter (China). Modeling and follow-up experimental programs had been approved by the Institutional Animal Care and Use Committee and China Council on Animal Care. 16 mice were randomly divided into 4 groups: control group (n = 4), NC group (n = 4), KLF6-SV1 group (n = 4) and siKLF6-SV1 group (n = 4). The corresponding A549 cells (10 × 6 each mouse) were injected subcutaneously into the back of the mouse, and the tumors were surgically removed and weighed when the average tumor volume reached about 1.5 cm3. After weighing, 1 sample was fixed in 10% neutral formaldehyde for streptavidin-perosidase (SP) staining, and the other four were stored under −80 °C for Western blot and quantitative real-time polymerase chain reaction (qRT-PCR) detection .
Co-culture system
Six-well plates and Transwell chamber were used for co-culture of lung cancer A549 cells/macrophages. A549 cells were seeded in six-well plates (5 × 105/well) and RPMI 1640 medium containing 10% FBS was added. THP-1 cells were seeded in Transwell (1.5 × 106/chamber) and induced to become M0 macrophages after 24 h induction using phorbol-12-myristate-13-acetate (PMA, 100 ng/mL), and then the cells were treated with macrophage colony-stimulating factor (M-CSF, 10 ng/mL) to induce to become M2 macrophages. The Transwell chamber was placed into six-well plate to establish the co-culture system of A549 cells/macrophages. Various cytokines and chemokines passed freely and cells failed to pass. The co-cultivation time was 24 h. The co-culture system without THP-1 cells were used as control.
SP staining
SP staining was performed to detect the CD163. Specimens were cut in 4-μm-thick section and deparaffinezed in xylene. 0.01 mol/L citrate buffer solution was used for antigen retrieval and 50 μL of peroxidase blocking solution was applied to block endogenous peroxidase activity. The primary antibody was added according to the kit instructions and incubated at 4 °C for 12 h. The secondary antibody was added and incubated at room temperature for 10 min. 100 μL of DAB was added for 5 min, counterstained, and the staining was observed under a microscope.
Cell counting kit-8 (CCK-8) assay
The CCK-8 assay was applied to test the cell viability and the kit was purchased from Tongren (Japan). Cells were pre-incubated at 37 °C in 5% CO2 atmosphere, and then CCK-8 reagent were added and cultured at 37 °C in 5% CO2 for 4 h. The optical density (OD) of each well at 450 nm was measured using a microplate reader (ELX 800, Bio-Teck, USA).
Enzyme-linked immunosorbent assay (ELISA) and chemical colorimetry
The concentrations ofM-CSFand granulocyte-macrophage colony stimulating factor (GM-CSF) were detected using ELISA. The kit was purchased from Cusabio Biotech Co., Ltd. (Wuhan, China) and the process was followed the manufacturer’s instructions.
Transwell assay
Transwell chamber and related reagents were purchased from Corning (USA). 500 pL of whole culture was added in the lower chamber, and then 5 × 104 cells were added and cultured for 4 h. The upper chamber was placed in the lower chamber. Finally, a cell suspension containing 5 × 104 cells was contained and was dropped into the upper chamber vertically. The cells were cultured at 37 °C in 5% CO2 for 6–8 h. After staining with Giemsa staining (Shanghai Gefan Biotechnology Co., Ltd., China), 5 high power fields were randomly select under the microscope to count the migration rate.
QRT-PCR
QRT-PCR was applied to detect the mRNA expression levels. The cells were triturated and lysed, and then the RNA was extracted by RNA extraction kit (Promega, Beijing, China). Reverse transcription kit (TaKaRa, Japan) was used to synthesize cDNA. Reverse transcription reactionr reaction conditions was 37 °C for 15 min and reverse transcriptase inactivation condition was 85 °C for 15 s. qRT-PCR was performed with the qRT-PCR kit (TaKaRa, Japan). PCR was performed by activating the DNA polymerase at 95 °C for 5 min, followed by 40 cycles of two-step PCR (95 °C for 10 s and 60 °C for 30 s) and a final extension at 75 °C for 10 min and held at 4 °C. RNase-free water were used as the templates of negative control. All primers were obtained from Genewiz (Suzhou, Jiangsu China) and listed in . The formula 2−ΔΔCT was implemented to analyze the mRNA expression levels.
Table 1. The sequences of primers.
Western blot
Proteins were tested by Western blot. Cells were lysed and the supernatant was collected by centrifugation at 12000 rpm at 4°C for 15 min. BCA assay was used to determine the protein concentration. SDS-PAGE gel was prepared applied to electrophoresis. The PVDF membrane (Bio-Rad, USA) was transferred by a Trans-Blot Transfer Slot (Bio-Rad, USA) and blocked with 5% fat-free milk for 2 h at room temperature. The primary antibody (anti-KLF6-SV1, FabGennix, KLF6SV1-101AP, dilution: 1:600; anti-, Abcam, ab, dilution: 1:800; anti-TWIST1, Abcam, ab50581, dilution: 1:800; anti-CCL2, Abcam, ab9669, dilution: 1:700; anti-E-cadherin, Abcam, ab6528, dilution: 1:800; anti-N-cadherin, Abcam, ab18203, dilution: 1:800; anti-MMP-9, Abcam, ab38898, dilution: 1:800; anti-CD163, Abcam, ab182422, dilution: 1:600; anti-CD68, Abcam, ab955, dilution: 1:700) was added according to the kit instruction, and the samples was shaked at room temperature for 2 h and incubated at 4°C for 12 h. The secondary antibody (mouse anti-human IgG, Abcam, ab1927, dilution: 1:10000; rabbit anti-human IgG, Abcam, ab6759, dilution: 1:8000; rabbit anti-goat IgG, Abcam, ab6741, dilution: 1:10000) was added and incubated at room temperature for 1.5 h. Chemiluminescence detection was carried out use ECL reagent (Huiying, Shanghai, China).
Statistical analysis
All the experimental data were presented asmean ± standard deviation (SD). Statistical analysis used SPSS 20 (SPSS, Inc., Chicago, IL, USA). The one-way analysis of variance (ANOVA) following Turkey’s multiple comparison was carried out to evaluate the differences between the experimental groups. The statistical significant was expressed as P < 0.05.
Results
Effects of KLF6-SV1on the expression levels of TWIST1 and CCL2
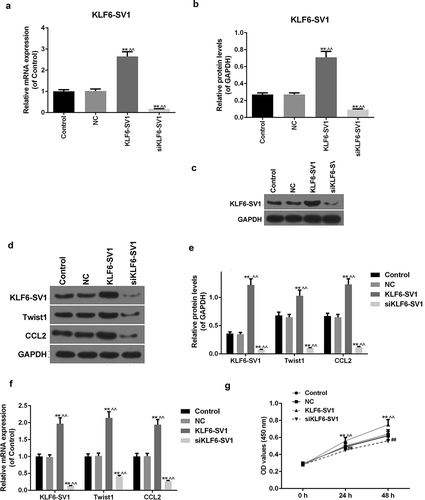
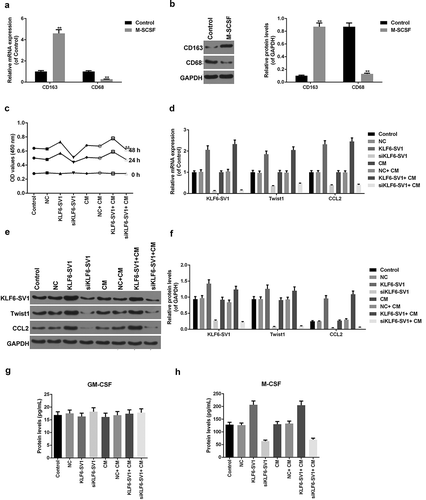
In this experiment, KLF6-SV1 gene overexpressed and underexpressed A549 cells were constructed respectively, qRT-PCR and Western blot were used to detect the expression of KLF6-SV1, TWIST1 and CCL2 mRNA and protein in each group. The results showed that the expression levels of KLF6-SV1 protein and mRNA were significantly increased or decreased after transfection with KLF6-SV1 or siKLF6-SV1 plasmids respectively (-)).This indicated that transfection experiments was successful and could be used for further studies. It was showed that TWIST1 and CCL2 mRNA and protein were increased in the KLF6-SV1 group and decreased in the siKLF6-SV1 group (-)). In addition, the study also found that up-regulation of KLF6-SV1 levels could promote cell growth (). This suggested that KLF6-SV1 might promoted the expression levels of TWIST1 and CCL2.
Figure 1. Effects of KLF6-SV1 on the expression levels of TWIST1 and CCL2.
(a-f) KLF6-SV1, TWIST1 and CCL2 proteins and mRNAs were tested by Western blot and qRT-PCR. (g) CCK-8 assay was used to tested the cell viability. *P < 0.05,**P < 0.01, versus control group; ^P < 0.05, ^^P < 0.01, versus NC group; #P < 0.05, ##P < 0.01, versus KLF6-SV1 group.
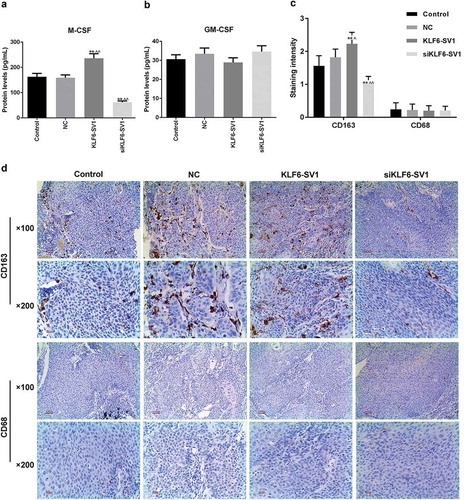
Effects of KLF6-SV1 on TAMs
To investigate the effects of different expression levels of KLF6-SV1 on TAMs, the expression levels of M-CSF and GM-CSF in tumors and the positive status of CD68 and CD163 in tissues were examined. Proteins expressed on the surface of macrophages can reflect their polarization. That CD163 was used as a marker for M2 and CD68 was used as a marker for M1 has been widely recognized. The results showed that KLF6-SV1 had no significant effects on GM-CSF, but could up-regulate the expression level of M-CSF (, )). SP staining results showed that the results of CD68 staining in the four groups were not different, while in the KLF6-SV1 group, the number of CD163-positive TAMs was significantly increased ((c, )). This suggested that KLF6-SV1 might induce macrophage polarization to M2.
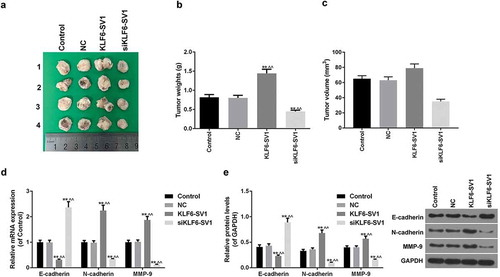
Effects of KLF6-SV1 on tumor weigh and EMT
By weighing the tumor in the four groups of mice, it was shown that KLF6-SV1 gene silencing inhibited tumor growth ((a-c)). The E-cadherin mRNA and protein in the KLF6-SV1 group were significantly lower than those in the control group and NC group, while N-cadherin and MMP-9 were significantly higher than those in the NC group. The results of the siKLF6-SV1 group were opposite to those in the KLF6-SV1 group (, )). This suggested that the KLF6-SV1 gene might affect EMT in lung cancer.
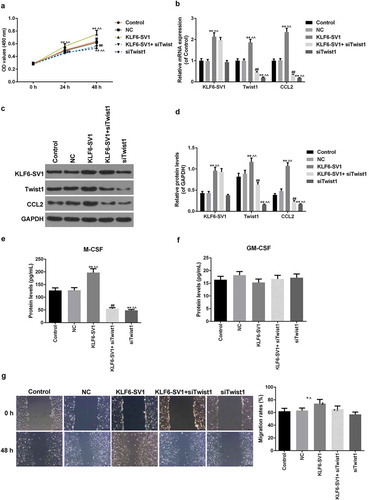
Effects of co-transfection of KLF6-SV1 and sitwist1 on A549 cells
To investigate that the KLF6-SV1 gene affects EMT by modulating the expression of TWIST1, co-transfection experiments were performed. The results showed that TWIST1 gene silencing down-regulated the effects of KLF6-SV1 on cell growth ()). Further experimental results also showed that low expression of TWIST1 gene had no significant effect on the expression level of KLF6-SV1 mRNA and protein, while TWIST1 gene silencing could down-regulate the expression of CCL-2 (-)).TWIST1 gene silencing had no significant effects on GM-CSF, but significantly down-regulated the high levels of M-GSF induced by KLF6-SV1 (-)). Transwell results showed that KLF6-SV1 overexpression promoted the migration ability of A549 cells, while TWIST1 gene silencing inhibited the migration-promoting effect of KLF6-SV1 ()). This suggested that KLF6-SV1 might regulate the migration of lung cancer cells by regulating the expression of TWIST1 and CCL-2.
Figure 4. Effects of co-transfection of KLF6-SV1 and siTWIST1 on A549 cells.
(a) CCK-8 assay was used to tested the cell viability. (b-d) KLF6-SV1, TWIST1 and CCL2 proteins and mRNAs levels at 48 h after tansfection were tested by Western blot and qRT-PCR. (e, f) ELISA was applied to detect the M-CSF and GM-CSF expressions at 48 h after tansfection. (g) Migration rate at 48 h after tansfection was detected using Transwell assay. *P < 0.05,**P < 0.01, versus control group; ^P < 0.05, ^^P < 0.01, versus NC group.
Effects of co-culture of A549 cells and macrophages on EMT of A549 cells
To explore the co-culture of A549 cells and macrophages on EMT of A549 cells, 7 experimental groups: control group, NC group (empty plasmid), KLF6-SV1group, siKLF6-SV1 group, CM group (M2 macrophages), NC+ CM group, KLF6-SV1+ CM group and siKLF6-SV1+ CM group.
By detecting the expression levels of the markers CD68 and CD163 in the macrophages, it was found that the levels of CD163 mRNA and protein increased and that of CD68 was decreased (, )). This suggested that THP-1 cells were successfully induced into M2 type macrophages by M-SCSF. The presence of M2 type macrophages did not significantly affect the cell viability of A549 cells with different KLF6-SV1 gene expression levels (). Co-culture of M2 macrophages had no significant effect on the mRNA and protein expression levels of KLF6-SV1, TWIST1 and CCL-2 (-)). The experimental results also showed that the presence of M2 type macrophages had no significant effect on the effects of M-CSF and GM-CSF induced by KLF6-SV1 (, )).This suggested that M2 macrophages did not affect the expression of KLF6-SV1, TWIST1 and CCL-2.
Figure 5. Effects of co-culture of A549 cells and macrophages on A549 cells.
(a, b) CD163 and CD68 proteins and mRNAs were tested by Western blot and qRT-PCR. (c) CCK-8 assay was used to tested the cell viability. (d-f) KLF6-SV1, TWIST1 and CCL2 proteins and mRNAs were tested by using Western blot and qRT-PCR. (g, h) ELISA was applied to detect the M-CSF and GM-CSF expressions. *P < 0.05,**P < 0.01, versus control group.
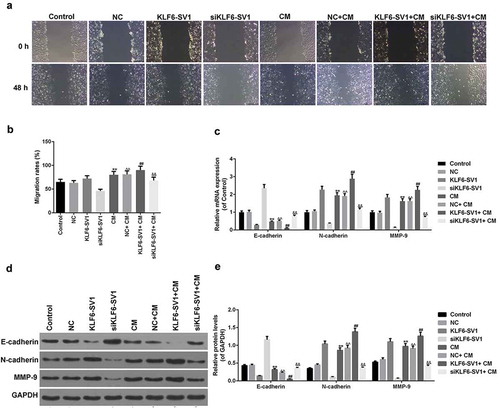
The effect of co-culture system on EMT of A549 cells was detected by Transwell test, it was showed that the migration rate in the NC+ CM group was significantly higher than that in the NC group, and the migration rate in the KLF6-SV1+ CM group was alsohigher than that in the KLF6-SV1 group, and that in the siKLF6-SV1+ CM group was alsohigher than in the siKLF6-SV1 group (, )). This suggesed that co-culture with M2 macrophages could promote the migration ability of A549 cells.The expression of EMT-related genes was detected by qRT-PCR and Western blot. The results showed that the expression levels of N-cadherin and MMP-9 mRNA and protein in the NC+ CM group, KLF6-SV1+ CM group and siKLF6-SV1+ CM group were significantly higher than those in the NC group, KLF6-SV1 group and siKLF6-SV1 group respectively, while E-cadherin showed the opposite trend (-)).This indicated that the co-culture system could up-regulate the EMT of A549 cells.
Figure 6. Effects of co-culture of A549 cells and macrophages on EMT of A549 cells.
(a, b) Migration rate was detected using Transwell assay. (c-e) E-cadherin, N-cadherin and MMP-9 proteins and mRNAs were tested by Western blot and qRT-PCR. *P < 0.05,**P < 0.01, versus control group; ^P < 0.05, ^^P < 0.01, versus NC group; #P < 0.05, ##P < 0.01, versus KLF6-SV1 group; &P < 0.05, &&P < 0.01, versus siKLF6-SV1 group.
Discussion
The transcriptional factors KLF familyinclude 18 members, and KLF is commonly expressed in cells to regulate proliferation, differentiation, and apoptosis.Citation26,Citation27 KLF6 is located near the telomere of chromosome 10p15.2 and is a tumor suppressor.Citation28 The deletion or mutation of KLF6 is the cause of hepatocellular carcinomas and breast cancer.Citation23,Citation29 The anti-cancer effects of KLF6 is associated with a series of related tumor pathways, in which KLF6 participates in the EMT process by regulating E-cadherin and other processes.Citation30 KLF6 is selectively cleaved due to loss of hybrids, somatic mutations, and so on to generate a knocking variant KLF6-SV1.Citation31 KLF6-SV1 has tumor effect, previous studies have shown that KLF6-SV1 is involved in the metastasis and invasion of lung cancer cells,Citation32 but the specific mechanism is not clear.
Tumor metastasis is regulated by the EMT process.Citation33 EMT is a complex process that receives multiple signaling pathways and regulatory factors, including Slug, Snail, and TWIST1.Citation34-Citation36 Among them, TWIST1 has many studies, which can increase the motility of cells by regulating E-cadherin-mediated cell-cell adhesion adhesion.Citation37 A recent study has aroused great interest, Marchelli et al. found for the first time that overexpression of TWIST1 up-regulated CCL2 levels, and CCL2 acted as a chemokine to induce macrophages.Citation38,Citation39 At the same time, recent studies have also shown that in the tumor microenvironment, TAMs are involved in the migration and invasion of tumor cells.Citation40,Citation41
KLF4 can be used as a marker for predicting bladder urothelial carcinoma and participate in the regulation of EMT by regulating the expression level of TWIST1.Citation42 Therefore, we speculated that KLF6-SV1 might also have a role in the involvement of lung cancer cells by regulating TWIST1. To explore the role of KLF6-SV1 in lung cancer, we constructed KLF6-SV1 overexpressing and silencing A549 cells, and explored the effects of different KLF6-SV1 expression levels on TWIST1 and CCL2. The results showed that KLF6-SV1 overexpression increased the expression levels of TWIST1 and CCL2 mRNA and protein, suggesting that KLF6-SV1 minght participate in the regulation of EMT by up-regulating the levels of TWIST1 and CCL2.
To further investigate the regulation of KLF6-SV1 on lung cancer EMT was related to TWIST1 and TAMs, we established in vivo experiments with high and low expression of KLF6-SV1 in A549 cells. The results showed that KLF6-SV1 had minimal effect on GM-CSF, while the expression level of KLF6-SV1 was positively correlated with the expression level of M-CSF. M-CSF can induce macrophage polarization to M2,Citation43 so we further studied the polarization of macrophages at different expression levels of KLF6-SV1. The results of SP staining experiments revealed that in the tumor tissues of KLF6-SV1 overexpressing A549 cells, the number of CD163 positively cell was high, while the number of positive CD68 cells was very small. Down-regulation of KLF6-SV1 expression decreased the positive level of CD163.There are markers on the surface of macrophages that can indicate the direction of polarization. Although there is no uniform standard at present, that CD163 was used as a marker for M2 and CD68 was used as a marker for M1 has been widely recognised.Citation42,Citation44,Citation45 It was showed in this study that the expression level of KLF6-SV1 was correlated with N-cadherin and MMP-9 mRNA and protein levels and negatively correlated with E-cadherin. The results of this study demonstrated that KLF6-SV1 had a regulatory effect on TWIST1, which might promote polarization of TAMs to M2. This suggested that KLF6-SV1 might up-regulate TWIST1 to promote EMT by promoting TAMs to M2 polarization.
To further demonstrate that KLF6-SV1 plays a role in promoting invasion by down-regulating TWIST1 and CCL2, we used KLF6-SV1 and siTWIST1 to perform co-transfection experiments on A549 cells. The results showed that TWIST1 gene silencing had no significant effect on the expression of KLF6-SV1, while low expression of TWIST1 further down-regulated the expression level of CCL2. Transwell results also showed that TWIST1 silencing inhibited the promotion of invasion by KLF6-SV1, suggesting that KLF6-SV1 is involved in the EMT process of lung cancer cells by regulating TWIST1 and CCL2.
Previous studies have shown that cells in the tumor microenvironment could participate in the EMT process of lung cancer, but there are few studies on the mechanism of regulating TAMs affecting lung cancer metastasis.Citation46,Citation47 This study shows that KLF6-SV1 participates in the EMT process by regulating TWIST1 and CCL2, and TWIST1 and CCL2 have the effect of inducing macrophages. Therefore, we speculated that the KLF6-SV1 gene might exert its role in regulating EMT through macrophages. In the next study, six-well plates and Transwell chamber were used for co-culture of lung cancer A549 cells and M2 macrophages. At the bottom of the Transwell chamber, there was a poly-fiber membrane with a pore size of 0.4 μm, the membrane had the function of blocking cells, but the cytokines, proteins and the like secreted by the cells could pass through the micropores freely.M2 type macrophages were induced by M-SCSF, and analysis of the co-culture solution revealed that CD163 levels were significantly increased and CD68 levels were decreased after induction, suggesting that macrophages in co-culture were M2 type. We first explored the effects of M2 macrophages on KLF6-SV1, TWIST1 and CCL2 protein and mRNA levels. The results showed that the presence of M2 macrophages had no significant effect on the expression levels of KLF6-SV1, TWIST1 and CCL2. Further studies have found that the presence of macrophages could increase the invasive ability of A549 cells, and in the presence of macrophages, overexpression of KLF6-SV1 could further up-regulate the invasion ability of A549 cells induced by macrophages, while the low expression of KLF6-SV1 showed an inhibitory effect on EMT. Further investigations have shown that under the condition of overexpression of KLF6-SV1, the presence of M2 macrophages further up-regulates N-cadherin and MMP-9 mRNA and protein expression and down-regulates E-cadherin.
There is limited research on the relationship between KLF and TWIST1. The latest study only find that in the urothelial carcinoma of bladder, knockdown of the KLF4 gene inhibit the EMT process by inhibiting the expression of TWIST1, but no mechanism for macrophages is reported.Citation42 KLF5 may be involved in the reduction of E-cadherin expression in ovarian cancer cells induced by complement component 3 (C3).Citation48 This study found for the first time that overexpression of KLF6-SV1 could promoted the expression of TWIST1. The role of TWIST1 in the regulation of EMT has been widely demonstrated. Macrophage polarization to M2 is found in a variety of tumors and is involved in immune escape, angiogenesis,Citation49-Citation51 and M2 macrophages can exert anti-inflammatory effects and participate in the EMT process by secretingcytokines.Citation52 This study suggested that M2 macrophages participate in the EMT process of lung cancer cells, and the increase of KLF6-SV1 mRNA and protein levels would further enhance the cell invasion ability. This might be due to the upregulation of TWIST1 and CCL2 expression levels by KLF6-SV1 and regulation of EMT by M2 macrophages. Overexpression of KLF6-SV1 up-regulated the level of TWIST1, and overexpression of TWIST1 induced macrophage polarization to M2 and promoted migration and invasion of lung cancer cells by regulating CCL2 and M-CSF. A factor can regulate multiple target genes, and the study by Hatami shows that overexpression of KLF6-SV1 in breast cancer causes up-regulation of MMP2, MMP3 and TWIST1.Citation25 This suggested that KLF6-SV1 may participate in tumor metastasis by regulating MMPs, and could also participate in EMT by regulating TWIST1 and macrophage polarization.
In conclusion, we first found that overexpression of KLF6-SV1 promoted the expression of TWIST1, and up-regulation of TWIST1 expression might promote the infiltration of M2 macrophages by up-regulating CCL2, thereby promoting the involvement of EMT in the metastasis of lung cancer cells. Targeting KLF6-SV1 and inhibiting the EMT process involved in macrophages might be a new approach to the treatment of lung cancer.
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
No animals are involved in this research.
Disclosure of interest
The authors declare no conflicts of interest.
Additional information
Funding
References
- Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. doi:10.1056/NEJMoa1501824.
- Wang T, Nelson RA, Bogardus A, Grannis FW Jr. Five-year lung cancer survival: which advanced stage nonsmall cell lung cancer patients attain long-term survival?. Cancer. 2010;116:1518–1525. doi:10.1002/cncr.24871.
- Meguid RA, Hooker CM, Harris J, Xu L, Westra WH, Sherwood JT, Sussman M, Cattaneo SM, Shin J, Cox S, et al. Long-term survival outcomes by smoking status in surgical and nonsurgical patients with non-small cell lung cancer: comparing never smokers and current smokers. Chest. 2010;138:500–509. doi:10.1378/chest.08-2991.
- Carnio S, Novello S, Mele T, Levra MG, Scagliotti GV. Extending survival of stage IV non-small cell lung cancer. Semin Oncol. 2014;41:69–92. doi:10.1053/j.seminoncol.2013.12.013.
- Voltolini L, Bongiolatti S, Luzzi L, Bargagli E, Fossi A, Ghiribelli C, Rottoli P, Gotti G. Impact of interstitial lung disease on short-term and long-term survival of patients undergoing surgery for non-small-cell lung cancer: analysis of risk factors. Eur J Cardiothorac Surg. 2013;43:e17–23. doi:10.1093/ejcts/ezs560.
- Cheung LW, Yung S, Chan TM, Leung PC, Wong AS. Targeting gonadotropin-releasing hormone receptor inhibits the early step of ovarian cancer metastasis by modulating tumor-mesothelial adhesion. Mol Ther. 2013;21:78–90. doi:10.1038/mt.2012.187.
- Yin M, Li X, Tan S, Zhou HJ, Ji W, Bellone S, Xu X, Zhang H, Santin AD, Lou G, et al. Tumor-associated macrophages drive spheroid formation during early transcoelomic metastasis of ovarian cancer. J Clin Invest. 2016;126:4157–4173. doi:10.1172/JCI87252.
- Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi:10.1016/j.cell.2010.03.015.
- Mittal K, Ebos J, Rini B. Angiogenesis and the tumor microenvironment: vascular endothelial growth factor and beyond. Semin Oncol. 2014;41:235–251. doi:10.1053/j.seminoncol.2014.02.007.
- Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol. 2007;7:41–51. doi:10.1038/nri1995.
- Yuan Z, Mehta HJ, Mohammed K, Nasreen N, Roman R, Brantly M, Sadikot RT, Mattei F. TREM-1 is induced in tumor associated macrophages by cyclo-oxygenase pathway in human non-small cell lung cancer. PLoS ONE. 2014;9:e94241. doi:10.1371/journal.pone.0094241.
- Chanmee T, Ontong P, Konno K, Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers. 2014;6:1670–1690. doi:10.3390/cancers6031670.
- Hao NB, Lu MH, Fan YH, Cao YL, Zhang ZR, Yang SM. Macrophages in tumor microenvironments and the progression of tumors. Clin Dev Immunol. 2012;2012:948098. doi:10.1155/2012/948098.
- Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. doi:10.12703/P.
- Joshi S, Singh AR, Zulcic M, Bao L, Messer K, Ideker T, Dutkowski J, Durden DL, Chrzanowska-Wodnicka M. Rac2 controls tumor growth, metastasis and M1-M2 macrophage differentiation in vivo. PLoS ONE. 2014;9:e95893. doi:10.1371/journal.pone.0095893.
- Festuccia WT, Pouliot P, Bakan I, Sabatini DM, Laplante M. Myeloid-specific Rictor deletion induces M1 macrophage polarization and potentiates in vivo pro-inflammatory response to lipopolysaccharide. PLoS ONE. 2014;9:e95432. doi:10.1371/journal.pone.0095432.
- Ye Y, Xu Y, Lai Y, He W, Li Y, Wang R, Luo X, Chen R, Chen T. Long non-coding RNA cox-2 prevents immune evasion and metastasis of hepatocellular carcinoma by altering M1/M2 macrophage polarization. J Cell Biochem. 2018;119:2951–2963. doi:10.1002/jcb.26509.
- Jia XH, Feng GW, Wang ZL, Du Y, Shen C, Hui H, Peng D, Li Z-J, Kong D-L, Tian J. Activation of mesenchymal stem cells by macrophages promotes tumor progression through immune suppressive effects. Oncotarget. 2016;7:20934–20944. doi:10.18632/oncotarget.8064.
- Velpula KK, Dasari VR, Tsung AJ, Dinh DH, Rao JS. Cord blood stem cells revert glioma stem cell EMT by down regulating transcriptional activation of Sox2 and Twist1. Oncotarget. 2011;2:1028–1042. doi:10.18632/oncotarget.367.
- Zhao N, Sun H, Sun B, Zhu D, Zhao X, Wang Y, Gu Q, Dong X, Liu F, Zhang Y, et al. miR-27a-3p suppresses tumor metastasis and VM by down-regulating VE-cadherin expression and inhibiting EMT: an essential role for Twist-1 in HCC. Sci Rep. 2016;6:23091. doi:10.1038/srep23091.
- D’Angelo RC, Liu XW, Najy AJ, Jung YS, Won J, Chai KX, Fridman R, Kim H-RC. TIMP-1 via TWIST1 induces EMT phenotypes in human breast epithelial cells. Mol Cancer Res. 2014;12:1324–1333. doi:10.1158/1541-7786.MCR-14-0105.
- Ito G, Uchiyama M, Kondo M, Mori S, Usami N, Maeda O, Kawabe T, Hasegawa Y, Shimokata K, Sekido Y. Kruppel-like factor 6 is frequently down-regulated and induces apoptosis in non-small cell lung cancer cells. Cancer Res. 2004;64:3838–3843. doi:10.1158/0008-5472.CAN-04-0185.
- Ozdemir F, Koksal M, Ozmen V, Aydin I, Buyru N. Mutations and Kruppel-like factor 6 (KLF6) expression levels in breast cancer. Tumour Biol. 2014;35:5219–5225. doi:10.1007/s13277-014-1678-6.
- Liang WC, Wang Y, Xiao LJ, Wang YB, Fu WM, Wang WM, Jiang H-Q, Qi W, Wan DC-C, Zhang J-F, et al. Identification of miRNAs that specifically target tumor suppressive KLF6-FL rather than oncogenic KLF6-SV1 isoform. RNA Biol. 2014;11:845–854. doi:10.4161/rna.29356.
- Hatami R, Sieuwerts AM, Izadmehr S, Yao Z, Qiao RF, Papa L, Look MP, Smid M, Ohlssen J, Levine AC, et al. KLF6-SV1 drives breast cancer metastasis and is associated with poor survival. Sci Transl Med. 2013;5:169ra12. doi:10.1126/scitranslmed.3004688.
- Black AR, Black JD, Azizkhan-Clifford J. Sp1 and kruppel-like factor family of transcription factors in cell growth regulation and cancer. J Cell Physiol. 2001;188:143–160. doi:10.1002/jcp.1111.
- Pei J, Grishin NV. A new family of predicted Kruppel-like factor genes and pseudogenes in placental mammals. PLoS ONE. 2013;8:e81109. doi:10.1371/journal.pone.0081109.
- Jeng YM, Hsu HC. KLF6, a putative tumor suppressor gene, is mutated in astrocytic gliomas. Int J Cancer Manag. 2003;105:625–629. doi:10.1002/ijc.11123.
- Boyault S, Herault A, Balabaud C, Zucman-Rossi J. Absence of KLF6 gene mutation in 71 hepatocellular carcinomas. Hepatology. 2005;41:681–2;author reply 2–3. doi:10.1002/hep.20588.
- Hatami R, Sieuwerts AM, Izadmehr S, Yao Z, Qiao RF, Papa L, Look MP, Smid M, Ohlssen J, Levine AC, et al. An oncogenic splice variant drives EMT and metastasis in breast cancer. Cancer Discov. 2013;3:16. doi:10.1158/2159-8290.CD-RW2013-027.
- Narla G, Difeo A, Reeves HL, Schaid DJ, Hirshfeld J, Hod E, Katz A, Isaacs WB, Hebbring S, Komiya A, et al. A germline DNA polymorphism enhances alternative splicing of the KLF6 tumor suppressor gene and is associated with increased prostate cancer risk. Cancer Res. 2005;65:1213–1222. doi:10.1158/0008-5472.CAN-04-4249.
- DiFeo A, Feld L, Rodriguez E, Wang C, Beer DG, Martignetti JA, Narla G. A functional role for KLF6-SV1 in lung adenocarcinoma prognosis and chemotherapy response. Cancer Res. 2008;68:965–970. doi:10.1158/0008-5472.CAN-07-2604.
- Rokavec M, Öner MG, Li H, Jackstadt R, Jiang L, Lodygin D, Kaller M, Horst D, Ziegler PK, Schwitalla S, et al. Corrigendum. IL-6R/STAT3/miR-34a feedback loop promotes EMT-mediated colorectal cancer invasion and metastasis. J Clin Invest. 2015;125:1362. doi:10.1172/JCI80323.
- Liu YN, Yin JJ, Abou-Kheir W, Hynes PG, Casey OM, Fang L, Yi M, Stephens RM, Seng V, Sheppard-Tillman H, et al. MiR-1 and miR-200 inhibit EMT via Slug-dependent and tumorigenesis via Slug-independent mechanisms. Oncogene. 2013;32:296–306. doi:10.1038/onc.2012.58.
- Kaufhold S, Bonavida B. Central role of Snail1 in the regulation of EMT and resistance in cancer: a target for therapeutic intervention. J Exp Clin Cancer Res. 2014;33:62. doi:10.1186/s13046-014-0062-0.
- Tao Y, Han T, Zhang T, Ma C, Sun C. LncRNA CHRF-induced miR-489 loss promotes metastasis of colorectal cancer via TWIST1/EMT signaling pathway. Oncotarget. 2017;8:36410–36422. doi:10.18632/oncotarget.16850.
- Shamir ER, Pappalardo E, Jorgens DM, Coutinho K, Tsai WT, Aziz K, Auer M, Tran PT, Bader JS, Ewald AJ. Twist1-induced dissemination preserves epithelial identity and requires E-cadherin. J Cell Biol. 2014;204:839–856. doi:10.1083/jcb.201306088.
- Low-Marchelli JM, Ardi VC, Vizcarra EA, van Rooijen N, Quigley JP, Yang J. Twist1 induces CCL2 and recruits macrophages to promote angiogenesis. Cancer Res. 2013;73:662–671. doi:10.1158/0008-5472.CAN-12-0653.
- Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–225. doi:10.1038/nature10138.
- Yaddanapudi K, Putty K, Rendon BE, Lamont GJ, Faughn JD, Satoskar A, Lasnik A, Eaton JW, Mitchell RA. Control of tumor-associated macrophage alternative activation by macrophage migration inhibitory factor. J Immunol. 1950;2013(190):2984–2993.
- Dwyer AR, Greenland EL, Pixley FJ. Promotion of Tumor Invasion by Tumor-Associated Macrophages: the Role of CSF-1-Activated Phosphatidylinositol 3 Kinase and Src Family Kinase Motility Signaling. Cancers. 2017;9(6).
- Tseng WC, Chuang CW, Yang MH, Pan CC, Tarng DC. Kruppel-like factor 4 is a novel prognostic predictor for urothelial carcinoma of bladder and it regulates TWIST1-mediated epithelial-mesenchymal transition. Urol Oncol. 2016;34:485.e15-.e24. doi:10.1016/j.urolonc.2016.07.002.
- Yang Y, Qin J, Lan L, Li N, Wang C, He P, Liu F, Ni H, Wang Y. M-CSF cooperating with NFkappaB induces macrophage transformation from M1 to M2 by upregulating c-Jun. Cancer Biol Ther. 2014;15:99–107. doi:10.4161/cbt.26718.
- Craig DG, Lee P, Pryde EA, Hayes PC, Simpson KJ. Serum neopterin and soluble CD163 as markers of macrophage activation in paracetamol (acetaminophen)-induced human acute liver injury. Aliment Pharmacol Ther. 2013;38:1395–1404. doi:10.1111/apt.12530.
- Weber M, Iliopoulos C, Moebius P, Buttner-Herold M, Amann K, Ries J, Preidl R, Neukam FW, Wehrhan F. Prognostic significance of macrophage polarization in early stage oral squamous cell carcinomas. Oral Oncol. 2016;52:75–84. doi:10.1016/j.oraloncology.2015.11.001.
- Zhang J, Cao J, Ma S, Dong R, Meng W, Ying M, Weng Q, Chen Z, Ma J, Fang Q, et al. Tumor hypoxia enhances Non-Small Cell Lung Cancer metastasis by selectively promoting macrophage M2 polarization through the activation of ERK signaling. Oncotarget. 2014;5:9664–9677. doi:10.18632/oncotarget.1856.
- Zhang Y, Wei Y, Jiang B, Chen L, Bai H, Zhu X, Li X, Zhang H, Yang Q, Ma J, et al. Scavenger Receptor A1 Prevents Metastasis of Non-Small Cell Lung Cancer via Suppression of Macrophage Serum Amyloid A1. Cancer Res. 2017;77:1586–1598. doi:10.1158/0008-5472.CAN-16-1569.
- Cho MS, Rupaimoole R, Choi HJ, Noh K, Chen J, Hu Q, Sood AK, Afshar-Kharghan V. Complement Component 3 Is Regulated by TWIST1 and Mediates Epithelial-Mesenchymal Transition. J Immunol. 2016;196:1412–1418. doi:10.4049/jimmunol.1501886.
- Zhang Y, Du W, Chen Z, Xiang C. Upregulation of PD-L1 by SPP1 mediates macrophage polarization and facilitates immune escape in lung adenocarcinoma. Exp Cell Res. 2017;359:449–457. doi:10.1016/j.yexcr.2017.08.028.
- Jetten N, Verbruggen S, Gijbels MJ, Post MJ, De Winther MP, Donners MM. Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis. 2014;17:109–118. doi:10.1007/s10456-013-9381-6.
- Oghumu S, Varikuti S, Terrazas C, Kotov D, Nasser MW, Powell CA, Ganju RK, Satoskar AR. CXCR3 deficiency enhances tumor progression by promoting macrophage M2 polarization in a murine breast cancer model. Immunology. 2014;143:109–119. doi:10.1111/imm.12293.
- Ke X, Wu M, Lou J, Zhang S, Huang P, Sun R, Huang L, Xie E, Wang F, Gu B. Activation of Toll-like receptors signaling in non-small cell lung cancer cell line induced by tumor-associated macrophages. Chin J Cancer Res. 2015;27:181–189. doi:10.3978/j.issn.1000-9604.2015.03.07.