ABSTRACT
Hepatocellular carcinoma (HCC), the most common aggressive malignancy of liver, is the third leading cause of cancer death across the world. Laminin gamma 1 (Lamc1), encodes laminin-γ1, an extracellular matrix protein involved in various progresses such as tumor cell proliferation and metabolism. In the present study, high expression of Lamc1 and PKM2 was observed in tumor tissues of HCC patients. In vitro, down-regulation of Lamc1 inhibited proliferation of HCC cells by promoting cell death, reduced glucose consumption and lactate production, accompanied by a decrease in the expression of glucose transporter 1 (GLUT1) and lactate dehydrogenase A (LDHA), and PTEN increased, as well as PTEN S380 and AKT S473/T308 phosphorylation decreased, while Lamc1 up-regulation had the opposite effect. The effects of PKM2 were similar to that of Lamc1 and markedly counteracted the effects of Lamc1 down-regulation. In addition, Lamc1-induced increase in PKM2 expression was strongly attenuated by a PI3K inhibitor, LY294002 or a si-p110 PI3K, with a significant decrease in GLUT1 and LDHA expression, as well as decreased AKT T308 phosphorylation. Thus, we speculated that Lamc1 was implicated in the progression of HCC probably by regulating PKM2 expression through PTEN/AKT pathway.
Introduction
Hepatocellular carcinoma (HCC), an aggressive malignancy of the liver, is the third leading cause of cancer death in the world and its incidence is steadily increasing across the whole world.Citation1 In recent years, hepatic resection or transplantation as well as radiofrequency ablation are considered to be potentially curative therapies for HCC.Citation2,Citation3 Although surgery is an effective method for the treatment of HCC, due to the high aggressiveness and frequent recurrence, the prognosis of HCC is still poor, and its survival rate is only 20% to 30% even after hepatic resection.Citation4
Warburg effect, defined over 80 y ago, is a unique metabolic phenotype in cancer cells, through this way, glucose metabolites are preferentially converted into nucleotides, amino acid and other cell structural blocks that meet the demands of tumor metabolism and growth.Citation5–Citation7 Accumulated studies have shown that the enhanced Warburg effect is mainly reflected in an increase in glucose consumption and lactate production, which is observed in many human cancers including HCC, and is closely related to tumor aggressiveness and poor prognosis of patients.Citation8–Citation11 Tumor-specific pyruvate kinase M2 (PKM2), one isoform of pyruvate kinase, is reported to regulate the final rate-limiting step of glycolysis. PKM2 is a special glycolytic enzyme that catalyzes the synthesis of pyruvate and ATP, thereby contributing to the Warburg effect, which contributes to the growth, survival, and metabolism of cancer cells.Citation12–Citation15 Phosphatase and tensin homologue deleted on chromosome 10 (PTEN) as a tumor suppressor antagonizes the action of PI3K-AKT pathway in many human cancers.Citation16 The PI3K-AKT pathway is well known to be the major signal cascades regulating glucose metabolism, and activation of AKT regulates various processes involved in cancer, such as cell cycle progression and growth, as well as controlling glucose consumption through the glucose-transporter 1 (GLUT1) transporter.Citation17
Laminin gamma 1 (Lamc1) gene encodes laminin-γ1, an extracellular matrix protein belonging to a family of laminins and involving in the assembly of basement membranes and various progresses including the growth and metastasis of tumors.Citation18–Citation20 Studies have closely related Lamc1 mRNA to the malignancy of HCC. Lamc1, reported to highly express in HCC tumor, promotes tumor metastasis and predicts the poor prognosis of HCC.Citation21 As a trans-regulator, Lamc1 promotes the migration and invasion of HCC cells by stimulating CD151 expression through competing for microRNA-124.Citation22 However, the effect of Lamc1 on Warburg effect in HCC remains unclear.
In this study, high expression of Lamc1 and PKM2 was detected in tumors of HCC patients. In HCC cells, Lamc1 down-regulation inhibited cell proliferation, reduced glucose consumption and lactate production, with decreased GLUT1 and LDHA, increased PTEN, as well as decreased PTEN S380 and AKT S473/T308 phosphorylation, while Lamc1 up-regulation had the opposite effect. The effects of PKM2 were similar to that of Lamc1 and markedly counteracted the effects of Lamc1 down-regulation. In addition, Lamc1-induced increase in PKM2 expression was strongly attenuated by a PI3K inhibitor, LY294002 or a si-p110 PI3K, concurrent with decreased GLUT1 and LDHA expression, and AKT T308 phosphorylation. Thus, we speculated that Lamc1 was implicated in the progression of HCC probably by regulating PKM2 expression through PTEN/AKT pathway.
Materials and methods
Tumor tissues and adjacent normal tissues
Forty tumor tissues and 20 matched adjacent normal tissues of HCC patients who were treated at People Hospital of LiShui were collected. The tissue samples were frozen in liquid nitrogen prior to use. All clinical specimens of humans were obtained with informed consent, and all experiments of this study were approved by the Ethics Committee of LiShui University. After RNA extraction, the expression of Lamc1 and PKM2 in these tissues was detected by real-time PCR.
Cell culture
Three human HCC cell lines of HepG2, Hep3B and Huh7, as well as a normal hepatocyte cell line of LO2 were purchased from Cell Bank of Chinese Academy of Science (Shanghai, China). The cells were cultured in a 37°C, 5% CO2 incubator with high glucose DMEM medium (HyClone, USA) containing 10% fetal bovine serum ([FBS], GIBCO, USA) and 1% double-antibiotic (penicillin and streptomycin, Solarbio, China). According to the demands of the cells, the medium was refreshed everyday or every 2 d during incubation.
Construction of the lentivirus
Specific siRNA designed for the target gene is synthesized by Genewiz Company (Shanghai, China) and inserted into Agel I/Ecol I restriction sites of a pLKO.1-Puro plasmid. The coding DNA sequence (CDS) regions of Lamc1 and PKM2 were, respectively synthesized to insert into EcoR I/BamH I restriction sites of pLVX-Puro plasmids. Constructed pLKO.1-Puro-siLamc1, pLVX-Puro-Lamc1 or pLVX-Puro-PKM2 (Addgen, USA) were co-transfected with packaging plasmids psPAX2 and pMD2G (Addgen) into 293T cells by Lipofectamine 2000 (Invitrogen). After 48 h of transfection, the packaged lentiviruses in the medium were collected.
Experimental grouping
To regulate Lamc1 level in HepG2, Hep3B and Huh7 cells lines, the cultured cells were divided to infect with lentivirus of Lamc1 interference (siLamc1-1, siLamc1-2 and siLamc1-3)/negative control (Vector), Lamc1 overexpression (oeLamc1)/Vector, or PKM2 overexpression (oePKM2)/Vector, while the cells treated with medium were as Control. After 48 h of infection, the expression of Lamc1 and PKM2 were quantified by real-time PCR and western blot analysis. To explore the effect of Lamc1 on these cells, assays of cell proliferation were performed at 0, 24, 48 and 72 h. Subsequently, biochemical detections of glucose uptake and lactate level, as well as western blot for genes expression were carried out.
To investigate the effect of PKM2 on HepG2, four groups were divided to respectively infect with Vector, siLamc1, oePKM2, and siLamc1 + oePKM2. The assays of cell proliferation, biochemical detections and western blot were performed 48 h later. For further study of mechanism of Lamc1, Huh7 cells were randomly divided and respectively infected with Vector + siNC, oeLamc1 + siNC, Vector + LY294002/si-p110 PI3K (a PI3K inhibitor), and oeLamc1 + LY294002/si-p110 PI3K. Western blot analysis for associated-genes expression was performed after infection.
Proliferation assay
Logarithmic growth phase cells were digested with 0.25% trypsin (Solarbio) into a cell suspension of 3 × 104 cells/mL. Each 100 μL of cell suspension was seeded in 96-well culture plates with three identical wells as duplicate wells, and cultured in a 37°C, 5% CO2 humidified incubator overnight. The next day, the cells were infected with lentivirus for 0, 24, 48 and 72 h, and then 100 µL of Cell Counting Kit 8 (CCK8, CCK8: serum-free medium = 1: 10, SAB) was added to each well. After 1 h of incubation, a microplate reader (Perlong, Beijing) was used to measure the optical density (OD) of the absorbance at 450 nm.
Real-time polymerase chain reaction (RT-PCR)
The total RNA of tissues or lentivirus-infected cells was isolated by Trizol reagent (Invitrogen, 1596-026). After quantified, the integrity of RNA was confirmed by electrophoresis using 1% agarose gel. Using a reverse transcriptase kit (Fermentas, #K1622), the RNA extraction was reversed into cDNA. And then with a SYBR Green PCR kit (Thermo, #K0223), RT-PCR reactions were conducted on a Real-time detector (ABI, ABI-7300, USA). Relative to GAPDH, the mRNA levels of Lamc1 and PKM2 were analyzed using the method of 2−△△CT. The primers used were as follows: Lamc1, 5ʹ ACTCCTAATCTTGGACCATAC 3ʹ and 5ʹ ACAACAGCACAACTTGAAC 3ʹ; PKM2, 5ʹ AGCAAGAAGGGTGTGAAC 3ʹ and 5ʹ CGGATGAATGACGCAAAC 3ʹ; GAPDH, 5ʹ AATCCCATCACCATCTTC 3ʹ and 5ʹ AGGCTGTTGTCATACTTC 3ʹ. In addition, the procedure of RT-PCR as follows: 95°C, 10 min (95°C, 15 s; 60°C, 45 s) × 40; 95°C, 15 s; 60°C, 1 min; 95°C, 15 s; 60°C, 15 s.Citation23
Western blot analysis
Treated-cells were lysed in RIPA buffer (Solarbio) containing protease and phosphatase inhibitors and incubated for 30 min on ice to homogenize fully. The proteins in the supernatant of lysates were collected after centrifuged for 10 min at 12000 g at 4°C, followed by quantified by a BCA protein kit (Thermo). After separation by SDS-PAGE electrophoresis, the proteins were semi-dry transferred by electroblotting onto polyvinylidene fluoride (PVDF) membranes (Millipore). Successfully transferred membranes were sheared, and then blocked in 5% skim milk (BD Biosciences, USA) at room temperature for 1 h. Following the incubation with primary antibodies against Lamc1 (1:2000, Santa Cruz, Sc-17751), PKM2 (1:100, Abcam, Ab38237), PTEN (1:1000, Cell Signaling Technology [CST], #9552), AKT (1:1000, CST, #9272), p-AKT (Ser473; 1:1000, CST, #9271), GLUT1 (1:8000, Abcam, Ab115730), LDHA (1:1000, Abcam, Ab101562), or GAPDH (1:2000, CST, #5174) overnight at 4°C with gentle shaking, the membranes were washed for 5–6 times and incubated with secondary antibodies (1:1000, Beyotime) for 1 h at room temperature. Subsequently, 5 min incubation of chemiluminescent reagent later, the target protein bands were visualized by an ECL imaging system (Tanon, Shanghai).
Biochemical detection
Logarithmic growth phase cells were inoculated in 6-well culture plates at a density of 5 × 105 cells/well, and then cultured in a 37°C, 5% CO2 incubator for 24 h. The next day, following 48 h infection of lentivirus, the cells were cultured with low glucose DMEM medium for 3 h. After washing of glucose-free Krebs-Ringer bicarbonate buffer (containing 2% BSA) at 37°C, the cells were incubated with glucose-free DMEM containing 100 µM 2-NBDG for 45 min. Finally, the cells were inoculated in 96-well plates after 3 times washing of iced PBS, and the fluorescence intensity was measured by a fluorescence microplate reader. In addition, after collected the supernatant of cultured medium, the lactate level was detected using a lactic acid kit in the guide of instructions.
Statistical analysis
The statistical analyses of all data were conducted by the software of GraphPad prism 7.0 (GraphPad Software, USA). The significant difference between two comparisons was analyzed by the method of student’s t-tests, while one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison were applied to multiple comparisons. The correlation between each two groups was determined through Pearson’s analysis. All data shown as mean ± SD were based on three independent experiments at least, and P < 0.05 was considered statistically significant.
Results
Lamc1 is significantly increased in tumors of HCC patients and associated with PKM2 expression
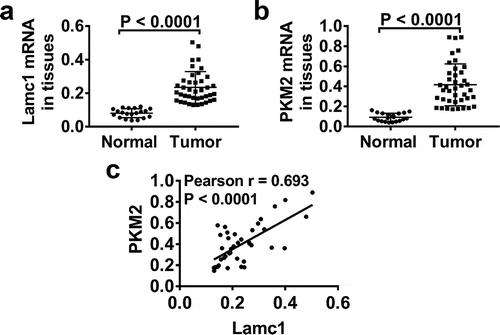
In order to explore whether Lamc1 regulates the Warburg effect, the expression levels of Lamc1 and PKM2, a key glycolytic gene, in tumors of HCC patients were detected. As shown in , compared with adjacent nontumour tissues, the mRNA levels of Lamc1 ()) and PKM2 ()) were significantly increased in tumors of HCC patients. Importantly, Pearson’s analysis showed a positive correlation between Lamc1 and PKM2 (). It suggested that Lamc1, associating with PKM2, may be implicated in HCC progression.
Figure 1. Lamc1 is significantly increased in tumors of HCC patients and associated with PKM2 expression Forty tumor and 20 adjacent normal tissues of HCC patients were collected. (a,b). After isolation of RNA, the expression of Lamc1 (a) and PKM2 (b) relative to GAPDH was detected by RT-PCR. (c) The correlation between Lamc1 and PKM2 was analyzed by Pearson’s analysis (P < 0.0001).
High expression levels of Lamc1 and PKM2 in HCC cell lines
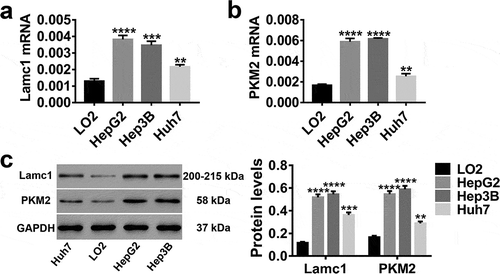
In vitro, LO2 (normal hepatocytes), HepG2, Hep3B and Huh7 (HCC cell lines) were applied, and the expression levels of Lamc1 and PKM2 in these cells were detected. As shown in , both mRNA and protein levels of Lamc1 ()) and PKM2 ()) were much higher in HCC cells than in normal hepatocytes, particular in HepG2 and Hep3B. It further evidenced the close correlation of Lamc1 and PKM2 with HCC.
Figure 2. High expression levels of Lamc1 and PKM2 in HCC lines RT-PCR and western blot were used to detect the expression of Lamc1 and PKM2 in LO2 (normal hepatocytes), HepG2, Hep3B and Huh7 (HCC cell lines) cells. (a,b) After the RNA extraction, the mRNA expression of Lamc1 (a) and PKM2 (b) relative to GAPDH were respectively detected. (c) After the protein extraction, the levels of Lamc1 and PKM2 protein were detected. With three repeated independent experiments, the data were expressed as mean ± SD, LO2 as a control, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
Down- and up-regualtion of Lamc1 in HCC cell lines by lentivirus infection
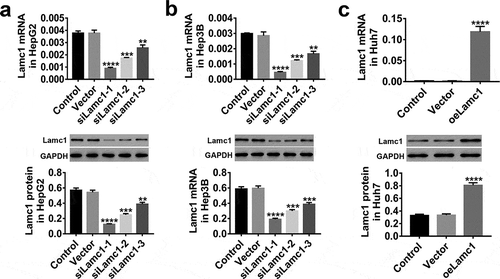
In HepG2, Hep3B and Huh7 cells, the expression of Lamc1 was regulated through lentivirus infection. The results shown in , Lamc1 expression were significantly down-regulated by siLamc1 lentivirus infection in HepG2 () and Hep3B () cells, and the effects of siLamc1-1 and siLamc1-2 were much better, while oe-Lamc1 significantly up-regulated the expression of Lamc1 in Huh7 cells (). Therefore, due to their effective regulation, siLamc1-1, siLamc1-2, and oeLamc1 were used for subsequent experiments.
Figure 3. Down- and up-regulation of Lamc1 in HCC cell lines by lentivirus infection. (a–c) After 48 h infection of lentiviruses siLamc1 and oeLamc1, the efficiency of siLamc1 or oeLamc1 regulating Lamc1 expression in HCC cells was determined by the assays of RT-PCR (upper) and western blot (lower). With three repeated independent experiments, the data were shown as mean ± SD, **P < 0.01, ***P < 0.001, and ****P < 0.0001 compared with Vector.
Lamc1 is required for the proliferation of HCC cells
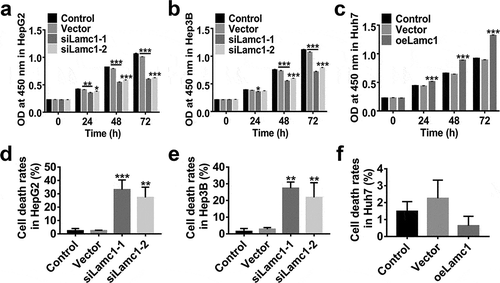
To assess the effect of Lamc1 on the proliferation of HCC cells, lentiviruses of siLamc1-1, siLamc1-2 and oeLamc1 were utilized to down- or up-regulate the expression of Lamc1. After 0, 24, 48 and 72 h of infection, the proliferation of HepG2, Hep3B and Huh7 cells were evaluated by CCK8 assays. Data shown in , down-regulation of Lamc1 potently suppressed the cell proliferation of HepG2 () and Hep3B (), on the contrary, Lamc1 up-regulation in Huh7 significantly promoted the cell proliferation (). In addition, the rates of cell death of Lamc1-silenced HepG2 () and Hep3B () cells were significantly increased, whereas Lamc1 up-regulation had no significant effect on cell death rates (). It indicated that down-regulation of Lamc1 in HCC cells inhibited cell proliferation by promoting cell death. Thus, Lamc1 was required for the proliferation of HCC cells.
Figure 4. Lamc1 is required for the proliferation of HCC cells. After infection with siLamc1 or oeLamc1 lentivirus, (a–c) the proliferation of HCC cells was evaluated by CCK8 assays at 0, 24, 48 and 72 h. (d–f). The cell death rates of HCC cells were evaluated by Trypan blue staining. With three repeated independent experiments, the data were shown as mean ± SD, *P < 0.05, **P < 0.01, and ***P < 0.001 compared with Vector.
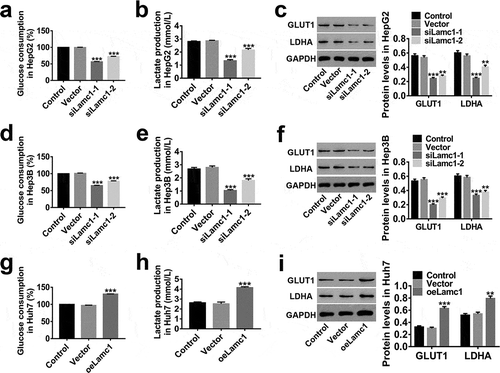
Down-regulation of Lamc1 inhibits the Warburg effect in HCC cells
We also investigate the effect of Lamc1 on Warburg effect in HCC cells to understand the mechanism of Lamc1 tumourigenic function. As shown in , a significant decrease in percentage of glucose consumption (relative to Control, )) and lactate production ()) was observed in Lamc1-silenced HCC cells, while up-regulation of Lamc1 had an opposite effect ()). It is agreement with previous studies that glucose consumption and lactate production increased significantly in cancer cells.Citation24,Citation25 GLUT1, a basic high-affinity glucose transporter, is critically important in malignant glucose metabolism.Citation26 It is reported that increased expression of GLUT1 offers more energy to malignant tumors.Citation27 Lactate dehydrogenase A (LDHA), regulating lactate production of Warburg effect, is reported to be involved in the progression of cancers.Citation28 These were consistent with our observation that the expression of GLUT1 and LDHA was positively regulated by Lamc1 in HCC cells (,)). These indicated that Lamc1 expression was critically important for Warburg effect and down-regulation of Lamc1 potently impairs Warburg effect of HCC.
Figure 5. Down-regulation of Lamc1 inhibits the Warburg effect in HCC cells. After down- and up-regulation of Lamc1, through biochemical detections, glucose consumption (a,d,g) and lactate production (b,e,h) of these cells were measured. Percentage of glucose consumption is relative to Control. (c,f,i). The protein levels of GLUT1 and LDHA was detected by western blot. With at least three independent experiments, the data were presented as mean ± SD, **P < 0.01, and ***P < 0.001 compared with Vector.
Lamc1 suppresses the cell proliferation and Warburg effect via regulating PKM2
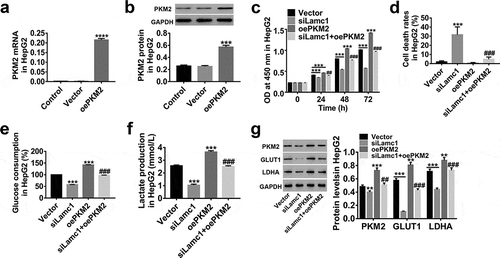
Studies have closely related a constitutively low PKM2 activity to Warburg effect.Citation29,Citation30 Here, lentiviruses of siLamc1 and oePKM2 were applied to explore the possible relationship between PKM2 and Lamc1. As shown in , the expression of PKM2 was significantly up-regulated by oePKM2 infection in HCC cells ()), and PKM2 up-regulation significantly promoted the cell proliferation () and elevated glucose consumption () and lactate production (), but the cell death rates was less changed (). In addition, we found that the effects of Lamc1 down-regulation on the proliferation, cell death and glucose metabolism of HCC cells was potently counteracted by PKM2 up-regulation. Similarly, PKM2 up-regulation counteracted the inhibition of GLUT1 and LDHA expression in Lamc1-silenced HCC cells (). It further confirmed that Lamc1 regulated the cell proliferation and Warburg effect of HCC probably by modulating PKM2 expression.
Figure 6. Lamc1 suppresses the cell proliferation and Warburg effect via regulating PKM2 (a,b). The efficiency of oePKM2 regulating PKM2 expression in HepG2 cells was determined by RT-PCR (a) and western blot (b). After co-infection of lentiviruses siLamc1 and oePKM2 in HCC cells, (c) the cell proliferation were assessed by CCK8 assays at 0, 24, 48 and 72 h. (d) The cell death rates in HepG2 were evaluated by Trypan blue staining. (e,f) The glucose consumption (e) and lactate production (f) of HCC cells were detected by biochemical detections. (g) The protein levels of PKM2, GLUT1 and LDHA were detected by western blot. With at least three independent experiments, the data were expressed as mean ± SD, **P < 0.01 and ***P < 0.001 compared with Vector, ##P < 0.01 and ###P < 0.001 compared with siLamc1.
Lamc1 regulates PKM2 expression through the phosphorylation of PTEN/AKT pathway
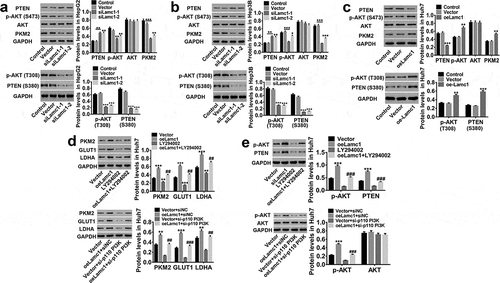
We then investigated the potential molecular mechanism by which Lamc1 regulates HCC cells by regulating PKM2 expression. As shown in , we found that down-regulating Lamc1 in HCC cells significantly inhibited PKM2 expression, decreased AKT S473/T308 phosphorylation and PTEN S380 phosphorylation, and increased PTEN, while AKT was unchanged (). On the contrary, up-regulation of Lamc1 had an opposite effect (). In addition, inhibition of PI3K by LY294002 or a si-p110 PI3K prevented Lamc1-induced increase in the protein levels of PKM2, GLUT1 and LDHA in HCC cells, accompanied by decreased AKT S473/T308 and unchanged AKT ()). PTEN as a tumor suppressor is capable to counteract PI3K-AKT action.Citation16 PI3K is the major substrate of PTEN, which is activated to form PIP3, and S380 is one of the phosphorylation sites of PTEN, regulating the stability of PTEN. The phosphorylation of PTEN S380 inhibits the phosphorylation of PIP2 to PIP3, and dephosphorylation of PIP3 inhibits PI3K/AKT signaling pathway. These results indicated that PTEN/AKT functioned upstream of PKM2 and was responsible for PKM2 expression, and through inhibition of PTEN/AKT pathway, Lamc1 suppressed the expression of PKM2 to further inhibit Warburg effect in HCC.
Figure 7. Lamc1 regulates PKM2 expression through the phosphorylation of PTEN/AKT pathway. (a/b/c) After Lamc1 down- and up-regulation, the protein levels of PTEN, p-PTEN (S380), p-AKT (S473/T308), AKT, PKM2 in HepG2 (a), Hep3B (b) and Huh7 (c) cells were detected by western blot analysis. (d/e) After treatment of oeLamc1 lentivirus and LY294002 or a si-p110 PI3K, the protein levels of PKM2, GLUT1, and LDHA, as well as p-AKT (T308) and AKT were detected. With three independent experiments, the data were shown as mean ± SD, *P < 0.05, **P < 0.01, and ***P < 0.001 compared with Vector, ##P < 0.01 and ###P < 0.001 compared with oeLamc1.
Discussion
HCC is the most common liver cancer causing death, and its incidence in both developing and developed countries is increasing. It has been reported that due to its high incidence and high mortality, the prognosis of HCC is poor.Citation31 In the present study, we found that Lamc1 was highly expressed in HCC tumors, and may regulate the cell proliferation and Warburg effect in HCC by regulating PKM2 expression through inactivation of PTEN/AKT pathway, which may be a new potential therapeutic target for HCC.
Lamc1, one of the extracellular matrix proteins, is a part of the tumor microenvironment and essential for growth, metastasis and survival of tumors.Citation32 Studies have reported that Lamc1 is an independent predictor of liver cancer prognosis,Citation21 which is consistent with our observation that Lamc1 was significantly elevated in HCC tissues or cell lines. For further study, the expression of Lamc1 was down- and up-regulated by lentivirus in HCC cells. We confirmed that down-regulation of Lamc1 significantly inhibited the proliferation of HCC cells by promoting cell death, and inhibited the Warburg effect, accompanied by decreased GLUT1 and LDHA, two key factors involved in Warburg effect, whereas Lamc1 up-regulation was opposite. In addition, PKM2 up-regulation potently enhanced the proliferation and Warburg effect of HCC cells, and the effects of Lamc1 down-regulation on HCC cells were counteracted by PKM2 up-regulation. It is consistent with the previous observations that PKM2 promotes tumor angiogenesis, the expression of PKM2 coincides with cancer cell glycolysis addiction and is associated with chromosome segregation and mitosis in tumor cells, decreasing PKM2 can partly inhibit the growth of liver cancer cells.Citation33,Citation34 Furthermore, significantly decreased PKM2 expression was observed in Lamc1-silenced HCC cells, as well as decreased AKT S473/T308 and PTEN S380 phosphorylation and increased PTEN. These showed that the expression of PKM2 was regulated by Lamc1 and there was a possible relationship between PKM2 and PTEN/AKT pathway. Inhibition of PI3K by LY294002 or a si-p110 PI3K significantly reduced Lamc1-induced increases in PKM2 expression, concurrent with decreased GLUT1 and LDHA expression, and AKT S473/T308. It demonstrated that PTEN/AKT functioned upstream of PKM2 and was responsible for PKM2 expression, and through the inhibition of PTEN/AKT pathway, Lamc1 suppressed the expression of PKM2 to further inhibit Warburg effect in HCC. Interestingly, it has been reported that through suppression of PKM2 activity, PARP14 promotes the Warburg effect in HCC,Citation35 which is inconsistent with our results. This may be due to a negative correlation between PKM expression and PKM2 activity; however, further validation is still needed. And MEG3 inhibits the growth of HCC cells through the negative regulating PKM2 and β-catenin activity.Citation36 PI3K-AKT pathway is a major signal cascade regulating glucose metabolism in human cancers. Through activation of PI3K-AKT pathway, Twist, a key regulator of EMT, promotes the glucose metabolism in breast cancer cells.Citation37 And Lamin A/C proteins are reported to positively involve in malignant behavior through PI3K-AKT pathway.Citation38 14-3-3β promotes HCC cell metastasis via PI3K-AKT-NF-κB pathway.Citation39 Based on these, we inferred that Lamc1 down-regulation inhibited the proliferation and Warburg effect of HCC cells probably by regulating PKM2 expression through inactivation of PTEN/AKT pathway.
In summary, we demonstrated that down-regulation of Lamc1 significantly suppressed proliferation and Warburg effect of HCC cells by regulating PKM2 expression through inactivation of AKT pathway. Targeting Lamc1 may provide a new potential therapeutic target for HCC.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. 2010. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. doi:10.1002/ijc.25516.
- Lau WY, Lai EC. Hepatocellular carcinoma: current management and recent advances. Hepatobiliary Pancreat Dis Int. 7;2008:237–257.
- Tiong L, Maddern GJ. 2011. Systematic review and meta‐analysis of survival and disease recurrence after radiofrequency ablation for hepatocellular carcinoma. Br J Surg. 98:1210–1224. doi:10.1002/bjs.7669.
- El-Serag HB, Marrero JA, Rudolph L and Reddy KR. 2008. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology. 134:1752. doi:10.1053/j.gastro.2008.01.074.
- Hsu PP, Sabatini DM. 2008. Cancer cell metabolism: warburg and beyond. Cell. 134:703–707. doi:10.1016/j.cell.2008.08.021.
- Heiden MGV, Thompson CB. 2009. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 324:1029. doi:10.1126/science.1160809.
- Warburg O. On the origin of cancer cells. Science. 123;1956:309–314.
- Kitamura K, Hatano E, Higashi T, Narita M, Seo S, Nakamoto Y, Yamanaka K, Nagata H, Taura K, Yasuchika K, et al. 2011. Proliferative activity in hepatocellular carcinoma is closely correlated with glucose metabolism but not angiogenesis. J Hepatol. 55:846–857. doi:10.1016/j.jhep.2011.01.038.
- Beyo D, F X L, Imbeaud S, Maurhofer O, Bioulac-Sage P, Zucman-Rossi J, Jf D, Jr I. 2013. Tissue metabolomics of hepatocellular carcinoma: tumor energy metabolism and the role of transcriptomic classification. Hepatology. 58:229–238. doi:10.1002/hep.26350.
- Beyoğlu D, Idle JR. 2013. The metabolomic window into hepatobiliary disease. J Hepatol. 59:842–858. doi:10.1016/j.jhep.2013.05.030.
- Gatenby RA, Gillies RJ. 2004. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 4:891–899. doi:10.1038/nrc1478.
- Dong G, Mao Q, Xia W, Youtao XU, Wang J, Lin XU, Jiang F. 2016. PKM2 and cancer: the function of PKM2 beyond glycolysis. Oncol Lett. 11:1980. doi:10.3892/ol.2016.4099.
- Wei L, Yang C, Zhang Y, Sheng L, Jian G, Xuan W, Mu J, Hu YP, Lin J, Ping D. 2016. Up-regulation of PKM2 promote malignancy and related to adverse prognostic risk factor in human gallbladder cancer. Sci Rep. 6:26351. doi:10.1038/srep26351.
- Zhang HS, Zhang FJ, Li H, Liu Y, Du GY and Huang YH. 2016. Tanshinone ⅡA inhibits human esophageal cancer cell growth through miR-122-mediated PKM2 down-regulation. Arch Biochem Biophys. 598:50–56. doi:10.1016/j.abb.2016.03.031.
- Liang J, Cao R, Zhang Y, Xia Y, Zheng Y, Li X, Wang L, Yang W, Lu Zand Yang W et al. 2016. PKM2 dephosphorylation by Cdc25A promotes the Warburg effect and tumorigenesis. Nat Commun. 7:12431. doi:10.1038/ncomms12431.
- Salmena L, Carracedo A and Pandolfi PP. 2008. Tenets of PTEN tumor suppression. Cell. 133:403. doi:10.1016/j.cell.2008.04.013.
- Hong SY, Yu FX, Luo Y and Hagen T. 2016. Oncogenic activation of the PI3K/Akt pathway promotes cellular glucose uptake by downregulating the expression of thioredoxin-interacting protein. Cell Signal. 28:377–383. doi:10.1016/j.cellsig.2016.01.011.
- Simonassmann P. 2013. The laminin family: founding members of the basement membrane. Cell Adh Migr. 7:44. doi:10.4161/cam.23276.
- Givant-Horwitz V, Davidson B, Reich R. 2005. Laminin-induced signaling in tumor cells. Cancer Lett. 223:1. doi:10.1016/j.canlet.2004.08.030.
- Patarroyo M, Tryggvason K, Virtanen I. Laminin isoforms in tumor invasion, angiogenesis and metastasis. Semin Cancer Biol. 2002;12(3):197–207. doi:10.1016/S1044-579X(02)00023-8.
- Zhang Y, Xi S, Chen J, Zhou D, Gao H, Zhou Z, Xu L, Chen M. 2017. Overexpression of LAMC1 predicts poor prognosis and enhances tumor cell invasion and migration in hepatocellular carcinoma. J Cancer. 8:2992. doi:10.7150/jca.21038.
- Yang ZP, Ma HS, Wang SS, Wang L, Liu T. 2017. LAMC1 mRNA promotes malignancy of hepatocellular carcinoma cells by competing for MicroRNA-124 binding with CD151. IUBMB Life. 69:595. doi:10.1002/iub.1642.
- Hong JY, Kang B, Kim A, Hwang S, Ahn J, Lee S, Kim J, Jh P, Ds C. 2011. Development of a highly sensitive real-time one step RT-PCR combined complementary locked primer technology and conjugated minor groove binder probe. Virol J. 8:1–6. doi:10.1186/1743-422X-8-1.
- Wind F, Negelein E. The metabolism of tumors in the body. J Gen Physiol. 8;1927:519–530.
- Braun AC. On the origin of the cancer cells. Am Sci. 58;1970:307–320.
- Han MW, Lee HJ, Cho KJ, Kim JS, Roh JL, Choi SH, Sy N, Sy K. 2012. Role of FDG-PET as a biological marker for predicting the hypoxic status of tongue cancer. Head Neck. 34:1395–1402. doi:10.1002/hed.21945.
- Yan SX, Luo XM, Zhou SH, Bao YY, Fan J, Lu ZJ, Liao XB, Huang YP, Tt W, Qy W. 2013. Effect of antisense oligodeoxynucleotides glucose transporter-1 on enhancement of radiosensitivity of laryngeal carcinoma. Int J Med Sci. 10:1375–1386. doi:10.7150/ijms.6855.
- Cui J, Shi M, Xie D, Wei D, Jia Z, Zheng S, Gao Y, Huang S, Xie K. 2014. FOXM1 promotes the warburg effect and pancreatic cancer progression via transactivation of LDHA expression. Clin Cancer Res. 20:2595–2606. doi:10.1158/1078-0432.CCR-13-2407.
- Hitosugi T, Kang S, Vander Heiden MG, Chung TW, Elf S, Lythgoe K, Dong S, Lonial S, Wang X, Gz C. 2009. Tyrosine phosphorylation inhibits PKM2 to promote the Warburg effect and tumor growth. Sci Signal. 2:ra73. doi:10.1126/scisignal.2000431.
- Anastasiou D, Yu Y, Israelsen WJ, Jiang JK, Boxer MB, Hong BS, Tempel W, Dimov S, Shen M, Jha A. 2012. Pyruvate kinase M2 activators promote tetramer formation and suppress tumorigenesis. Nat Chem Biol. 8:839–847. doi:10.1038/nchembio.1060.
- Stefaniuk P, Janusz C, Wiercinska-Drapalo A. 2010. Present and future possibilities for early diagnosis of hepatocellular carcinoma. World J Gastroenterol. 16:418. doi:10.3748/wjg.v16.i4.418.
- Zee JAVD, Eijck CHV, Hop WC, Biermann K, Dicheva BM, Seynhaeve AL, Koning GA, Am E, Tlt H. 2012. Tumour basement membrane laminin expression predicts outcome following curative resection of pancreatic head cancer. Br J Cancer. 107:1153–1158. doi:10.1038/bjc.2012.373.
- Heiden MGV, Lc C, Cb T. 2009. Understanding the Warburg Effect: the metabolic requirements of cell proliferation. Science. 324:1029. doi:10.1126/science.1160809.
- Jiang Y, Li X, Yang W, Hawke DH, Zheng Y, Xia Y, Aldape K, Wei C, Guo F, Chen Y. 2014. PKM2 regulates chromosome segregation and mitosis progression of tumor cells. Mol Cell. 53:75–87. doi:10.1016/j.molcel.2013.11.001.
- Iansante V, Choy PM, Fung SW, Liu Y, Chai JG, Dyson J, Rio AD, D’Santos C, Williams R, Chokshi S. 2015. PARP14 promotes the Warburg effect in hepatocellular carcinoma by inhibiting JNK1-dependent PKM2 phosphorylation and activation. Nat Commun. 6:7882. doi:10.1038/ncomms8882.
- Zheng Q, Lin Z, Xu J, Lu Y, Meng Q, Wang C, Yang Y, Xin X, Li X, Pu H, et al. 2018. Long noncoding RNA MEG3 suppresses liver cancer cells growth through inhibiting β-catenin by activating PKM2 and inactivating PTEN. Cell Death Dis. 9:253. doi:10.1038/s41419-018-0305-7.
- Yang L, Hou Y, Yuan J, Tang S, Zhang H, Zhu Q, Du Y, Zhou M, Wen S, Xu L, et al. 2015. Twist promotes reprogramming of glucose metabolism in breast cancer cells through PI3K/AKT and p53 signaling pathways. Oncotarget. 6:25755–25769. doi:10.18632/oncotarget.4697.
- Kong L, Schäfer G, Bu H, Zhang Y, Zhang Y, Klocker H. Lamin A/C protein is overexpressed in tissue-invading prostate cancer and promotes prostate cancer cell growth, migration and invasion through the PI3K/AKT/PTEN pathway. Eur Urol Suppl. 11;2012:751.
- Tang Y, Lv P, Sun Z, Han L, Zhou W. 2016. 14-3-3β promotes migration and invasion of human hepatocellular carcinoma cells by modulating expression of MMP2 and MMP9 through PI3K/Akt/NF-κB pathway. PLoS One. 11:e0146070. doi:10.1371/journal.pone.0146070.
