ABSTRACT
Cancer is a severe lethal disease. Currently, immunotherapy has become an effective alternative therapeutic approach for cancers. Cytokine-induced killer (CIK) cells have a higher proliferation rate, increased efficacy with few side-effects, and non-MHC-restricted killing after co-culturing with dendritic cells (DCs). Therefore, it has been widely studied and applied in the treatment of cancers. In our study, we explored the antitumor effects of CIK cells co-culturing with DCs pulsed with non-cell derived targeting peptides, which could specifically bind to certain tumor cells. Our results indicated that targeting peptide-loaded DCs could enhance the differentiation and cytotoxicity of CIK cells. Moreover, CIK cells, which were treated with specific targeting peptide-loaded DCs, could effectively and specifically kill tumor cells in vitro and in vivo, as long as tumor cells were pre-coated with the specific binding peptides. In conclusion, targeting peptides could guide DC-CIK to effectively and specifically kill tumor cells which were pre-coated with these targeting peptides and non-cell derived targeting peptide-loaded-DC-CIK may work as a novel means for cancer therapy.
Introduction
Cancer is one of the major causes of death around the world. Although the death rates of cancer over two decades has continuously declined, the overall survival rate remains particularly low.Citation1 Most patients with advanced local invasion and/or long-distance metastasis cannot be cured by conventional treatments such as surgery, radiation, or chemotherapy. Meanwhile, most of these cancer therapies are associated with adverse events. Therefore, it is necessary to develop new and promising therapeutic approaches to improve treatment efficacy and reduce patients suffering. Immunotherapy has emerged as the fourth most important treatment modality among the available therapeutic regimens for malignant tumors recently.Citation2,Citation3 One kind of the immunotherapies, the adoptive cellular immunotherapy of dendritic cells co-culturing with cytokine-induced killer cells (DC-CIK), has been widely studied and applied as an important option for cancer treatment.Citation4,Citation5
CIK cells are generated ex vivo by inducing peripheral blood mononuclear cells with the specific media formulations and have a marked ability to proliferate abundantly in vitro.Citation6 Moreover, CIK cells are a group of heterogeneous cells consisting of CD3+CD8+, CD3+CD56+ cells. CIK cells, with a characteristic CD3+CD56+ phenotype, have both the powerful antitumor ability of T lymphocytes and the non-major histocompatibility complex (MHC)-restricted cytotoxicity of NK cells. Therefore, CIK cells have a cytotoxic effect on various tumor cells including multidrug-resistant tumor cells.Citation7–Citation11 The lytic activity of CIK cells against tumor cells is mainly attributed to the high expression of the CD3+CD56+. Recently, studies have shown that CIK cells co-culturing with DCs (DC-CIK) can lead to a better antitumor activity on all sorts of cancers.Citation12–Citation15
The cytotoxic effect of CIK against various tumor cells is mediated by perforin and FasL. However, due to the lack of tumor specificity, CIK has relatively low cytotoxicity on some tumors.Citation16,Citation17 DCs, as the most potent antigen-presenting cells (APCs), have been generally used to overcome this hurdle. DCs are the major APCs that not only can capture and process antigens including tumor-associated antigens but also directly trigger natural killer (NK) cells function and activate antigen-specific cytotoxic T lymphocyte (CTL) responses against cancer, including the secretion of cytokines to initiate the cellular and humoral immune responses. CIK cells which are co-culturing with DCs can lead to a higher potential to prevent tumor growth and recurrence in cancer patients than CIK cells alone.Citation18–Citation20
It is one of the most effective strategies for tumor immunotherapy that antigenic peptides are applied to pulse DCs and make DCs more powerful to activate CIK cells (or T cells) specifically.Citation21–Citation23 Antigenic peptides, coming from some malignant tumor-specific antigens, are captured by DCs, and are bound with the MHC molecules on the surface of DCs through antigen processing. DCs loaded by antigenic peptides have the stronger activation effect to CIK cells, which directly kill malignant tumor cells with the overexpressed associated-specific antigens.
In our study, the non-cell derived targeting peptides would be applied to mimic antigenic peptides and the specific cytotoxicity of targeting peptides-DC-CIK on tumor cells pre-coated with targeting peptides would be verified. A1, the specific binding peptide for lung cancer cell line A549, and HCBP1, the specific binding peptide for H460 tumor sphere cells, were used to pulse DCs and the targeting peptide-loaded DCs were co-culturing with CIK cells followed in this report.Citation24,Citation25 As a result, we verified that targeting peptides could guide the specific cytotoxic effect on tumor cells through DC-CIK system and deduced that targeting peptides could mimic antigenic peptides and activate the specific DC-CIK cytotoxicity effect. Our discovery may provide great inspiration in cancer treatment.
Results
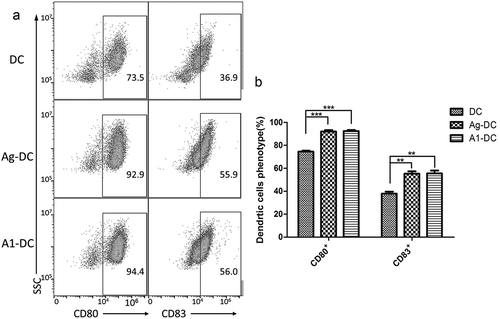
A1 targeting peptide promoted the differentiation of DCs
The phenotypes of DCs were analyzed by identifying the expression of differentiation clusters (CD80, CD83) on the cell membrane using flow cytometry (FCM). To examine whether A1 targeting peptide promoted DCs differentiation and maturation in vitro, A1 targeting peptide was added to the DCs culture media from day 3. The MDA-MB-231 tumor cells lysates (Ag MAD-MB-231), used as the reference antigens, were also added to the DCs culture media from day 3. On day 7, A1-DC, Ag MDA-MB-231-DC, and DC cells were harvested and the expression of CD80 and CD83 were analyzed by FCM. The results showed that the A1-DC cells expressed higher levels of CD80 and CD83 compared with DC (P < 0.01) (). In brief, it can be concluded that A1 targeting peptides could increase the expression of the differentiation clusters that act as co-stimulatory molecules to promote DCs differentiation.
Figure 1. A1 peptide promoted the differentiation of DCs. (a). FCM analysis of CD80 and CD83 expression after 7 days in culture on DC, Ag-DC cells, A1-DC cells. (b). The quantification of CD80 and CD83 expression from the FCM data was given as mean ± SEM from three independent experiments. **P < 0.01, ***P < 0.001.
A1 targeting peptide-treated DCs could enhance the differentiation and cytotoxicity of CIK cells
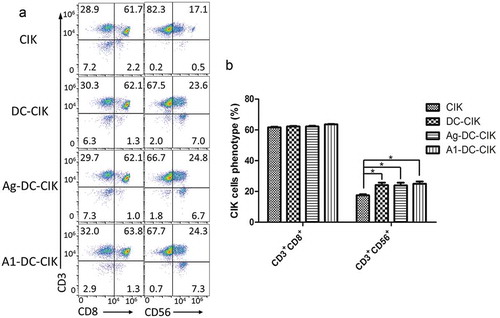
The CIK cells are a group of heterogeneous cells. Therefore, the phenotypes of CIK cells were analyzed by referring to the proportions of cells expressing CD3+CD56+ and CD3+CD8+ in the cell population. Moreover, the higher the CD3+CD56+ were expressed, the stronger the cytotoxicity of CIK cells was on tumor cells.
The expressions of CD3+CD8+ and CD3+CD56+ in CIK, DC-CIK, Ag MDA-MB-231-DC-CIK, and A1-DC-CIK cells were analyzed by FCM. The proportion of CD3+CD56+ cells increased when the CIK cells were co-cultured with DCs, Ag MDA-MB-231-DC or A1-DC cells (P < 0.05) (). There were no obvious differences for the population of CD3+CD8+ cells in the groups of CIK, DC-CIK, Ag MDA-MB-231-DC-CIK or A1-DC-CIK, which all expressed a higher level of CD3+CD8+ (P > 0.05) (). Overall, our results showed that A1-DC cells could enhance the differentiation and cytotoxicity of CIK cells.
Figure 2. A1 peptide-treated DCs could enhance the differentiation and cytotoxicity of CIK cells. (a). CIK cells cultured on 14 day were collected and incubated with CD3-FITC, CD8-APC and CD56-PE antibodies. The phenotypes of these cells were analyzed by FCM as described in Materials and methods. The figure showed the FCM data for the CD3+CD8+ and CD3+CD56+ expression after 14 days in culture on CIK, DC-CIK, Ag-DC-CIK and A1-DC-CIK. (b). The quantification of CD3+CD8+ and CD3+CD56+ expression from the FCM data was given as mean ± SEM from three independent experiments. *P < 0.05.
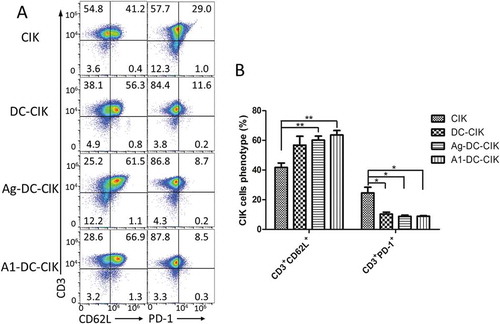
Additionally, the memory and suppression features of cells were also examined through analyzing the expressions of CD3+CD62L+ and CD3+PD-1+ in CIK, DC-CIK, Ag MDA-MB-231-DC-CIK, and A1-DC-CIK cells. The results showed that there were 66.9% CD3+CD62L+ cells in the A1-DC-CIK group, compared with 41.2% in CIK group, 56.3% in DC-CIK group and 61.5% in Ag MDA-MB-231-DC-CIK group. For the CD3+PD-1+ cells, the ratio is 8.5% in A1-DC-CIK group, which was the lowest among all groups ().
Figure 3. Co-culturing of A1 peptide-treated-DC with CIK cells induced the production of memory T lymphocytes and down-regulation of co-inhibitory molecules. A. FCM analysis of CD62L and PD-1 expression after 14 days culture. B. The quantification of CD62L and PD-1 expression. Data were given as mean ± SEM from three independent experiments. *P<0.05, **P<0.01.
A1 targeting peptide-treated-DC-CIK cells had specific cytotoxicity on A549 cells coated with A1 targeting peptides in vitro
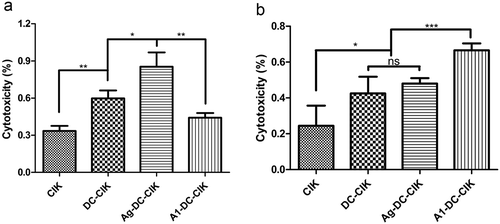
The specific cytotoxicity of A1-DC-CIK to A549 cells pre-coated with A1 targeting peptides was examined with CCK-8 kit. As shown in , effector cells Ag MDA-MB-231-DC-CIK cells exhibited the highest cytotoxic activity on MDA-MB-231 cells compared with the other groups (P < 0.05). Meanwhile, the cytotoxicity of A1-DC-CIK cells on A549 cells coated with A1 targeting peptides was the highest compared with that of the other cells (). These results showed that Ag MDA-MB-231-DC-CIK and A1-DC-CIK cells did have a specific cytotoxic effect on tumor cells, either which the tumor lysates came from or which the binding peptides were coated on.
Figure 4. A1 peptide-treated-DC-CIK cells exhibited specific cytotoxicity on A549 cells coated with A1 peptides in vitro.CIK, DC-CIK, Ag-DC-CIK or A1-DC-CIK effector cells were cultured for 14 days. The resulting effector cell populations were used to examine cytotoxicity on MDA-MB-231 and A549 cells. The target cells were incubated with A1 polypeptide (50 μg/mL) for an hour before mixed with the effector cells. The cytotoxicity of the effector cells against target cells was examined with CCK8 kit. (a). MDA-MB-231 cells were incubated with effector cells for 24 hour at effector-to-target (E: T) ratios of 20:1. (b). A549 cells were incubated for 24 hour with effector cells at E: T ratios of 20:1. Data were given as mean ± SEM from three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001.
A1 targeting peptide-treated-DC-CIK cells could inhibit the tumor growth in xenografts mouse models
The in vivo specific cytotoxic effect of A1-DC-CIK cells was evaluated in the xenograft mouse model. The A549-luc cells were injected into the mice subcutaneously to develop the xenograft mouse model. A week later, the different effector cells were intravenously injected into the tail of the mice in the corresponding group. One hour before the injection of effector cells, A1 targeting peptides were injected into the tumor mass of mice in each group.
The tumor volumes and the average radiance (p/s/cm2/str) of mice in each group were recorded. The results from the ROI analysis of tumor bioluminescence signals exhibited that the A1-DC-CIK cells obviously retarded the tumor growth. The Ag MDA-MB-231-DC-CIK and DC-CIK cells did not present obvious cytotoxic effect on tumors compared with CIK cells only ( and )). Data from vernier calipers measurement displayed the same tendency as those from the average radiance on the assessment of tumor volume alteration ().
Figure 5. A1 peptide-treated-DC-CIK cells could inhibit the tumor growth in a xenografts mouse models. (a). Representatives bioluminescence images of tumor-bearing mice in each group. (b). Three weeks after cell therapy, the record of bioluminescent signal changes of tumor mass for each group were compared. *P < 0.05. (c). The record of tumor volume changes of each group treated mice. *P < 0.05. (d). IFN-γ and GZMB immune staining images of tumor tissues from A1-DC-CIK, Ag-DC-CIK, DC-CIK and PBS groups. E. Immunohistochemical quantitative analysis of IFN-γand GZMB in D. Data were given as mean ± SEM from three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001.
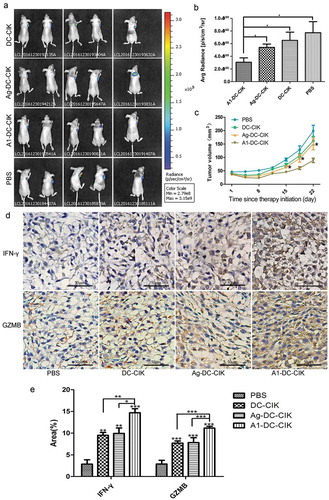
To further verify that the inhibitory effects on tumor growth were mediated by T cells, the expression of interferon-γ (IFN-γ) and Granzyme B (GZMB) in tumor tissues was examined. As we know, activated T cells would secrete more cytokines, such as IFN-γ and GZMB to the TME to initiate the killing of tumor cells.Citation25,Citation26 The results from IHC () showed that the expression of IFN-γ and GZMB increased significantly in the A1-DC-CIK cells group compared with those in Ag MDA-MB-231-DC-CIK, DC-CIK and CIK cells groups, which could explain why A1-DC-CIK cells could kill tumor cells more efficiently.
HCBP1 targeting peptide-treated-DC-CIK cells exhibited specific cytotoxicity on H460 sphere cells coated with HCBP1 targeting peptides in vitro
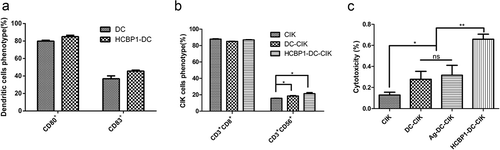
To verify whether other cell-targeting peptides could guide the specific cytotoxicity effect on tumor cells through DC-CIK system as well, HCBP1, which could specifically bind with H460 sphere cells, was applied to repeat some of the experiments mentioned above. As shown in , both HCBP1-DC cells and DC cells expressed higher levels of CD80 and CD83 in cytokine enriched media. The proportion of CD3+CD56+ cells increased after CIK cells were co-cultured with DCs or HCBP1-DC cells, indicating that HCBP1-DC cells could enhance the differentiation and cytotoxicity of CIK cells (). The specific cytotoxicity effect of HCBP1-DC-CIK cells on tumor cells coated with HCBP1 targeting peptides was evaluated. The ratios of dead cells to the whole population of H460 sphere cells were 65.82 ± 2.77% in the HCBP1-DC-CIK cells, 31.68 ± 5.41% in the Ag MDA-MB-231-DC-CIK cells, 27.76 ± 4.38% in the DC-CIK cells and 12.80 ± 1.55% in the CIK cells, respectively (). Therefore, the approach that targeting peptides could guide the specific cytotoxicity effect on tumor cells through DC-CIK system was proven to be effective for the tumor treatment.
Figure 6. HCBP1 peptide-treated-DC-CIK cells had specific cytotoxicity on H460 sphere cells coated with HCBP1 peptides in vitro. (a). The quantification of CD80 and CD83 expression of DCs, HCBP1-DC cells for 7 days in culture is given as mean ± SEM from three independent experiments. (b). The quantification of CD3+CD8+ and CD3+CD56+ expression of CIK, DC-CIK and HCBP1-DC-CIK for 14 days in culture are given as mean ± SEM from three independent experiments. *P < 0.05. C. H460 sphere cells were incubated with CIK, DC-CIK, Ag-DC-CIK or HCBP1-DC-CIK cells for 24 h at E: T ratios of 20:1. The target cells were incubated with HCBP1 polypeptide (50 μg/mL) for an hour before mixed with the effector cells. Data were given as mean ± SEM from three independent experiments. *P < 0.05, **P < 0.01.
Discussion
Currently, immunotherapy has become an effective and promising alternative therapeutic approach for cancers.Citation27–Citation29 As an emerging adoptive immunotherapy, DC-CIK cells immunotherapy has become an important adjunctive therapeutic approach for the traditional treatments to cancers such as surgery, radiotherapy, and chemotherapy. It has been reported that tumor peptides or lysates of tumor cell are able to pulse DCs, and the anti-tumor activity of CIK is enhanced by co-culturing with the DCs.Citation30–Citation33 In our study, non-cell derived targeting peptides were used to pulse DCs. Targeting peptide-pulsed DCs were co-culturing with CIK cells and the cytotoxic effect of targeting peptide-treated-DC-CIK cells on tumor cells which had been coated with targeting peptides was explored. The results showed that the targeting peptides pulsed DCs could promote the proliferation and differentiation of CIK cells in vitro. Furthermore, the specific cytotoxicity on tumor cells pre-coated with targeting peptides by the targeting peptide-treated-DC-CIK cells was verified by in vitro and in vivo experiments. All the data indicated that the application of targeting peptide-treated-DC-CIK cells may be a potentially effective means for the treatment of cancers.
DCs are the most potent antigen-presenting cells. The antigen-presenting ability of DCs is hundreds of times more efficient than other antigen-presenting cells, such as B lymphocytes and macrophages.Citation34,Citation35 Tumor antigens or antigenic peptides from tumor lysates, which are captured, processed and presented by DCs, can boost the expression of differentiation clusters, such as CD80, CD83, which act as co-stimulatory molecules to promote the differentiation of DCs. Antigenic peptide, a peptide from some malignant tumor-specific antigens, can identify the desired epitope through process and presentation of DCs. DCs loaded by antigenic peptides have the stronger activation effect to CTLs, which could directly kill malignant tumor cells expressing the associated-specific antigens.Citation36 However, the immunotherapy to cancers guided by tumor lysates can induce many severe side effects such as cytokine release syndrome, macrophage activation syndrome,tumor lysis syndrome, neurotoxicity, which impacts its therapeutic effect and limits its widespread application.Citation37–Citation39 To balance the pros and cons effect of cell lysates and peptides on the tumor therapy, we believe that the peptide may be a better candidate to guide CIK for tumor therapy effectively. In our study, A1 targeting peptides or HCBP1 targeting peptides were specific binding peptides for cancer cells.Citation24,Citation25 The targeting peptide-treated-DC cells expressed higher levels of CD80 compared with the ones cultured in cytokine enriched media alone, which meant that targeting peptide might mimic the antigenic peptides and could be captured, processed and presented by DCs.
Targeting peptide-pulsed DCs co-culturing with CIK cells led to a significant increase in the proliferation and cytotoxic activity of CIK cells in vitro. Many cytokines, for example, IL-6, IL-10 or IL-12, are secreted from DCs or CIK cells after these two kinds of cells interact with each other.Citation31 The cytotoxic effect of CIK cells due to secretion of cytokines is non-MHC restrictive. Besides this, antigen-pulsed DC-activated CIK cells can trigger MHC restrictive cytotoxicity to enhance the specific cytotoxic effect.Citation31 Therefore, targeting peptide-pulsed-DC-CIK cells could specifically kill tumor cell pre-coated with targeting peptides. Furthermore, in our study, we found the expression level of CD62L got increased and PD-1 got decreased on CD3+ T cells in DC-CIK, Ag MDA-MB-231-DC-CIK, and A1-DC-C-CIK group, which meant that DC-activated CIK cells might gain the memory property to inhibit the regrowth of cancers and evade from the inhibitory effect from the tumor microenvironment. For the future application of targeting peptides to DC-CIK immunotherapy on cancers in a clinic, our study leaves an open question how to deliver targeting peptides to tumor loci and allow peptides to coat on the surface of tumor cells before activated CIK cells were administrated.
To summarize our study, we established a system in which targeting peptide-treated-DC-CIK cells were applied to specifically and efficiently kill tumor cells pre-coated with targeting peptides. Therefore, targeting peptide-treated-DC-CIK based immunotherapies may be a potentially effective treatment for cancers.
Materials and methods
Cell culture
Human non-small lung cancer cell line A549 and human breast cancer cell line MDA-MB-231 were purchased from the Chinese Academy of Sciences Cell Bank (Shanghai, China). A549 cells were cultured in RPMI-1640 (Hyclone, Utah, USA) supplemented with 10% FBS (Hyclone) and 1% penicillin-streptomycin solution (Hyclone). MDA-MB-231 cells were cultured in DMEM (Hyclone) supplemented with 10% FBS and 1% penicillin-streptomycin solution (Hyclone). H460 spheres were obtained through the method published beforeCitation24 and were cultured in the serum-free DMEM: Nutrient Mixture F-12 (DMEM-F12)(Hyclone) supplemented with 20 ng/ml basic fibroblast growth factor (PeproTech, NJ, USA), 20 ng/ml epidermal growth factor (PeproTech) and 2% B27 (Gibco, Grand Island, NY, USA). Adherent cells were harvested when they were subconfluent and H460 spheres were harvested every 6 days. All the cells were cultured at 37°C, in 5% CO2.
All anti-human CD3-FITC, CD3-PE, CD8-APC, CD56-PE, CD62L-APC, PD-1-FITC, CD80-FITC, CD83-APC antibodies were obtained from BD Bioscience (San Jose, CA, USA). Cytokines recombinant human interleukin-2 (IL-2), recombinant human interleukin-4 (IL-4), recombinant human interleukin-15 (IL-15), recombinant human interferon-γ (IFN-γ), recombinant human Granzyme B(GZMB), recombinant mutant human tumor necrosis factor-α (TNF-α), recombinant human granulocyte/macrophage colony-stimulating factor (GM-CSF) were purchased from Peprotech (Rocky Hill, NJ, USA). Anti-Human CD3 functional grade purified antibody was obtained from eBioscience (San Diego, CA, USA). The cell proliferation assay kit (Cell Counting Kit-8) was purchased from Dojindo Molecular Technologies (Rockville, MD, USA). The A1 peptide (WFCSWYGGDTCVQ) and HCBP1 peptide (GGLGCFPEGEMACWWSGGSGK) used in our paper were synthesized by Shanghai Biotech bioscience and technology Co., Ltd. (Shanghai, China).
Generation of dendritic cells and CIK cells
The local Bioethics Committee has approved the study protocol to draw blood from healthy volunteers after written informed consent for the purpose of generating DCs and CIK cells against lung adenocarcinoma. According to standard procedures, peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll/Hypaque density gradient centrifugation. PBMCs were cultured at a density of 3 × 106 cells/ml in RPMI-1640 complete culture medium (RPMI-1640 medium supplemented with 10% fetal bovine serum, 1% penicillin-streptomycin, 2 mM L-glutamine and 50 μM 2-mercaptoethanol) for 2 h at 37°C in a humidified 5% CO2 incubator. The adherent PBMCs were cultured in RPMI-1640 complete culture medium with 1000 U/ml GM-CSF and 500 U/ml IL-4 to generate DCs. Subsequently, the medium was replaced every 3 days. On day 6, 1000 U/ml of TNF-α was added to the media to promote the maturation of DCs. To generate CIK cells, the non-adherent PBMCs were cultured in RPMI-1640 complete culture medium containing 1000 U/ml IFN-γ on the initial day. After 24 h of incubation, 200 ng/ml CD3 monoclonal antibody and 1000 U/ml IL-2 were added. Fresh medium with IL-2 and 50 ng/mL IL-15 were replenished every 3 days. From day 7 in culture, CIK cells were sub-cultured in fresh RPMI-1640 complete culture medium containing 50 ng/mL IL-15 and 1000 U/ml IL-2 every 2 days.
Tumor lysate (Ag MDA-MB-231) preparation
MDA-MB-231 tumor lysates were obtained by three rapid freeze-thaw cycles. Specifically, confluent cultures of MDA-MB-231 were digested with 0.25% pancreatic enzyme for 3 min, washed with PBS and resuspended in PBS at a density of 1.0 × 107 cells/ml. The cells in suspension were frozen in liquid nitrogen and lysed by three freeze-thaw cycles. Then, the cell lysis was centrifuged for 10 min at 3000 rpm. The supernatant was collected and sterilized through a 0.22 μm filter membrane. The protein concentration was calculated by the BCA method. The whole-cell lysate was named as Ag MDA-MB-231 and stored at −80°C.
Tumor lysates or peptide pulsing DCs
Subsequent to 3 days of culture, the DCs were divided into three groups. One group was termed the A1 (HCBP1)-DC group, comprising cells supplemented with the targeting peptides at a final concentration of 30 μg/ml. The second group was termed the AgMDA-MB-231-DC group, consisting of cells supplemented with the MDA-MB-231 cell lysates, as aforementioned, at a final concentration of 200 μg/ml. The last group was termed the DC group, which comprised cells without the antigen. The three cell groups were incubated at 37°C in a 5% CO2 atmosphere.
CIK cells and DCs co-culturing
On day 7, CIK cells were co-cultured with autologous 7-day DCs, as aforementioned, at a cell ratio of 10:1. All the groups were cultured at 37°C in a humidified 5% CO2 atmosphere and sub-cultured every 2 days in RPMI-1640 complete culture medium with 1000 U/ml IL-2 and 50 ng/mL IL-15.
Flow cytometry
The DCs cultured for 7 days and CIK cells for 14 days were collected, respectively. After being washed in PBS and resuspended at 1 × 106 cells per 100 μL, anti-human CD80-FITC, CD83-APC antibodies were added to the DCs; anti-human CD3-FITC, CD3-PE, CD8-APC, CD56-PE, CD62L-APC, and PD-1-FITC antibodies were added to the CIK cells. A mouse IgG (PE/FITC/APC) (BD) was used as a negative control in all the assays. After incubation for 30 min at 4°C, cells were washed twice in PBS and analyzed by flow cytometry on an AccuriTM C6 (BD Biosciences, USA) by the manufacturer’s recommendations.
In vitro cytotoxicity assay
The in vitro cytotoxicity of the A1-DC-CIK, HCBP1-DC-CIK, Ag MDA-MB-231-DC-CIK, DC-CIK and CIK cells against target cells was detected by a CCK8 kit. The A1-DC-CIK, HCBP1-DC-CIK, Ag MDA-MB-231-DC-CIK, DC-CIK, and CIK cells were harvested on day 13 after induction and were used as effector cells. The human breast cancer cell line MDA-MB-231, human non-small lung cancer cell line A549 and H460 spheres cells were used as target cells. The effector and target cells were incubated in U-bottomed 96-well plates at a ratio of 20:1 for 24 h at 37°C in a 5% CO2 atmosphere. The groups that contained effector and target cells were the experimental groups, while the control groups comprised only effector or target cells or 1640-RPMI culture medium. The target cells were incubated with A1 or HCBP1 targeting peptide (50 μg/mL) for an hour before mixing with the effector cells. The CCK8 assay was carried out to detect cell viability, and optical density (OD) was read at 450 nm. The assays were carried in triplicate and the cytotoxic activity was calculated as follows: Cytotoxic activity, % = [1 – (OD experimental group – OD corresponding effector cells)/(OD target cells – OD culture medium] × 100%.
In vivo cytotoxicity assay
The human non-small lung cancer cell line A549 were transfected with the pLVX-puro/luciferase plasmid, followed by the selection with puromycin (1 μg/mL), and clones with stable expression were established through the monoclonal cultivation. Female BALB/c nude mice at the age of five weeks (SLAC Laboratory Animal) were used in the experiment. The luciferase-expressing A549 cells (A549-luc) in 0.1 mL PBS with 2 × 106 tumor cells were injected subcutaneously into the backs of the mice. One week later, the BALB/c mice were randomly divided into four groups, the A1-DC-CIK, Ag MDA-MB-231-DC-CIK, DC-CIK experimental groups, and the PBS control group. And 6 × 106 A1-DC-CIK, Ag MDA-MB-231-DC-CIK, DC-CIK cells in 0.2 ml PBS, or 0.2 ml PBS, were intravenously injected into the tail of the mice in the corresponding group every three days for 3 times. One hour before the injection of effector cells, A1 targeting peptide was injected into the tumor in each group. The tumor volumes and the weights of mice were measured every 3 ~ 4 days. Tumor volume = 1/2 × a × b2 (a is the length, b is the width). The mice were photographed in an IVIS Lumina II system (PerkinElmer) and ROIs of bioluminescence were analyzed to evaluate tumor growth every week. All the mice were killed on day 45 and the tumor mass was collected. The expression of IFN-γ and GZMB in the tumor mass was determined by immunohistochemistry (IHC). All animal studies were approved by a local Ethics Committee for Animal Experiments.
Statistical analysis
All data were analyzed with GraphPad Prism 5 statistical software. The values were expressed as means ± SEM. Student’s t-tests and ANOVA were used for statistical analysis. P < 0.05 were considered to be significant. The results of flow cytometry were analyzed by FlowJo 10.
Disclosure of potential conflicts of interest
The authors declare no conflict of interest.
Acknowledgments
This work was funded by the National Natural Science Foundation of China (No. 81372361, 81602552), Natural Science Foundation of Jiangsu province (No. BE2015647), Science and Technology Bureau of Suzhou (No.SS201649).
Additional information
Funding
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018; 68:7–30. doi: 10.3322/caac.21442.
- Hontscha C, Borck Y, Zhou H, Messmer D, Schmidtwolf IG. Clinical trials on CIK cells: first report of the international registry on CIK cells (IRCC). J Cancer Res Clin Oncol. 2011;137:305–310. doi:10.1007/s00432-010-0887-7.
- Zhan HL, Gao X, Pu XY, Li W, Li ZJ, Zhou XF, Qiu JG. A randomized controlled trial of postoperative tumor lysate-pulsed dendritic cells and cytokine-induced killer cells immunotherapy in patients with localized and locally advanced renal cell carcinoma. Chin Med J. 2012;125:3771–3777. doi:10.3760/cma.j.issn.0366-6999.2012.21.004.
- Anichini A, Tassi E, Grazia G, Mortarini R. The non-small cell lung cancer immune landscape: emerging complexity, prognostic relevance and prospective significance in the context of immunotherapy. Cancer Immunol Immunother. 2018;67:1011–1022. doi:10.1007/s00262-018-2147-7.
- Introna M, Correnti F. Innovative clinical perspectives for CIK cells in cancer patients. Int J Mol Sci. 2018:19. doi:10.3390/ijms19020358.
- Rutella S, Iudicone P, Bonanno G, Fioravanti D, Procoli A, Lavorino C, Foddai ML, Lorusso D, Martinelli E, Vacca M. Adoptive immunotherapy with cytokine-induced killer cells generated with a new good manufacturing practice-grade protocol. Cytotherapy. 2012;14:841–850. doi:10.3109/14653249.2012.681038.
- Chu H, Du F, Jiang L, Wang Z, Gong Z, Lian P, Li P, Chen J. The efficacy of CIK-based immunotherapies for advanced solid tumors. Technol Cancer Res Treat. 2017;16:577–585. doi:10.1177/1533034616659163.
- Meng Y, Yu Z, Wu Y, Du T, Chen S, Meng F, Su N, Ma Y, Li X, Sun S, et al. Cell-based immunotherapy with cytokine-induced killer (CIK) cells: from preparation and testing to clinical application. Hum Vaccin Immunother. 2017;13:1–9. doi:10.1080/21645515.2017.1285987.
- Mesiano G, Todorovic M, Gammaitoni L, Leuci V, Giraudo Diego L, Carnevale-Schianca F, Fagioli F, Piacibello W, Aglietta M, Sangiolo D. Cytokine-induced killer (CIK) cells as feasible and effective adoptive immunotherapy for the treatment of solid tumors. Expert Opin Biol Ther. 2012;12:673–684. doi:10.1517/14712598.2012.675323.
- Shi L, Zhou Q, Wu J, Ji M, Li G, Jiang J, Wu C. Efficacy of adjuvant immunotherapy with cytokine-induced killer cells in patients with locally advanced gastric cancer. Cancer Immunol Immunother. 2012;61:2251–2259. doi:10.1007/s00262-012-1289-2.
- Zhang J, Zhu L, Wei J, Liu L, Yin Y, Gu Y, Shu Y. The effects of cytokine-induced killer cells for the treatment of patients with solid tumors: a clinical retrospective study. J Cancer Res Clin Oncol. 2012;138:1057–1062. doi:10.1007/s00432-012-1179-1.
- He L, Wang J, Chang D, Lv D, Li H, Zhang H. Clinical value of Pro-GRP and T lymphocyte subpopulation for the assessment of immune functions of lung cancer patients after DC-CIK biological therapy. Exp Ther Med. 2018;15:1580–1585. doi:10.3892/etm.2017.5520.
- Song H, Liu S, Zhao Z, Sun W, Wei X, Ma X, Zhao P, Gao D. Increased cycles of DC/CIK immunotherapy decreases frequency of Tregs in patients with resected NSCLC. Int Immunopharmacol. 2017;52:197–202. doi:10.1016/j.intimp.2017.09.014.
- Zhang J, Li H, Gao D, Zhang B, Zheng M, Lun M, Wei M, Duan R, Guo M, Hua J, et al. A prognosis and impact factor analysis of DC-CIK cell therapy for patients with hepatocellular carcinoma undergoing postoperative TACE. Cancer Biol Ther. 2018:1–9. doi:10.1080/15384047.2018.1433501.
- Zhang X, Du M, Zhang Q, Chen H, Zhao L, Li H, Guo W. In vivo anti-tumor effect of DC-CIK cells on human lymphoma cell line Raji. Pak J Pharm Sci. 2017;30:1075–1079.
- Qiu Y, Yun MM, Dong X, Xu M, Zhao R, Han X, Zhou E, Yun F, Su W, Liu C. Combination of cytokine-induced killer and dendritic cells pulsed with antigenic α-1,3-galactosyl epitope-enhanced lymphoma cell membrane for effective B-cell lymphoma immunotherapy. Cytotherapy. 2015;18:91. doi:10.1016/j.jcyt.2015.09.012.
- Zou Y, Li F, Hou W, Sampath P, Zhang Y, Thorne SH. Manipulating the expression of chemokine receptors enhances delivery and activity of cytokine-induced killer cells. Br J Cancer. 2014;110:1992. doi:10.1038/bjc.2014.140.
- Li QY, Shi Y, Huang DH, Yang T, Wang JH, Yan GH, Wang HY, Tang XJ, Xiao CY, Zhang WJ, et al. Cytokine-induced killer cells combined with dendritic cells inhibited liver cancer cells. Int J Clin Exp Med. 2015;8:5601–5610.
- Wang Y, Xu Z, Zhou F, Sun Y, Chen J, Li L, Jin H, Qian Q. The combination of dendritic cells-cytotoxic T lymphocytes/cytokine-induced killer (DC-CTL/CIK) therapy exerts immune and clinical responses in patients with malignant tumors. Exp Hematol Oncol. 2015;4:32. doi:10.1186/s40164-015-0027-9.
- Zhao X, Ji C-Y, Liu G-Q, Ma D-X, Ding H-F, Xu M, Xing J. Immunomodulatory effect of DC/CIK combined with chemotherapy in multiple myeloma and the clinical efficacy. Int J Clin Exp Pathol. 2015;8:13146.
- Liu Y, Zheng Z, Zhang Q, Zhou X, Feng Y, Yan A. FOLFOX regimen plus dendritic cells-cytokine-induced killer cells immunotherapy for the treatment of colorectal cancer: a meta-analysis. Onco Targets Ther. 2017;10:2621–2633. doi:10.2147/ott.s138011.
- Sun WW, Dou JX, Zhang L, Qiao LK, Shen N, Zhao Q, Gao WY. Killing effects of Huaier Granule combined with DC-CIK on nude mice transplanted with colon carcinoma cell line. Oncotarget. 2017;8:46081–46089. doi:10.18632/oncotarget.17687.
- Yamanaka R, Homma J, Yajima N, Tsuchiya N, Sano M, Kobayashi T, Yoshida S, Abe T, Narita M, Takahashi M. Clinical evaluation of dendritic cell vaccination for patients with recurrent glioma: results of a clinical phase I/II trial. Clin Cancer Res. 2005;11:4160–4167. doi:10.1158/1078-0432.CCR-05-0120.
- Dong B, Wang A, Yuan L, Chen L, Pu K, Duan W, Yan X, Zhu Y. Peptide-fluorescent bacteria complex as luminescent reagents for cancer diagnosis. PLoS One. 2013;8:e54467. doi:10.1371/journal.pone.0054467.
- Wang A, Chen L, Pu K, Zhu Y. Identification of stem-like cells in non-small cell lung cancer cells with specific peptides. Cancer Lett. 2014;351:100–107. doi:10.1016/j.canlet.2014.05.004.
- Li C, Zhang N, Zhou J, Ding C, Jin Y, Cui X, Pu K, Zhu Y. Peptide blocking of PD-1/PD-L1 interaction for cancer immunotherapy. Cancer Immunol Res. 2018;6:178–188. doi:10.1158/2326-6066.cir-17-0035.
- Ott PA. Immunotherapy: immune-modified response criteria - an iterative learning process? Nat Rev Clin Oncol. 2018. doi:10.1038/nrclinonc.2018.36.
- Seya T, Takeda Y, Takashima K, Yoshida S, Azuma M, Matsumoto M. Adjuvant immunotherapy for cancer: both dendritic cell-priming and check-point inhibitor blockade are required for immunotherapy. Proc Jpn Acad Ser B Phys Biol Sci. 2018;94:153–160. doi:10.2183/pjab.94.011.
- Zhu J, Li R, Tiselius E, Roudi R, Teghararian O, Suo C, Song H. Immunotherapy (excluding checkpoint inhibitors) for stage I to III non-small cell lung cancer treated with surgery or radiotherapy with curative intent. Cochrane Database Syst Rev. 2017;12:Cd011300. doi:10.1002/14651858.CD011300.pub2.
- Cheng XY, Li JL. Biological activity of cytotoxic dendritic cells cocultured with cytokine-induced killer cells and their effect on acute leukemia cells. Genet Mol Res. 2015;14:13208–13214. doi:10.4238/2015.October.26.17.
- Li SJ, Zhang LS, Chai Y, Zhang YF, Zhang YM, Zeng PY, Wu CY. Killing activity of co-cultured cytokine-induced killer cells and dendritic cells against multi-drug resistant tumor cell lines. Zhonghua Zhong Liu Za Zhi. 2007;29:733–737.
- Liu Y, Liu H, Liu H, He P, Li J, Liu X, Chen L, Wang M, Xi J, Wang H, et al. Dendritic cell-activated cytokine-induced killer cell-mediated immunotherapy is safe and effective for cancer patients >65 years old. Oncol Lett. 2016;12:5205–5210. doi:10.3892/ol.2016.5337.
- Shan CC, Shi LR, Ding MQ, Zhu YB, Li XD, Xu B, Jiang JT, Wu CP. Cytokine-induced killer cells co-cultured with dendritic cells loaded with the protein lysate produced by radiofrequency ablation induce a specific antitumor response. Oncol Lett. 2015;9:1549–1556. doi:10.3892/ol.2015.2977.
- Clark GJ, Silveira PA, Hogarth PM, Hart DNJ. The cell surface phenotype of human dendritic cells. Semin Cell Dev Biol. 2018. doi:10.1016/j.semcdb.2018.02.013.
- Ivanov S, Merlin J, Lee MKS, Murphy AJ, Guinamard RR. Biology and function of adipose tissue macrophages, dendritic cells and B cells. Atherosclerosis. 2018;271:102–110. doi:10.1016/j.atherosclerosis.2018.01.018.
- Shimato S, Natsume A, Wakabayashi T, Tsujimura K, Nakahara N, Ishii J, Ito M, Akatsuka Y, Kuzushima K, Yoshida J. Identification of a human leukocyte antigen-A24–restricted T-cell epitope derived from interleukin-13 receptor α2 chain, a glioma-associated antigen. J Neurosurg. 2008;109:117–122. doi:10.3171/JNS/2008/109/7/0117.
- Ma Y, Xu Y-C, Tang L, Zhang Z, Wang J, Wang H-X. Cytokine-induced killer (CIK) cell therapy for patients with hepatocellular carcinoma: efficacy and safety. Exp Hematol Oncol. 2012;1:11. doi:10.1186/2162-3619-1-11.
- Ogba N, Arwood NM, Bartlett NL, Bloom M, Brown P, Brown C, Budde EL, Carlson R, Farnia S, Fry TJ, et al. Chimeric antigen receptor T-cell therapy. J Natl Compr Canc Netw. 2018;16:1093–1106. doi:10.6004/jnccn.2018.0073.
- Zou WP. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi:10.1038/nri1806.
