ABSTRACT
Vastatin, a fragment derived from type VIII collagen, is one of the least studied collagen-derived matrikines. Vastatin can be detected in serum but little is known regarding the relevance of serum vastatin in colorectal cancer (CRC). In this study, serum vastatin was measured (ELISA) in 67 healthy controls and 48 CRC patients prior to resection and compared to clinicopathological parameters and serum biomarkers of stromal reactivity (C3M, VICM). Impact of resection and chemotherapy were evaluated by comparing baseline values with a 3-month follow-up sample (n = 23). Serum vastatin was detectable in 114 of 115 subjects. At baseline vastatin was elevated in CRC compared to controls (P < 0.001) with a diagnostic accuracy (AUROC) of 0.865, p < 0.0001. Vastatin correlated with age in controls but not in patients with CRC; no association was seen with clinicopathological parameters. Vastatin was independently associated with C3M (stepwise linear regression coefficient 0.25, p = 0.046). Overall, no difference was seen in vastatin levels between baseline and follow-up. In conclusion, vastatin is elevated in serum from patients with CRC and correlate with interstitial matrix degradation (C3M). This indicates that vastatin is linked to stromal reactivity and suggests that vastatin has biomarker potential in CRC. The association with clinicopathological parameters and treatment effect needs further evaluation.
Introduction
The tumor microenvironment and associated stromal reactivity contribute to tumor manifestation, growth and metastasis and rely heavily on the vasculature and angiogenic processes.Citation1,Citation2 Remodeling of the basement membrane (BM), the thin non-cellular sheet-like protein structure that is an anchoring substrate for epithelial and endothelial cells and provides mechanical and biological support for maintaining cell polarity and behavior, is an important part of tumorigenesis.Citation3,Citation4
The importance of the intestinal BM is gaining increasing attention in gastrointestinal research, and particularly in colorectal cancer (CRC) in which the turnover of BM is altered. One example of altered BM remodeling is reflected by the highly increased release of fragments of type IV collagen (the major BM component) to the circulation of patients with CRC.Citation5 Similar findings have been reported with other gastrointestinal disorders as well.Citation6 This not only supports that BM remodeling is important in GI disorders but also that specific BM derived protein fragments may have non-invasive biomarker potential in CRC.
Type IV, VIII, XV and XVIII collagens are all components of BM. Interestingly, these collagens have matrikines (bioactive peptide fragments) derived from proteolytic cleavage of their non-collagenous (NC-1) domain.Citation7 The best described matrikine is endostatin (the NC-1 domain of type XVIII collagen a1 chain), which has shown endogenous anti-angiogenic and anti-tumor effects.Citation8 Interestingly, serum endostatin has previously shown promising biomarker potential in CRC.Citation9
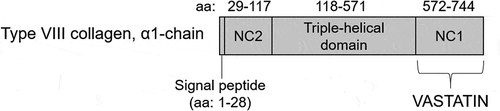
The type VIII collagen derived matrikine vastatin (the C-terminal on the NC-1 domain of type VIII collagen a1 chain) is one of the least studied BM collagen-derived matrikines. The type VIII collagen a1 chain and vastatin is schematically illustrated in . Vastatin/type VIII collagen is expressed by vascular smooth muscle cells in response to vascular injury and has been linked to angiogenic and pro-tumorigenic processes.Citation10–Citation16 A serum biomarker has previously been developed by our research group which after careful subsequent evaluation was shown to reflect serum vastatin.Citation17
Figure 1. Schematic illustration of the type VIII collagen a1-chain and vastatin with amino acid (aa) sequence. The proteases responsible for generating vastatin remains to be established. NC: non-collagenous domain.
The aim of this study was to investigate the impact and relevance of serum vastatin in healthy controls and patients with CRC in relation to age, gender and clinicopathological parameters including tumor location, tumor stage/class and venous invasion (VI) in the patients with malignant disease. Moreover, the impact of tumor resection and adjuvant chemotherapy was analyzed. Finally, the link between serum vastatin and stromal reactivity biomarkers was evaluated.
Results
Demographics and clinicopathological parameters
The demographics and clinicopathological parameters of the healthy controls and patients with CRC are shown in . Patients with CRC were older and included more females than the control group. No differences were seen on BMI. Tumors were located in in the colon in 85% of cases and in the rectum in 15% of cases. Most patients (52%) were in TNM stage 2, 15% were in TNM stage 1, 19% in TNM stage 3, 8% in TNM stage 4 and 6% were uncharacterized by stage. According to TNM classes 49% of patients were in T3N0M0. Lastly, 56% of patients presented with venous invasion (VI).
Table 1. Subject characteristics.
Serum vastatin in healthy controls and baseline CRC samples and impact of age and gender
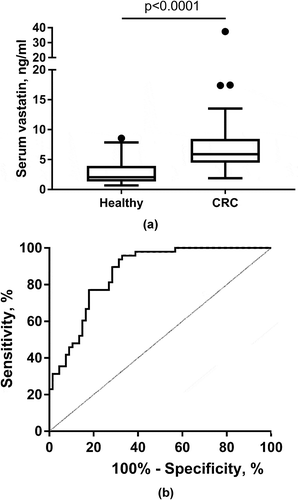
All patients except one healthy subject had detectable serum vastatin levels (≥ 0.68 ng/ml). The pre-resection (baseline) levels of serum vastatin in the healthy control group and in the patients with CRC are presented in ). Patients with CRC presented with significantly higher (p < 0.0001) vastatin levels in serum compared to the control group. In the control group vastatin levels ranged from 0.68–8.57 ng/ml with a median value of 2.06 ng/ml, whereas in the patients with CRC vastatin levels ranged from 1.88–37.41 ng/ml with a median value of 5.90 ng/ml. In support, as shown in ), the clinical accuracy for identifying patients with CRC was good with an AUROC of 0.865 (P < 0.0001), and with the optimal serum vastatin cut-point level for separating CRC from controls at 3 ng/ml.
Figure 2. Serum vastatin in healthy controls and colorectal cancer (CRC) patients (a) and ROC curve analysis (b) for evaluating clinical accuracy of serum vastatin in CRC patients vs. the control group. In B), the area under the ROC curve was calculated to be AUC 0.865 (95%CI: 0.788–0.921), p < 0.0001. Optimal criterion value was 3 ng/ml with a sensitivity of 96% and specificity of 67%. A: Data presented as Tukey plots.
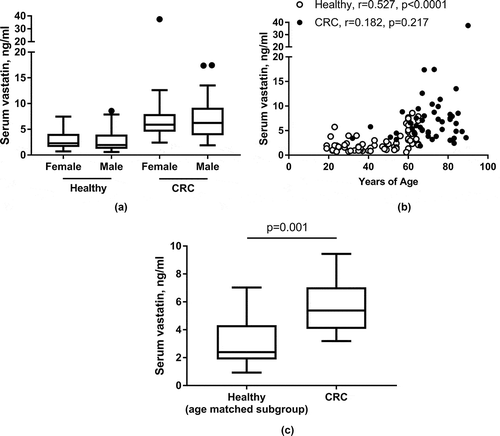
Next, we evaluated the association between serum vastatin levels and demography (gender and age). As shown in ), no difference could be detected in serum vastatin levels when dividing either the control group or the patients with CRC by gender. A significant correlation between serum vastatin and age was seen in the healthy control group (rSpearman = 0.527, p < 0.0001) whereas no correlation was seen for patients with CRC (rSpearman = 0.217, p = 0.217) ()). To evaluate further the influence of age on the difference between controls and patients with CRC, a sub-analysis was performed on age-matched subjects covering the age-span 30–65 years. As shown in ), significantly (p = 0.001) elevated levels was found in patients with CRC compared to the age-matched controls.
Figure 3. Serum vastatin according to gender (a) and age (b). In a subgroup analysis (c) of age-matched CRC patients (n = 13) and controls (n = 13) covering the age-span 30–65 years of age, significant difference in serum vastatin levels were maintained. CRC: colorectal cancer. A/C: Data presented as Tukey plots. B: Spearman correlation coefficient, r.
Serum vastatin in CRC samples and relationship with clinicopathological parameters
Serum vastatin in baseline CRC samples were evaluated in relation to tumor location (rectum vs. colon), TNM stage/class and venous invasion (VI). Results are presented in . No significant differences were seen in serum vastatin levels between TNM stages/classes, the presence or absence of VI and tumor location.
Table 2. Serum vastatin in CRC samples and relationship with clinico-pathological parameters.
Serum vastatin in CRC samples and correlation with serum biomarkers of stromal reactivity
To evaluate the association with serum vastatin and stromal reactivity in patients with CRC, we measured two biomarkers (C3M and VICM) reflecting stromal reactivity and which both have previously been described to be released from CRC tumor biopsies in ex vivo culture.Citation18 Serum vastatin correlated positively with serum C3M (rSpearman = 0.435, p = 0.002) whereas no correlation was seen between serum vastatin and serum VICM (rSpearman = 0.117, p = 0.430).
Independent associations between serum vastatin levels in CRC and demographic/clinicopathological features as well as stromal biomarker levels
A stepwise multiple linear regression analysis was performed to evaluate the independent associations between serum vastatin levels in patients with CRC and clinicopathological features (age, BMI, gender, tumor stage, tumor type, VI) as well as stromal reactivity biomarker levels (C3M, VICM). Of the factors included in the analysis, only C3M levels were independently associated with serum vastatin levels in patients with CRC ().
Table 3. Stepwise multiple linear regression model for evaluating the independent association of clinicopathological parameters and stromal biomarkers with serum vastatin in patients with CRC.
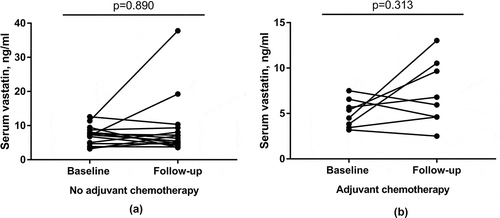
Impact of tumor resection and adjuvant chemotherapy on serum vastatin levels
By comparing serum vastatin levels in pre-resection/pre-chemotherapy baseline samples with a 3-month post-resection/post-chemotherapy follow-up sample, we evaluated the impact of tumor resection only ()) and tumor resection including post-operative adjuvant chemotherapy ()). In both groups there was no significant differences between baseline serum vastatin levels and 3-month follow-up. Still, in individual patients more than 2-fold changes were detected.
Discussion
In this study we evaluated the impact and relevance of measuring the matrikine vastatin (the NC1 domain of human type VIII collagen a1 chain) in serum from healthy controls and patients with CRC prior to tumor resection. Serum vastatin was detectable in both healthy controls and patients with CRC, with significantly elevated levels found in CRC. In support, it has been shown that another well-characterized matrikine, endostatin (the NC1 domain of human type XVIII collagen a1 chain), which is also involved in BM remodeling and angiogenesis, is also elevated in serum from patients with CRC.Citation9
To evaluate further the biomarker potential of serum vastatin in CRC we analyzed the relationship between serum vastatin levels and clinicopathological parameters including age, BMI, gender, TNM stage, tumor type and VI. Overall, serum vastatin was associated with age in healthy controls. Especially, the elderly part of the healthy controls appeared to have relatively high serum vastatin levels. This is not a surprise, as tissue turnover has been shown to be quantitively different in older vs younger animals (rats) and humans.Citation19,Citation20 The reason behind this age-related increase in serum vastatin levels cannot be explained from the present study and warrants further investigation. However, it might be associated with increased prevalence of (undiagnosed) cardiovascular disease as has been described for endostatin,Citation21,Citation22 and/or the general increase in mild, moderate and severe co-morbidities that are associated with becoming older.Citation23 Nonetheless, it is important to consider the age of patients in future evaluations of the clinical applicability of measuring serum vastatin.
In this study, no association was seen between serum vastatin and CRC tumor type, TNM stage/class, and VI which are well-known prognostic factors of CRCs.Citation24 This indicates that serum vastatin is not simply a surrogate read-out of prognosis or tumor burden, but rather provides information in terms of important BM remodeling processes (and tumor angiogenesis). Gene-expression analysis of CRC has previously identified the COL8A1 gene to be associated with progression and prognosis of CRC.Citation25 Our data highlights that specific fragments (or matrikines) derived from a collagen of interest, may provide unique value compared to gene-expression analysis of the same collagen.
The proteases responsible for generating vastatin is currently not known. However, in vitro cleavage of type VIII collagen occurs by human neutrophil elastase potentially linking vastatin to chronic inflammation.Citation26,Citation27 Tumor angiogenesis, and hence BM remodeling, has been linked to both tumor inflammation and desmoplasia.Citation28,Citation29 This suggest a link between stromal reactivity and vastatin and led to the evaluation of an association between serum vastatin and the stromal reactivity biomarkers C3M and VICM. Both C3M and VICM have been found secreted from CRC tumor tissue in ex vivo culture and both associate with gastrointestinal disorders and CRC when measured in serum.Citation5,Citation6,Citation18,Citation30
No correlation was seen between serum vastatin and VICM. VICM is released from tissue-resident activated macrophages and released to the circulation.Citation31 Enhanced tumor angiogenesis and release of pro-angiogenic factors have been associated with infiltration and activation of macrophages in cancer.Citation32 However, the present findings indicate that macrophages do not associate with vastatin. In contrast to VICM, C3M was correlated positively and independently associated with serum vastatin levels. C3M measures MMP-degraded type III collagen, a major interstitial matrix component, and an important part of the fibrosis/desmoplasia associated ECM remodeling and stromal reactivity.Citation33–Citation35 Interestingly, type VIII has been linked to induction of MMP expression and ECM remodeling associated with fibrosis.Citation36–Citation39 In support, indications from CRC suggests that type VIII collagen drives tumor progression through regulation of the focal adhesion-related pathway, a well-known contributor to fibroblast activation and associated interstitial collagen remodeling.Citation25 Hence, these findings indicate that vastatin correlate with interstitial matrix degradation that is part of the desmoplastic reaction. Taken together, vastatin is linked to stromal reactivity and has biomarker potential in CRC.
In the present study, no impact of tumor resection and adjuvant chemotherapy on serum vastatin levels was seen. Overall, this suggests that serum vastatin does not only originate from the primary tumor, but rather that serum vastatin could be indicative of other pathological conditions unspecific for CRC, such as inflammatory bowels disease that has been linked with both angiogenic alterations and CRC.Citation40,Citation41
One interesting aspect of the biomarker potential of serum vastatin is related to response/resistance to anti-angiogenic therapies. CRC is one of the most studied cancers in relation to angiogenesis and anti-angiogenic treatment, and vastatin as a biomarker may therefore be highly relevant in this indication.Citation42 Resistance to anti-angiogenic therapies has been directly associated with vascular remodeling processes and an increased expression of angiogenic factors, which contribute to vessel stabilization.Citation43–Citation45 Still, a better understanding of resistance mechanism is needed and biomarkers associated with vascular BM remodeling, such as serum vastatin, may predict the behavior of individual tumors in this context.Citation46
A limitation to this study includes a relatively small number of patients, despite support from a power calculation for comparing serum vastatin levels in the included patients and controls. Hence, the findings should be validated in larger cohorts. Moreover, the design was cross-sectional with limited clinicopathological information available, warranting longitudinal investigations in well-described clinical cohorts. Because age could be a confounding factor, a major limitation to this study is related to the age difference between controls and patients with CRC. However, when conducting a sub-analysis of age-matched subjects, this still resulted in significantly elevated serum vastatin levels in CRC compared to controls. Additional investigations are needed to fully evaluate the impact of age.
In summary, this study evaluated the impact and relevance of serum vastatin – the NC1 domain of human type VIII collagen a1 chain and a so-called matrikine – in healthy controls and patients with CRC. Serum vastatin was elevated in patients with CRC highlighting the relevance of BM remodeling in this disease, albeit the link to demographic and clinicopathological features needs further investigations. Interestingly, serum vastatin correlated with a surrogate measure of interstitial matrix turnover (C3M) indicating that vastatin is linked to stromal reactivity of the tumor.
Material & Methods
Patients and serum collection
The patients with CRC were included in the present study subsequent to informed consent and approval by the Ethical Committee of the Capital Region of Denmark (Copenhagen, Denmark; approval no. H-1–2014-048) in compliance with the Helsinki Declaration. Tumor resections were performed at Bispebjerg Hospital, Copenhagen, Denmark between November 2014 and October 2015. Exclusion criteria were i) if under the age of 18 years, ii) pregnancy, iii) a psychotic disorder or dementia and iv) any prior anti-tumor treatment. Tumor stage/class was defined from the AJCC/UICC TNM Classification and Stage groupings system Citation47. Medical staff at Bispebjerg Hospital, Copenhagen, Denmark, collected serum samples. Times of serum collection were 1 day prior to surgery (baseline, n = 48) and 3 months post-treatment (follow-up, n = 23). The follow-up visit was voluntary and loss to follow-up was primarily either inability to draw blood or come to hospital due to geographic reasons. Of the 48 patients, 16 were treated with adjuvant standard of care chemotherapy with 8 of these patients being included with a follow-up visit blood sample draw. Control serum samples (n = 67) were acquired from commercial vendors (Valley BioMedical, Winchester, VA, USA and Proteogenex, Inglewood, CA, USA). All control subjects had filed informed consent according to vendor’s information.
Serum vastatin assay (PRO-C8)
The levels of vastatin was measured in serum by the competitive enzyme-linked immunosorbent assay (ELISA) PRO-C8. The technical evaluation of this assay is described elsewhere.Citation17 In brief, a biotinylated synthetic target peptide was dissolved in an optimized assay buffer that was added to a 96-well streptavidin coated plate. The plate incubated for 30 min at 20°C and was washed five times in wash-buffer. All incubation steps were done by agitating the plate with 300 rounds per minute. The target peptide calibrator, or serum sample (diluted 1:2), and a solution of horseradish peroxidase (HRP) conjugated monoclonal antibody specific to the target peptide-sequence of interest was added the wells and the plate incubated for 20 h at 4°C. The plate was washed five times in wash-buffer and tetramethylbenzidine was added and the plate incubated for 15 min at 20°C in dark. The reaction was stopped by adding 1% H2SO4 and the OD450–650nm was measured. A four-parametric mathematical fit model was used to plot a calibration curve and calculate unknown values (in serum). Data were analyzed using the SoftMax Pro v.6.3 software. Values were determined from duplicate measures and only accepted if the coefficient of variation (%CV) was < 20%, as recommended by the manufacturer (Nordic Bioscience, Herlev, Denmark). Samples with PRO-C8 levels below the detection limit (0.68 ng/ml) were assigned the value of 0.68 ng/ml (n = 1).
Serum stromal biomarker assays (C3M and VICM)
Levels of C3M (matrix metalloprotease (MMP) degraded type III collagen)Citation48, and VICM (MMP degraded and citrullinated vimentin)Citation49 are surrogate measures of tissue remodeling and stromal reactivity and were measured in baseline serum from the patients with CRC (n = 48) as previously described and recommended by the manufacturer (Nordic Bioscience, Herlev, Denmark) in a similar fashion as to described for PRO-C8. Both C3M and VICM have been found secreted from CRC tumor tissue in ex vivo culture.Citation18
Statistical analysis
A Mann Whitney U-test or Kruskal-Wallis test was used to compare serum vastatin levels with respect to healthy vs. CRC, gender groups and clinicopathological parameters. A power calculation for comparing two means (estimated control group mean = 3, standard deviation = 1.5; estimated increase in cancer = 4 (+ 1), standard deviation 2; type 1 error risk (α) = 0.05) confirmed that measurements in 48 patients diagnosed with colorectal cancer and 67 healthy controls were sufficient to validate the use of serum vastatin in CRC with a power of 86.5% (type-2 error risk = 0.135). To evaluate the ability of serum vastatin to separate patients with CRC from controls, the area under receiver operating characteristics (AUROC) curve was calculated. Correlations between serum vastatin levels, age and stromal biomarkers (C3M, VICM) were evaluated by the Spearman rank correlation coefficient (r). A multiple linear regression analysis (stepwise method) was used to evaluate the independent association of clinicopathological parameters and stromal biomarkers with serum vastatin in patients with CRC. The impact of tumor resection and/or adjuvant chemotherapy was evaluated in paired samples with the Wilcoxon signed-rank test. In all tests, a p-value of less than 0.05 was considered statistically significant. MedCalc Statistical Software v14.8.1 and Graphpad Prism v7.04 were used to perform statistical analysis and plots.
Disclosure of Potential Conflicts of Interest
Nicholas Willumsen and Morten A Karsdal are employed at Nordic Bioscience involved in biomarker discovery and assay development. Morten A Karsdal owns stocks at Nordic Bioscience. Lars N Jorgensen declares no conflicts of interest
Acknowledgments
We acknowledge the Danish Research Foundation for supporting this study.
References
- Farnsworth RH, Lackmann M, Achen MG, Stacker SA. Vascular remodeling in cancer. Oncogene. 2014;33:3496–3505. doi:10.1038/onc.2013.304.
- Ciombor KK, Goldberg RM. Update on anti-angiogenesis therapy in colorectal cancer. Curr Colorectal Cancer Rep. 2015;11:378–387. doi:10.1007/s11888-015-0292-3.
- Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer. 2003;3:422–433. doi:10.1038/nrc1094.
- Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol. 2011;3:12.
- Kehlet SN, Sanz-Pamplona R, Brix S, Leeming DJ, Karsdal MA, Moreno V. Excessive collagen turnover products are released during colorectal cancer progression and elevated in serum from metastatic colorectal cancer patients. Sci Rep. 2016;6:30599.
- Mortensen JH, Godskesen LE, Jensen MD, Van Haaften WT, Klinge LG, Olinga P, Dijkstra G, Kjeldsen J, Karsdal MA, Bay-Jensen AC, et al. Fragments of citrullinated and MMP-degraded Vimentin and MMP-degraded type III Collagen are novel serological biomarkers to differentiate Crohn’s disease from ulcerative colitis. J Crohns Colitis. 2015;9:863–872.
- Karsdal MA, Nielsen SH, Leeming DJ, Langholm LL, Nielsen MJ, Manon-Jensen T, Siebuhr A, Gudmann NS, Rønnow S, Sand JM, et al. The good and the bad collagens of fibrosis - Their role in signaling and organ function. Adv Drug Deliv Rev. 2017;121:43–56.
- O’Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285.
- Kantola T, Väyrynen JP, Klintrup K, Mäkelä J, Karppinen SM, Pihlajaniemi T, Autio-Harmainen H, Karttunen TJ, Mäkinen MJ, Tuomisto A. Serum endostatin levels are elevated in colorectal cancer and correlate with invasion and systemic inflammatory markers. Br J Cancer. 2014;111:1605–1613.
- Sibinga NE, Foster LC, Hsieh CM, Perrella MA, Lee WS, Endege WO, Sage EH, Lee ME, Haber E. Collagen VIII is expressed by vascular smooth muscle cells in response to vascular injury. Circ Res. 1997;80:532–541.
- Xu R, Yao Z-Y, Xin L, Zhang Q, Li T-P, Gan R-B. NC1 domain of human type VIII collagen (alpha 1) inhibits bovine aortic endothelial cell proliferation and causes cell apoptosis. Biochem Biophys Res Commun. 2001;289:264–268. doi:10.1006/bbrc.2001.5970.
- Li X, Wang Z, Tong H, Yan Y, Li S. Effects of COL8A1 on the proliferation of muscle-derived satellite cells. Cell Biol Int. 2018;42:1132–1140. doi:10.1002/cbin.v42.9.
- Li Y, Li J, Woo YM, Shen Z, Yao H, Cai Y, Lin MC, Poon WS. Enhanced expression of Vastatin inhibits angiogenesis and prolongs survival in murine orthotopic glioblastoma model. BMC Cancer. 2017;17:126. doi:10.1186/s12885-017-3125-8.
- Ma ZH, Ma JH, Jia L, Zhao YF. Effect of enhanced expression of COL8A1 on lymphatic metastasis of hepatocellular carcinoma in mice. Exp The Med. 2012;4:621–626. doi:10.3892/etm.2012.652.
- Koon N, Schneider-Stock R, Sarlomo-Rikala M, Lasota J, Smolkin M, Petroni G, Zaika A, Boltze C, Meyer F, Andersson L, et al. Molecular targets for tumour progression in gastrointestinal stromal tumours. Gut. 2004;53:235–240. doi:10.1136/gut.2003.021238.
- Zhao Y, Jia L, Mao X, Xu H, Wang B, Liu Y. siRNA-targeted COL8A1 inhibits proliferation, reduces invasion and enhances sensitivity to D-limonence treatment in hepatocarcinoma cells. IUBMB Life. 2009;61:74–79. doi:10.1002/iub.v61:1.
- Hansen NUB, Willumsen N, Sand JMB, Larsen L, Karsdal MA, Leeming DJ. Type VIII collagen is elevated in diseases associated with angiogenesis and vascular remodeling. Clin Biochem. 2016;49:903–908. doi:10.1016/j.clinbiochem.2016.05.023.
- Willumsen N, Bager C, Bay‑Jensen A, Kehlet S, Harling H, Leeming D, Karsdal M, Jorgensen L. Unique insight into microenvironmental changes in colorectal cancer: exï vivo assessment of matrix metalloprotease‑mediated molecular changes in human colorectal tumor tissue and corresponding non‑neoplastic adjacent tissue. Oncol Lett. 2017;13:3774–3780. doi:10.3892/ol.2017.5900.
- Karsdal MA, Genovese F, Madsen EA, Manon-Jensen T, Schuppan D. Collagen and tissue turnover as a function of age: implications for fibrosis. J Hepatol. 2016;64:103–109. doi:10.1016/j.jhep.2015.08.014.
- Kehlet SN, Willumsen N, Armbrecht G, Dietzel R, Brix S, Henriksen K, Karsdal MA. Age-related collagen turnover of the interstitial matrix and basement membrane: implications of age- and sex-dependent remodeling of the extracellular matrix. PLoS ONE. 2018;13:e0194458. doi:10.1371/journal.pone.0194458.
- Mitsuma W, Kodama M, Hanawa H, Ito M, Ramadan MM, Hirono S, Obata H, Okada S, Sanada F, Yanagawa T, et al. Serum endostatin in the coronary circulation of patients with coronary heart disease and its relation to coronary collateral formation. Am J Cardiol. 2007;99:494–498. doi:10.1016/j.amjcard.2006.09.095.
- Carlsson AC, Ruge T, Sundstrom J, Ingelsson E, Larsson A, Lind L, Arnlov J. Association between circulating endostatin, hypertension duration, and hypertensive target-organ damage. Hypertension. 2013;62:1146–1151. doi:10.1161/HYPERTENSIONAHA.113.02250.
- Piccirillo JF, Vlahiotis A, Barret LB, Flood KL, Spitznagel EL Jr., Steyerberg EW. The changing prevalence of comorbidity across the age spectrum. Crit Rev Oncol Hematol. 2008;67:124–132. doi:10.1016/j.critrevonc.2008.01.013.
- Betge J, Pollheimer MJ, Lindtner RA, Kornprat P, Schlemmer A, Rehak P, Vieth M, Hoefler G, Langner C. Intramural and extramural vascular invasion in colorectal cancer. Cancer. 2012;118:628–638. doi:10.1002/cncr.26523.
- Shang J, Wang F, Chen P, Wang X, Ding F, Liu S, Zhao Q. Co-expression network analysis identified COL8A1 is associated with the progression and prognosis in human colon adenocarcinoma. Dig Dis Sci. 2018;63:1219–1228. doi:10.1007/s10620-018-4996-5.
- Döring G. The role of neutrophil Elastase in chronic inflammation. Am J Respir Crit Care Med. 1994;150:S114–7. doi:10.1164/ajrccm.150.5.7832867.
- Kittelberger R, Neale TJ, Francky KT, Greenhill NS, Gibson GJ. Cleavage of type VIII collagen by human neutrophil elastase. BBA - Mol Basis Dis. 1992;1139:295–299. doi:10.1016/0925-4439(92)90103-T.
- Candido J, Hagemann T. Cancer-related inflammation. J Clin Immunol. 2013;33:79–84.
- Vong S, Kalluri R. The role of stromal myofibroblast and extracellular matrix in tumor angiogenesis. Genes Cancer. 2011;2:1139–1145. doi:10.1177/1947601911423940.
- van Haaften WT, Mortensen JH, Karsdal MA, Bay-Jensen AC, Dijkstra G, Olinga P. Misbalance in type III collagen formation/degradation as a novel serological biomarker for penetrating (Montreal B3) Crohn’s disease. Aliment Pharmacol Ther. 2017;46:26–39. doi:10.1111/apt.14092.
- Bay-Jensen AC, Guo X, Mortensen JH, Karsdal MA. VICM is a novel biomarker of macrophage activity evaluated in a phase IIb clinical trial of mavrilimumab. Arthritis Rheumatol. 2015;67(suppl):10.
- Ono M. Molecular links between tumor angiogenesis and inflammation: inflammatory stimuli of macrophages and cancer cells as targets for therapeutic strategy. Cancer Sci. 2008;99:1501–1506. doi:10.1111/j.1349-7006.2008.00853.x.
- Karsdal MA, Nielsen MJ, Sand JM, Henriksen K, Genovese F, Bay-Jensen A-C, Smith V, Adamkewicz JI, Christiansen C, Leeming DJ. Extracellular matrix remodeling: the common denominator in connective tissue diseases. Possibilities for evaluation and current understanding of the matrix as more than a passive architecture, but a key player in tissue failure. Assay Drug Dev Technol. 2013;11:70–92. doi:10.1089/adt.2012.474.
- Egeblad M, Rasch MG, Weaver VM. Dynamic interplay between the collagen scaffold and tumor evolution. Curr Opin Cell Biol. 2010;22:697–706. doi:10.1016/j.ceb.2010.08.015.
- Cox TR, Erler JT. Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis Model Mech. 2011;4:165–178. doi:10.1242/dmm.004077.
- Adiguzel E, Hou G, Sabatini PJB, Bendeck MP. Type VIII collagen signals via β1 integrin and RhoA to regulate MMP-2 expression and smooth muscle cell migration. Matrix Biol. 2013;32:332–341. doi:10.1016/j.matbio.2013.03.004.
- Hou G, Mulholland D, Gronska MA, Bendeck MP. Type VIII collagen stimulates smooth muscle cell migration and matrix metalloproteinase synthesis after arterial injury. Am J Pathol. 2000;156:467–476. doi:10.1016/S0002-9440(10)64698-6.
- Lopes J, Adiguzel E, Gu S, Liu SL, Hou G, Heximer S, Assoian RK, Bendeck MP. Type VIII collagen mediates vessel wall remodeling after arterial injury and fibrous cap formation in atherosclerosis. Am J Pathol. 2013;182:2241–2253. doi:10.1016/j.ajpath.2013.02.011.
- Lopes J, Hou G, Bendeck M. Type VIII collagen mediates atherosclerotic plaque development and fibrous cap formation. Can J Cardiol. 2013;29:S171. doi:10.1016/j.cjca.2013.07.262.
- Binion DG, Rafiee P. Is inflammatory bowel disease a vascular disease? Targeting angiogenesis improves chronic inflammation in inflammatory bowel disease. Gastroenterology. 2009;136:400–403. doi:10.1053/j.gastro.2008.12.029.
- Kim ER, Chang DK. Colorectal cancer in inflammatory bowel disease: the risk, pathogenesis, prevention and diagnosis. World J Gastroenterol. 2014;20:9872.
- Botrel TEA, Clark LG de O, Paladini L, Clark OAC. Efficacy and safety of bevacizumab plus chemotherapy compared to chemotherapy alone in previously untreated advanced or metastatic colorectal cancer: a systematic review and meta-analysis. BMC Cancer. 2016;16:677.
- Gladbender J, Cooney E, Kandel J, Yamashiro D. Vascular remodeling and clinical resistance to antiangiogenic cancer therapy. Drug Resist Updat. 2004;7:289–300.
- Qian C-N, Tan M-H, Yang J-P, Cao Y. Revisiting tumor angiogenesis: vessel co-option, vessel remodeling, and cancer cell-derived vasculature formation. Chin J Cancer. 2016;35:10.
- Rahbari NN, Kedrin D, Incio J, Liu H, Ho WW, Nia HT, Edrich CM, Jung K, Daubriac J, Chen I, et al. Anti-VEGF therapy induces ECM remodeling and mechanical barriers to therapy in colorectal cancer liver metastases. Sci Transl Med. 2016;8:360.
- Vasudev NS, Reynolds AR. Anti-angiogenic therapy for cancer: current progress, unresolved questions and future directions. Angiogenesis. 2014;17:471–494.
- Edge S, Compton C. The American joint committee on cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474.
- Barascuk N, Veidal SS, Larsen L, Larsen DV, Larsen MR, Wang J, Zheng Q, Xing R, Cao Y, Rasmussen LM, et al. A novel assay for extracellular matrix remodeling associated with liver fibrosis: an enzyme-linked immunosorbent assay (ELISA) for a MMP-9 proteolytically revealed neo-epitope of type III collagen. Clin Biochem. 2010;43:899–904.
- Vassiliadis E, Oliveira CP, vares-da-Silva MR, Zhang C, Carrilho FJ, Stefano JT, Rabelo F, Pereira L, Kappel CR, Henriksen K, et al. Circulating levels of citrullinated and MMP-degraded vimentin (VICM) in liver fibrosis related pathology. Am J Transl Res. 2012;4:403–414.