ABSTRACT
Background: Ovarian cancer (OC) is the gynecologic malignant tumor with high mortality. Accumulating evidence indicates that M2-like tumor-associated macrophages (TAMs) can secret EGF to participate in ovarian cancer growth, migration, and metastasis. An EGF-downregulated lncRNA, LIMT (lncRNA inhibiting metastasis), was identified as a critical regulator of mammary cell migration and invasion. Nevertheless, whether EGF secreted from M2-like TAMs regulates LIMT expression in ovarian cancer progression remains largely unknown.
Methods: The human OC cell lines OV90 and OVCA429 were recruited in this study. The differentiation of the human monocyte cell line THP-1 into M2-like TAMs was confirmed using flow cytometry within the application of phorbol 12-myristate 13-acetate (PMA). ELISA was performed to detect EGF concentration in co-culture system of M2-like TAMs and OC cell lines. Moreover, CCK-8, flow cytometry and immunofluorescence staining of Ki67 were performed to assess the capacity of cell proliferation. Besides, cell migration and invasion were determined by wound healing and transwell assays. Furthermore, the expression levels of epithelial-mesenchymal transition (EMT) markers and EGFR/ERK signals were analyzed by qRT-PCR and western blot. Female athymic nude mice (8–12 weeks of age; n = 8 for each group) were recruited for in vivo study.
Results: In the present study, THP-1 cells exhibited the phenotype markers of M2-like TAMs with low proportion of CD14+ marker and high proportion of CD68+, CD204+, CD206+ markers within the application of PMA. After co-culturing with M2-like TAMs, EGF concentration in the supernatants was significantly increased in a time-dependent manner. Besides, OC cells presented better cell viability, higher cell proliferation, and stronger migration and invasion. The expression of EMT-related markers N-cadherin, Vimentin and EGFR/ERK signals were markedly up-regulated, while E-cadherin was significantly decreased. However, these effects induced by co-culture system were reversed by the application of AG1478 (an EGFR inhibitor) or LIMT overexpression. Furthermore, the endogenous expression of LIMT was decreased in OC cell lines compared with the control group. Also, the in vivo experiments verified that the inhibition of EGFR signaling by AG1478 or overexpression of LIMT effectively repressed the tumor growth.
Conclusion: Taken together, we demonstrated that EGF secreted by M2-like TAMs might suppress LIMT expression via activating EGFR-ERK signaling pathway to promote the progression of OC.
Introduction
Ovarian cancer (OC) is the gynecologic malignant tumor with high mortality. Due to non-specific symptoms in the early stage of the disease and the lack of effective screening techniques, a large number of patients are diagnosed at advanced stages causing a poor prognosis, with a 5-year survival rate of only 29%.Citation1 At present, the main method for the treatment of epithelial ovarian cancer is still surgery and platinum-based chemotherapy. Despite continuous efforts to improve the disease diagnosis and treatment, there has been no significant improvement of OC patients’ poor prognosis due to intraperitoneal and extensive pelvic implantation metastasis.Citation2 Therefore, it is essential to elucidate the specific target and reveal the potential mechanism of OC metastasis, which is also vital for developing novel drugs and improving the survival rate for OC patients.
It has been widely recognized that the occurrence and development of ovarian cancer is a result of genetic alterations.Citation3,Citation4 However, recent studies pointed out that tumor microenvironment (TEM) also plays an important role in the progression of ovarian cancer.Citation5 Actually, tumor-associated macrophages (TAMs) are an essential component of TEM.Citation6 The precursor cells of macrophages are the mononuclear cells in systemic circulation which ooze out of blood vessels and reach various tissues of the body, ultimately differentiating into macrophages under the influence of local microenvironment. Under the stimulation of different factors, the tissue macrophages will be polarized into different phenotypes, forming a type of heterogeneous immune cells that exert immunostimulatory or immunosuppressive effects. TAMs refers to the mononuclear cells in systemic circulation that exude from the blood vessels and gradually form immature macrophages after capturing signal substances released by tumor cells, then enter the tumor tissue, and are further polarized into different phenotypes.Citation7 Macrophages can be broadly divided into two categories: (a) classical activated macrophages, i.e., M1 macrophages, which have the function of killing tumor cells and pathogenic microorganisms; and (b) alternatively activated macrophages, i.e., M2 macrophages, which promote tissue repair and blood vessel formation. The main difference between these two types of macrophages is the substances they secrete. M1 macrophages secrete reactive oxygen species, nitrogen intermediates, and various inflammatory factors, while M2 macrophages secrete a large number of growth factors (e.g., epidermal growth factor (EGF), fibroblast growth factor, and vascular endothelial growth factor).Citation8 M2 macrophages are generally the major phenotype of TAMs. In the microenvironment of multiple malignant tumors, TAMs play an immunosuppressive role and promote the infiltration, proliferation, angiogenesis, and metastasis of tumor cells, thereby closely associated with the prognosis of tumor patients.Citation6,Citation7 There are the phenomena of TAMs aggregation in ovarian cancer microenvironment and its number associated with the poor prognosis of ovarian cancer.Citation9,Citation10 However, the role of TAMs in the OC metastasis is not yet clear.
Long non-coding RNAs (lncRNAs) are the non-protein-coding RNAs, whose transcripts are longer than 200 nucleotides in length.Citation11 The mutations or expression abnormalities of lncRNAs are closely associated with several types of malignant tumors,Citation12,Citation13 in which they might significantly regulate tumor metastasis and influence overall patient survival. Gao et al.Citation14 found that lncRNA HOST2 was highly expressed in OC, and overexpression of HOST2 in OC contributed to OC metastasis. In addition, lncRNA HOTAIR was also reported to be highly expressed in the OC tissue.Citation15 Similarly, high expression level of HOTAIR is closely associated with the occurrence and development, invasion, metastasis, and prognosis of OC. Recently, a hitherto uncharacterized EGF-downregulated lncRNA, LIMT (lncRNA inhibiting metastasis), was identified as a regulator of mammary cell migration and invasion.Citation16 It has been shown that depletion of LIMT enhances tumor metastasis formation in vivo. However, the expression and underlying mechanism of LIMT in OC remains elusive. As an important part of the tumor microenvironment, it has been reported that M2-like TAMs could release a large number of EGF, thereby promoting the infiltration, proliferation, angiogenesis, and metastasis of tumor cells.Citation8 Therefore, we hypothesized that EGF secreted by M2-like TAMs may activate the EGFR-ERK pathway in ovarian cancer cells, which inhibited the expression of LIMT and eventually promoted the metastasis of ovarian cancer. In this study, the effects of M2-like TAMs on EGF/EGFR signaling pathway was observed. What’s more, the expression of LIMT in OC and the relationship of LIMT and EGF/EGFR signaling were defined, which help to better understand OC progression and provide novel therapeutic targets for OC treatment.
Materials and methods
Cell culture
The human monocyte cell line THP-1 (ATCC, USA) was maintained in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS) and 2 mM L-glutamine. THP-1 cells (2 × 105/ml) were differentiated to M2-like TAMs using 320 nM phorbol 12-myristate 13-acetate (PMA, Sigma-Aldrich) for 24 h.Citation17 The human normal ovarian epithelial cells IOSE80 and human ovarian cancer (OC) cell lines OV90 and OVCA429 (ATCC, USA) were cultured in Dulbecco’s modified Eagle’s medium (Sigma‐Aldrich, USA) supplemented with 10% fetal bovine serum (FBS), 100 units/mL penicillin, 100 μg/mL streptomycin, and 2 mM glutamine. All the cells were incubated at 37°C and 5% CO2. The morphology of the differentiated cells was observed and photographed using an Olympus microscope (DSX110, Japan). The differentiated THP1 cells (M2-like TAMs) and OV90 or OVCA429 cells were co-cultured in CO2 independent medium supplemented with 0.5 mM L-glutamine and 2.5 g/L of D-glucose.
Cell characterization by flow cytometry
The cells were harvested by treatment with 0.1% trypsin-EDTA, and detached cells were washed with cold PBS (pH 7.3). The cells were labeled with FITC-conjugated antibodies CD206 or CD68 or CD14, and PE-conjugated antibody CD204 (Abcam, USA). For CD68 staining, the cells were fixed and permeabilized with a BD Cytofix ⁄CytopermTM Fixation ⁄ Permeabilization Solution Kit (BD Biosciences, USA). Cells were then incubated with FITC-CD68 mAb. For surface markers (CD14, CD206, and CD204), the cells were incubated with FITC-CD14, FITC-CD206 or PE-CD204 mAb without permeabilization step. The cells were examined using a FACSCanto II cytometer (BD Biosciences, Germany), and the data were analyzed using FlowJo software (FlowJo, USA).
Cell transfection
The lentiviral vector LV-HULC (GenePharma, Shanghai, China) were used for the LIMT overexpression in OC cells. The vector including the negative sequence which does not target any gene as a control. Lentivirus was produced in 293T cells. OV90 and OVCA429 cells at exponential stage (5 × 104 cells/well) were transfected with virus particles in the presence of 5 μg/ml polybrene at MOI (multiplicities of infection) of 100.
Enzyme-linked immunosorbent assay
After co-culturing M2-like TAMs with OV90 or OVCA429 cells, the supernatants were collected at different indicated intervals. The EGF concentration was detected by enzyme-linked immunosorbent assays (ELISA). ELISA assay kits for EGF were performed following the supplier’s instructions (R&D, USA).
Cell viability assay
The Cell Counting Kit-8 (CCK-8) assay was used to measure the cell viability according to the manufacturer's protocols (Beyotime, China). OV90 or OVCA429 cells (1 × 104) were seeded into 96-well plates and cultured with the 100 μl conditioned medium of M2-like TAMs for 48 h. Then, the medium was removed and 100 μl of CCK-8 solution was added to each well followed with 4 h incubation at 37°C, 5% CO2 atmosphere. Finally, the absorbance at 450 nm was detected using a 96-well plate reader.
Cell cycle analysis
Cell Cycle was identified using the Propidium Iodide ReadyProbes® Reagent (Thermo Fisher Scientific, USA). OV90 or OVCA429 cells (1 × 105) were seeded in the low chamber of 24-well transwell apparatus with 0.4-um pore size (Corning, USA) and M2-like TAMs were seeded in the upper chamber as the condition of co-culture for 48 h. OV90 or OVCA429 cells in the low chamber were collected and washed with PBS. Then, the cells were fixed with 70% ethanol. After stained by 300 μl propidium iodide (Thermo Scientific, USA) in darkness, the cells were immediately analyzed by FACSCanto II cytometer (BD Biosciences, Germany).
Immunofluorescence staining
Immunofluorescence staining for Ki67 was performed to assess the cell proliferation according to the manufacturer’s instruction. Cells were incubated with primary antibody against Ki67 (Abcam, USA; 1:300). A corresponding secondary antibody with fluorophores Alexa 488 (Abcam, USA; 1:1000) was added for 1 h at room temperature. And then cells were counterstained with 10 mg/ml DAPI.
Wound healing assay
OV90 or OVCA429 cells (5 × 104) were seeded in the low chamber of 24-well transwell apparatus with 0.4-um pore size (Corning, USA) and M2-like TAMs were seeded in the upper chamber as the condition of co-culture for 48 h at 37°C. The confluent OV90 or OVCA429 cells were scratched with a 200 µl pipette tip and then plates were washed with fresh medium to remove non-adherent cells and then photographed for different indicated time. Wound area was determined using an inverted microscope (IX71; Olympus, Japan).
Cell invasion assay
Cell invasion assay was performed using a Transwell system (Corning, USA). The upper chamber of the Transwell was coated with extracellular matrix (BD Biosciences, USA). Cells were trypsinized, and 1 × 105 cells were seeded into the upper chamber with serum-free opti-MEM media. The low chamber was filled with M2-like TAMs. After incubation for 48 h, cells on the lower side of the filter were fixed in 3.8% formaldehyde for 20 min and stained with 0.1% crystal violet solution. The number of cells in five randomly selected fields was counted under a phase-contrast microscope (Olympus, Japan) and analyzed statistically.
RNA extraction and qRT-PCR
RNA was isolated from OC cells with TRIzol reagent (Invitrogen, USA) and RNeasy Plus Micro Kit (QIAGEN, USA) according to the manufacturer’s instructions. Then, reverse transcription was conducted to synthesize the cDNA by utilizing the SuperScript® IV First-Strand Synthesis System (Invitrogen, USA). qRT-PCR was performed in Applied Biosystems 7500 Real-Time PCR System (Applied Biosystems, USA), using 20 ng template in 25 µL reaction volume with 2 x Power SYBR® Green PCR Master Mix (Invitrogen, USA). Amplification conditions were as follows: 95°C for 10 min followed by 45 cycles consisting of 95°C for 15 s, 58°C for 30 s and 68°C for 60 s. The gene expression levels for all samples were normalized to β-actin expression using the comparative Ct method. All data are displayed as the mean ± SD of three independent experiments.
Western blot analysis
Cells were harvested and incubated in the RIPA buffer (Sigma–Aldrich, USA). Protein concentrations were determined using the BCA protein assay kit (Thermo Fisher Scientific, USA). Proteins (30 μg) were separated by 10% SDS-PAGE and transferred onto polyvinylidene difluoride (PDVF) membranes (Millipore, Bedford, MA). After blocking, the membranes were then incubated with primary antibodies against E-cadherin, N-cadherin, Vimentin, and β-actin (1:1000; Abcam, USA). β-actin was loaded as an internal reference. Bands were then treated with Horseradish peroxidase (HRP)-conjugated secondary antibody (1:2000; Abcam, USA). Bands were developed using chemiluminescence substance (Thermo Scientific, USA).
Animal models
Female athymic nude mice (8–12 weeks of age; n = 8 for each group) were purchased from the Cancer Institute of the Chinese Academy of Medical Science. OV90 cells (1 x 106 cells/animal) cells were injected into the peritoneal cavity of mice. Seven days after injection, mice were randomly divided into the control, LIMT, and AG1478 groups. The control and LIMT encoding lentivirus, and AG1478 were intraperitoneally injected twice a week in the OC mice model, respectively. Tumor weight and number and location of tumor nodules were recorded until for 5 weeks. All animal experiments were conducted in accordance with the Animal Welfare Act and were approved by the Ethics Committee of the Third Xiangya Hospital of Central South University.
Statistical analysis
Data were analyzed with Prism 5.0 (GraphPad Software, USA). All experiments were performed in triplicates and data were expressed as the means ± standard deviation (SD). One-way analysis of variance (ANOVA) with multiple comparisons using Dunnett’s test was applied for multiple comparisons. P < 0.05 was considered significantly different.
Results
PMA induced the differentiation of THP-1 cells into M2-like TAMs
THP-1 cells were treated with PMA for differentiating into M2-like TAMs. As shown in , cell adhesion, spread morphology, and blocked proliferation were observed in the PMA treated group, which represents typical features of macrophages. In addition, the cell biomarkers, including CD14 (monocytes), CD206 (macrophages), CD68 (M2 TAMs), and CD204 (M2 TAMs), were detected after PMA treatment. The flow cytometry analysis () showed that the level of CD206, CD68, and CD204 were significantly enhanced after the PMA treatment, while that of CD14 was dramatically decreased. All these results indicated that PMA could induce THP-1 cells differentiating into M2-like TAMs.
Figure 1. PMA induced the differentiation of THP-1 cells into M2-like TAMs. (a) THP-1 cells morphology after PMA treatment. Scale bar, 50 µm. (b) Expression of cell markers, including CD14 (monocytes), CD68 (macrophages), CD206 (M2 TAMs), and CD204 (M2 TAMs), detected after PMA treatment by flow cytometry analysis.
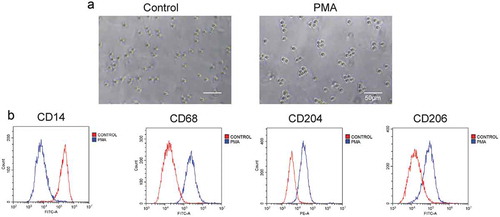
M2like TAMs secreted EGF and promoted cell proliferation, invasion, and migration of OC cells
In order to investigate the roles of M2-like TAMs in OC metastasis, M2-like TAMs were co-cultured with OV90 or OVCA429 cells for different intervals. Firstly, the ELISA assay showed that EGF concentration in the supernatants was obviously increased in a time-dependent manner (). Following that, we further explored the biological functions of M2-like TAMs on OC cell lines. The results of CCK-8 showed that after co-culturing with M2-like TAMs, the cell viability of OC cells increased dramatically (). Furthermore, the flow cytometry analysis and immunofluorescence staining of Ki67 were conducted to determine the cell cycle and cell proliferation, as shown in , ), we observed that OC cells presented higher cell proliferation after co-culturing with M2-like TAMs. In addition, the transwell and wound healing assays were subjected to assess the cell invasion and migration. And the results indicated that the capacity of cell invasion and migration was markedly increased within the guidance of M2-like TAMs (). Therefore, these findings indicated that M2-like TAMs could secret EGF and they might play a positive role in the promotion of OC development.
Figure 2. M2-like TAMs secreted EGF and the effects on cell proliferation, migration, and invasion. In order to investigate the role of M2-like TAMs in OC metastasis, M2-like TAMs were co-cultured with OV90 or OVCA429 cells for different intervals. And we cultured OC cells alone as the control groups. (a) EGF concentration in the supernatants was determined by ELISA assay. **P < 0.01 vs. Control (6 h). (b) Cell viability of OC cells when co-cultured with M2 TAMs detected by CCK-8 assay. *P < 0.05 and **P < 0.01 vs. Control. (c) Flow cytometry analysis of OC cell cycle after co-cultivation. **P < 0.01 vs. Control. (d) Inmmunofluorescence staining of Ki67 in OC cells when co-cultured with M2-like TAMs. Scale bar, 400 µm. (e) The invasion of OC cells after co-culturing with M2-like TAMs detected by transwell invasion assay. *P < 0.05 and **P < 0.01 vs. Control. (f) The migration of OC cells after co-culturing with M2-like TAMs detected by wound healing assay.*P < 0.05 vs. Control.
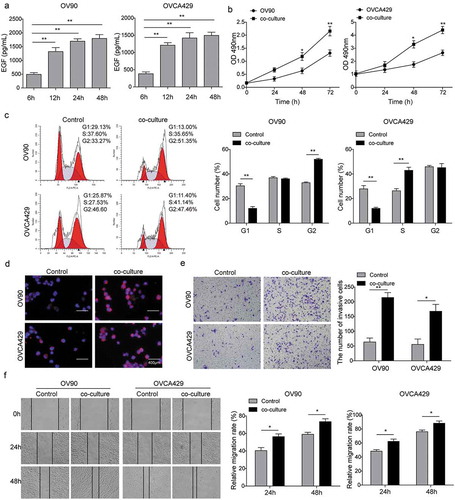
EGF secreted by M2-like TAMs promoted cell proliferation, migration, and invasion via activating EGFR-ERK signaling pathway
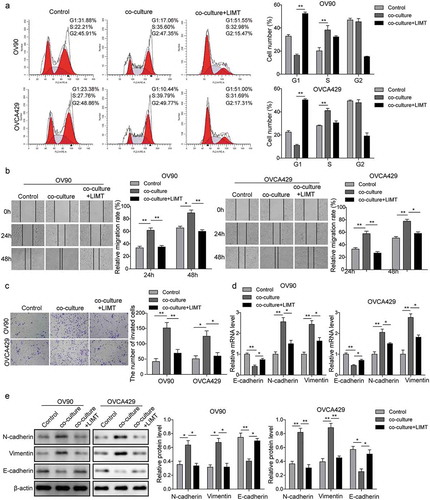
Based on the previous study, we observed that M2-like TAMs could secret EGF in a time-dependent manner in a co-culture system. It has been proved that EGR stimulates signaling pathway through EGF receptor (EGFR). To further certify whether EGF secreted by M2-like TAMs participate in the regulation of OC metastasis and the underlying mechanism, AG1478 (an EGFR inhibitor, 10 μM) was applied for pre-treatment OC cells. As shown in , the flow cytometry of cell cycle showed that AG1478 treatment arrested cell cycle at G1 phase, indicating that the promotion of cell proliferation induced by co-culture system was blocked by AG1478. Meanwhile, the migration and invasion of OC cells were also measured using wound healing and transwell assays. The results also presented that the application of AG1478 obviously decreased the number of migratory and invasive cells, which was opposite with the co-culture group (). Furthermore, qRT-PCR and western blot analyses were subjected to measure the expression of the proteins. As for the EMT related proteins, the qRT-PCR () and western blot () showed that AG1478 treatment up-regulated the expression of E-cadherin but down-regulated the expression of N-cadherin and vimentin both in mRNA and protein levels. Furthermore, the activation of EGFR/ERK signals was also blocked by AG1478 (). Taken together, our results suggested that EGF secreted from M2-like TAMs might be involved in cell proliferation, migration, and invasion through regulating EGFR/ERK signaling pathway.
Figure 3. EGF secreted by M2-like TAMs promoted cell proliferation, migration, and invasion via EGFR/ERK signaling pathway. OC cells were incubated with 10 uM AG1478 for 24 h and then were co-cultured with M2-like TAMs. (a) Flow cytometry analysis of the OC cell cycle. *P < 0.05 vs. Control. **P < 0.01 vs. Co-culture. (b) The migration of OC cells under different conditions was detected by wound healing assay. **P < 0.01 vs. Control. *P < 0.05 and **P < 0.01 vs. Co-culture. (c) The invasion of OC cells under different conditions was measured by transwell invasion assay. **P < 0.01 vs. Control. *P < 0.05 vs. Co-culture. (d&E) The expression levels of epithelial-mesenchymal transition (EMT) and EGFR-ERK signaling pathway related proteins measured by qRT-PCR (d) and western blot (e). *P < 0.05 and **P < 0.01 vs. Control. *P < 0.05 and **P < 0.01 vs. Co-culture.
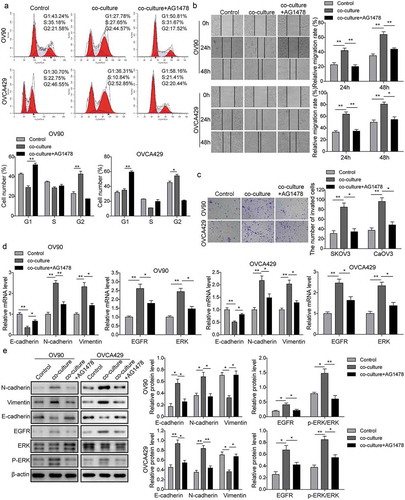
EGF secreted by M2-like TAMs inhibited LIMT expression in OC cells
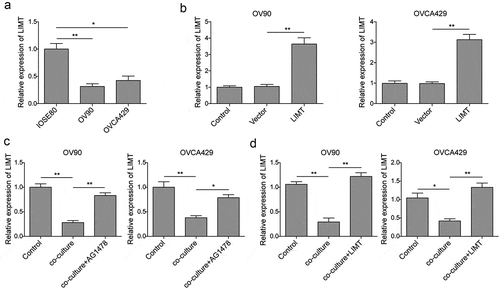
LIMT, a highly conserved lncRNA, has been proved to be a hitherto uncharacterized EGF-downregulated lncRNA. Whereas, the exact expression and mechanisms of LIMT in ovarian cancer remain unclear. To further explore whether there is a correlation between EGF and LIMT, firstly, the endogenous expression of LIMT in OC cells was determined by qRT-PCR. The results showed that LIMT was significantly decreased in OC cells compared with that of control (). Next, cells were transfected with LIMT encoding lentivirus for following detection (), presented a marked increase of LIMT expression level. Additionally, we also detected the expression of LIMT in a co-culture system following exposure with AG1478 or LIMT overexpression. Our data showed that co-culture OC cells with M2-like TAMs obviously caused the down-regulation of LIMT, while pre-treatment with AG1478 or LIMT encoding lentivirus, the inhibition of LIMT expression was reversed (). All these results indicated that EGF might be a key growth factor secreted by M2-like TAMs to repress LIMT expression via EGFR signal.
Figure 4. EGF secreted by M2-like TAMs inhibited LIMT expression in OC cells. (a) The endogenous expression levels of LIMT in OC cell lines and normal cells determined by qRT-PCR. *P < 0.05 and **P < 0.01 vs. IOSE80. (b) The expression levels of LIMT was determined by qRT-PCR in OC cells transfected with LIMT encoding lentivirus. **P < 0.01 vs. Vector. (C&D) The expression levels of LIMT in AG1478 pre-treated (c) or LIMT encoding lentiviru transfected (d) OC cell lines after co-culturing with M2-like TAMs. *P < 0.05 and **P < 0.01 vs. Control. *P < 0.05 and **P < 0.01 vs. Co-culture.
M2-like TAMs induced OC cell metastasis via inhibiting LIMT expression
Since downregulation of LIMT was observed in OC cells, we tested that overexpression of LIMT would attenuate OC progression in vitro. For this, cells were transfected with LIMT encoding lentivirus before co-culturing with M2-like TAMs. As expected, the positive effects of M2-like TAMs on OC cells proliferation, migration and invasion were significantly inhibited by LIMT overexpression (). Consistently, the results of qRT-PCR and western blot analyses of EMT related proteins also showed a similar trend (). These data supported a role for LIMT in inhibiting OC progression in vitro.
Figure 5. The effects of LIMT overexpression on M2-like TAMs induced OC cell metastasis in vitro. OV90 or OVCA429 cells were pre-treated with LIMT encoding lentivirus before incubating with M2-like TAMs. And we cultured OC cells alone as the control groups. (A) Flow cytometry analysis of OC cell cycle. **P < 0.01 vs. Control. **P < 0.01 vs. Co-culture. (B) The migration of OC cells under different conditions detected by wound healing assay.*P < 0.05 and **P < 0.01 vs. Control, *P < 0.05 and **P < 0.01 vs. Co-culture. (C) The invasion of OC cells under different conditions was detected by transwell invasion assay. *P < 0.05 and **P < 0.01 vs. Control, *P < 0.05 and **P < 0.01 vs. Co-culture. (D&E) The expression levels of epithelial-mesenchymal transition (EMT) related proteins measured by qRT-PCR (D) and western blot (E). *P < 0.05 and **P < 0.01 vs. Control, *P < 0.05 and **P < 0.01 vs. Co-culture.
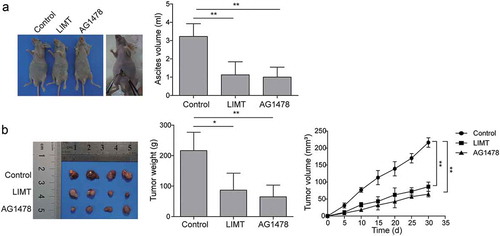
LIMT plays a critical role in the OC tumorigenesis
To determine whether LIMT regulates OC tumorigenesis in vivo, nude mice model bearing OC tumor were established and treated with intraperitoneal injection of LIMT encoding lentivirus, control lentivirus, and AG1478. Ascites formation is a common event in OC. The results showed that LIMT markedly decreased the ascites formation in mice bearing OC cancer tumors when compared with the control group (). A consistent pattern was also observed in the AG1478 group (). In addition, the results showed that intraperitoneal injection of LIMT into nude mice or AG1478 treatment dramatically decreased the number of tumor nodules and tumor weight () in comparison with the control group. The growth of tumors was also significantly suppressed (). The results confirmed that LIMT was a vital lncRNA of inhibiting OC tumorigenesis modulated by EGFR signaling.
Figure 6. The role of LIMT in OC tumorigenesis in vivo. Nude mice model bearing OC tumor were established and treated with intraperitoneal injection of LIMT encoding lentivirus, control lentivirus, and AG1478. (A) The ascites formation in mice bearing OC cancer tumors after LIMT encoding lentivirus, and AG1478 treatment. **P < 0.01 vs. Control. (B) The number of tumor nodules, tumor weight and tumor growth among different groups. *P < 0.05 and **P < 0.01 vs. Control.
Discussion
Ovarian cancer is a kind of gynecologic malignant tumor that has a high mortality with a 5-year survival rate being only about 29%.Citation1 Most researches on the mechanism of the occurrence and development of ovarian cancer focuses on the genetic alterations of ovarian cancer cells. However, more and more studies have found that tumor microenvironment plays an important role in the progression of ovarian cancer.Citation5 Among the various immune cells infiltrated by human ovarian cancer tissues, TAMs have the largest quantity.Citation18 Ovarian cancer cells secrete a variety of cytokines that make the mononuclear cells in the blood gather in ovarian cancer tissues and differentiated into macrophages. Also, ovarian cancer can secrete a large number of macrophage colony stimulating factor-1 (CSF-1), thus inducing macrophages to polarize towards M2 macrophages.Citation19 Through conducting immunohistochemical staining for CD68 and the specific marker molecules of M2 macrophages CD163 or CD206, it can be known that the larger the number of M2 macrophages,Citation20 the worse the prognosis of patients with advanced ovarian cancer. By performing immunohistochemical analysis of both M1 and M2 macrophages in ovarian cancer, Zhang W et al.Citation21 found that the patients who had a high proportion of M1/M2 macrophages saw a better prognosis, which suggests that M2 TAMs play an important role in the progression of ovarian cancer. Therefore, in the present study, to obtain the M2-like TAM, THP-1 cells were differentiated in vitro into macrophages by PMA treatment. The M2-like TAMs were successfully obtained confirmed by the cell morphology of macrophage and biomarkers including CD14 (monocyte), CD206 (macrophages), CD68 (M2 TAMs), and CD204 (M2 TAMs).
TAMs have been reported to promote the metastasis and invasion of ovarian cancer cells through multiple mechanisms.Citation22 In vitro experiments showed that the invasiveness of human ovarian cancer cells co-cultured with macrophages was enhanced, and this process was achieved through the activation of JNK and NF-kB signaling pathways.Citation23 Macrophages can also promote the invasiveness of ovarian cancer cells by expressing SR-A.Citation24 It was found by in vivo experiments that the SR-A−/- macrophages had a reduced stimulatory effect on the invasiveness of ovarian cancer cells, thereby slowing the progression of ovarian cancer. Furthermore, Yin et al.Citation9 also showed that TAMs promote spheroid formation and tumor growth by secreting EGF in an OC mouse model. Activation of EGFR on tumor cells by EGF in turn up-regulated VEGF/VEGFR signaling in surrounding tumor cells in order to promote the tumor cell proliferation and migration. These findings suggest that EGF secreted from TAMs plays a critical role in promoting ovarian cancer metastasis. In order to clarify the role of EGF in the regulation of OC metastasis, M2-like TAMs were co-cultured with OC cells. Similarly, our data also presented an increasing level of EGF in the supernatant of the co-culture system of M2-like TAMs and OC cells. Besides, the capacity of OC cells proliferation, migration, and invasion was significantly promoted. Curiously, however, the cell cycle of OV90 cells showed a different change under the co-culture with M2-like TAMs, of which phase displayed an inconsistent change in DNA chrysalis and replication. These remind us that there might be some other mechanism getting involved in regulating the cell cycle. Interestingly, with the application of AG1478 (an EGFR inhibitor) for further experiments, our results revealed that the EGF secreted by M2 TAM might activate the EGFR-ERK signaling pathway, thus promoting the OC progression. All these studies demonstrated that TAMs could promote the progression of ovarian cancer through multiple pathways.
The invasiveness and metastasis of tumor cells are ascribed to their phenotypic changes, which involve a trans-differentiation process called EMT.Citation25 It is a multi-gene, multi-step, multi-stage complex process, referring to the morphological transformation of epithelial cells towards fibroblast or mesenchymal phenotype and the obtainment of migration ability. EMT is characterized by the enhanced abilities of migration, invasiveness, and apoptosis tolerance.Citation26 Although EMT is not the only mechanism of metastasis, it is one of the most important mechanisms in the initiation of tumor metastasis. The activation of potential EMT processes enhances the ability of tumor cells to invade and metastasize towards distant organs or tissues.Citation27 In this study, the expression of EMT related protein E-cadherin was significantly down-regulated, while N-cadherin and vimentin were up-regulated by co-culturing with M2-like TAMs in both OC cells. This indicates that M2-like TAMs can induce the EMT of OC cells, and further initiate the tumor metastasis.
Long non-coding RNAs (lncRNAs) are emerging as vital regulators in the pathogenesis of multiple cancers, including ovarian cancer. LIMT is a newly identified lncRNA, which was originally denoted as LINC01089 or LOC338799.Citation16 Sas-Chen et al.Citation23 found that LIMT, a hitherto uncharacterized EGF-downregulated lncRNA, of which depletion enhances breast cancer metastasis. Nevertheless, there has been no report published regarding the exact expression, biological role, and underlying mechanism of LIMT in ovarian cancer. In our previous study, M2-like TAMs could secret EGF in a time-dependent manner. Besides, EGF signaling route was also reporter to serve as a regulator in the OC EMT process.Citation28 Moreover, the activation of EGF downstream signaling pathway is based on the EGF receptor (EGFR). Therefore, we further investigated whether LIMT also depends on prior activation of the EGFR signaling pathway in OC. Interestingly, we observed that EGF secreted by M2-like TAMs inhibited LIMT expression via activating EGFR-ERK signal pathway in OC cells. This pattern can be efficiently reversed when applying with EGFR inhibitor, AG1478 or LIMT overexpression, which suggests that the activation of the EGFR-ERK pathway affected the metastasis of ovarian cancer via suppressing LIMT expression. Ascites formation is a common event in OC. As expected, our in vivo study found that LIMT overexpression and AG1478 treatment markedly decreased the ascites formation in mice bearing OC cancer tumors when compared with the control group. In addition, the number of tumor nodules and tumor weight were observed to be dramatically decreased in comparison with the control group. These in vivo results further confirmed that LIMT plays a vital role in inhibiting OC tumorigenesis modulated by EGFR signaling.
Although no targeted drug for the lncRNA in TAMs with increased specificity has been found, lncRNA has been reported as a target for tumor treatment. Wu et al.Citation29 found that ovarian cancer cells had seen decreased migration and invasion capacity after knocking down lncRNA MALAT1. The suppression of lncRNA HOTAIR with small interfering RNAs was found to reduce the metastasis of ovarian cancer.Citation30 These studies suggest that discovering the differentially expressed lncRNAs in ovarian cancer could provide more precise targets for cancer treatment against TAMs, and therefore is of profound significance for improving the prognosis of ovarian cancer.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Reid BM, Permuth JB, Sellers TA. Epidemiology of ovarian cancer: a review. Cancer Biol Med. 2017;14:9–32. doi:10.20892/j.issn.2095-3941.2016.0084.
- Goff BA. Advanced ovarian cancer: what should be the standard of care? J Gynecol Oncol. 2013;24:83–91. doi:10.3802/jgo.2013.24.1.83.
- Testa U, Petrucci E, Pasquini L, Castelli G, Pelosi E. Ovarian cancers: genetic abnormalities, tumor heterogeneity and progression, clonal evolution and cancer stem cells. Medicines (Basel). 2018;5:16. doi: 10.3390/medicines5010016.
- Sadikovic B, Al-Romaih K, Squire JA, Zielenska M. Cause and consequences of genetic and epigenetic alterations in human cancer. Curr Genomics. 2008;9:394–408. doi:10.2174/138920208785699580.
- Ghoneum A, Afify H, Salih Z, Kelly M, Said N. Role of tumor microenvironment in ovarian cancer pathobiology. Oncotarget. 2018;9:22832–22849. doi:10.18632/oncotarget.25126.
- Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. doi:10.1038/nm.3394.
- Fujimura T, Kambayashi Y, Fujisawa Y, Hidaka T, Aiba S. Tumor-associated macrophages: therapeutic targets for skin cancer. Front Oncol. 2018;8:3. doi:10.3389/fonc.2018.00003.
- Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. doi:10.12703/P.
- Yin M, Li X, Tan S, Zhou HJ, Ji W, Bellone S, Xu X, Zhang H, Santin AD, Lou G, et al. Tumor-associated macrophages drive spheroid formation during early transcoelomic metastasis of ovarian cancer. J Clin Invest. 2016;126:4157–4173. doi:10.1172/JCI87252.
- Ke X, Zhang S, Wu M, Lou J, Zhang J, Xu T, Huang L, Huang P, Wang F, Pan S. Tumor-associated macrophages promote invasion via Toll-like receptors signaling in patients with ovarian cancer. Int Immunopharmacol. 2016;40:184–195. doi:10.1016/j.intimp.2016.08.029.
- Kung JT, Colognori D, Lee JT. Long noncoding RNAs: past, present, and future. Genetics. 2013;193:651–669. doi:10.1534/genetics.112.146704.
- Li Y, Li W, Liang B, Li L, Wang L, Huang H, Guo S, Wang Y, He Y, Chen L, et al. Identification of cancer risk lncRNAs and cancer risk pathways regulated by cancer risk lncRNAs based on genome sequencing data in human cancers. Sci Rep. 2016;6:39294. doi:10.1038/srep39294.
- Chen Y, Yu X, Xu Y, Shen H. Identification of dysregulated lncRNAs profiling and metastasis-associated lncRNAs in colorectal cancer by genome-wide analysis. Cancer Med. 2017;6:2321–2330. doi:10.1002/cam4.1168.
- Gao Y, Meng H, Liu S, Hu J, Zhang Y, Jiao T, Liu Y, Ou J, Wang D, Yao L, et al. LncRNA-HOST2 regulates cell biological behaviors in epithelial ovarian cancer through a mechanism involving microRNA let-7b. Hum Mol Genet. 2015;24:841–852. doi:10.1093/hmg/ddu502.
- Qiu JJ, Wang Y, Ding JX, Jin HY, Yang G, Hua KQ. The long non-coding RNA HOTAIR promotes the proliferation of serous ovarian cancer cells through the regulation of cell cycle arrest and apoptosis. Exp Cell Res. 2015;333:238–248. doi:10.1016/j.yexcr.2015.03.005.
- Sas-Chen A, Aure MR, Leibovich L, Carvalho S, Enuka Y, Körner C, Polycarpou-Schwarz M, Lavi S, Nevo N, Kuznetsov Y, et al. LIMT is a novel metastasis inhibiting lncRNA suppressed by EGF and downregulated in aggressive breast cancer. EMBO Mol Med. 2016;8:1052–1064. doi:10.15252/emmm.201606198.
- Chanput W, Mes JJ, Wichers HJ. THP-1 cell line: an in vitro cell model for immune modulation approach. Int Immunopharmacol. 2014;23:37–45. doi:10.1016/j.intimp.2014.08.002.
- Colvin EK. Tumor-associated macrophages contribute to tumor progression in ovarian cancer. Front Oncol. 2014;4:137. doi:10.3389/fonc.2014.00137.
- Kawamura K, Komohara Y, Takaishi K, Katabuchi H, Takeya M. Detection of M2 macrophages and colony-stimulating factor 1 expression in serous and mucinous ovarian epithelial tumors. Pathol Int. 2009;59:300–305. doi:10.1111/j.1440-1827.2009.02369.x.
- Barros MH, Hauck F, Dreyer JH, Kempkes B, Niedobitek G. Macrophage polarisation: an immunohistochemical approach for identifying M1 and M2 macrophages. PLoS One. 2013;8:e80908. doi:10.1371/journal.pone.0080908.
- Zhang M, He Y, Sun X, Li Q, Wang W, Zhao A, Di W. A high M1/M2 ratio of tumor-associated macrophages is associated with extended survival in ovarian cancer patients. J Ovarian Res. 2014;7:19. doi:10.1186/1757-2215-7-19.
- Pogge von Strandmann E, Reinartz S, Wager U, Muller R. Tumor-host cell interactions in ovarian cancer: pathways to therapy failure. Trends Cancer. 2017;3:137–148. doi:10.1016/j.trecan.2016.12.005.
- Hagemann T, Wilson J, Kulbe H, Li NF, Leinster DA, Charles K, Klemm F, Pukrop T, Binder C, Balkwill FR. Macrophages induce invasiveness of epithelial cancer cells via NF-kappa B and JNK. J Immunol. 2005;175:1197–1205.
- Neyen C, Pluddemann A, Mukhopadhyay S, Maniati E, Bossard M, Gordon S, Hagemann T. Macrophage scavenger receptor a promotes tumor progression in murine models of ovarian and pancreatic cancer. J Immunol. 2013;190:3798–3805. doi:10.4049/jimmunol.1203194.
- Roche J. The epithelial-to-mesenchymal transition in cancer. Cancers (Basel). 2018;10. doi:10.3390/cancers10110400.
- Kim DH, Xing T, Yang Z, Dudek R, Lu Q, Chen YH. Epithelial mesenchymal transition in embryonic development, tissue repair and cancer: A comprehensive overview. J Clin Med. 2017;7. doi:10.3390/jcm7010001.
- van Zijl F, Krupitza G, Mikulits W. Initial steps of metastasis: cell invasion and endothelial transmigration. Mutat Res. 2011;728:23–34. doi:10.1016/j.mrrev.2011.05.002.
- Park GB, Ko HS, Kim D. Sorafenib controls the epithelialmesenchymal transition of ovarian cancer cells via EGF and the CD44HA signaling pathway in a cell typedependent manner. Mol Med Rep. 2017;16:1826–1836. doi:10.3892/mmr.2017.6773.
- Wu L, Wang X, Guo Y. Long non-coding RNA MALAT1 is upregulated and involved in cell proliferation, migration and apoptosis in ovarian cancer. Exp Ther Med. 2017;13:3055–3060. doi:10.3892/etm.2017.4304.
- Zhang Z, Cheng J, Wu Y, Qiu J, Sun Y, Tong X. LncRNA HOTAIR controls the expression of Rab22a by sponging miR-373 in ovarian cancer. Mol Med Rep. 2016;14:2465–2472. doi:10.3892/mmr.2016.5572.
