ABSTRACT
Hepatocellular carcinoma (HCC) is a primary malignancy of the liver and occurs predominantly in patients with underlying chronic liver disease and cirrhosis. HCC is now the third leading cause of cancer deaths worldwide, with over 500,000 people affected. However, there is no complete effective (ideal) treatment for liver cancer yet, and the new methods are expected to be discovered. Herein, for the first time, we report the anti-HCC effects of copper-cysteamine nanoparticles (Cu-Cy NPs), a new type of photosensitizers. An in vitro 3-(4,5-dimethylthiazol- 2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay shows that Cu-Cy NPs could significantly reduce the activity of HepG2 cells at a very low dose after a short time of ultraviolet radiation. In addition, we found that cell death was induced by Cu-Cy NPs, which is associated with cellular apoptosis. This implied that apoptosis might be the main mechanism of the Cu-Cy’s anti-HCC activity. Furthermore, we found that Cu-Cy NPs obviously inhibited the tumor growth in vivo. More interestingly, we found that the soluble Cu-Cy NPs were able to enter exosomes which were secreted by tumor cells, and exosomes could be used to deliver Cu-Cy NPs to target tumor cells. All these observations suggest that Cu-Cy NPs have a good potential for cancer treatment.
Introduction
Hepatocellular carcinoma (HCC) is one of the six most malignant tumors in the world.Citation1,Citation2 The latest global statistics report shows that there are 0.74 million cases of HCC and 0.69 million deaths per year.Citation1,Citation3 China has the highest global incidence of HCC (54% of the worldwide total).Citation1,Citation4,Citation5 Hepatocellular carcinoma has a high incidence rate and an extremely poor prognosis, with a 5-year survival rate of less than 5%.Citation3,Citation6,Citation7 Drug therapy is one of the most commonly used treatment methods in HCC. Typical chemotherapeutics include 5-fluorouracil (5-FU) and doxorubicin.Citation8,Citation9 Although drugs have a certain curative effect on treating HCC, some studies have shown that these anticancer drugs cause side-effects within the human body.Citation10 Therefore, new treatment methods and new drugs for liver cancer are urgently needed.
Photodynamic therapy (PDT) is a new and promising method that is a potential alternative to conventional chemotherapy. Photosensitizers used for PDT and activated by light will react with the oxygen molecules present in tissues and generate cytotoxic singlet oxygen.Citation11 Some advantages of PDT over other techniques include some degree of selectivity of drug binding to tumor tissue, the absence of systemic toxicity of the drug alone, the ability to focus the light on the tumor region, the possibility of treating multiple lesions simultaneously and the ability to re-treat a tumor to improve the response.Citation12,Citation13 The PDT outcome is directly related to the efficiency of singlet oxygen generation which affected by photosensitizer, light, and oxygen.Citation14 For PDT to work properly, the light must be effectively delivered to the photosensitizer. Commonly, light sources are lasers, light-emitting diodes, and halogen or arc lamps in some cases. However, light delivery is challenging for the treatment of deep cancers because light cannot penetrate deeply into tissue.Citation15 Therefore, PDT is commonly used to treat superficial lesions (e.g., skin cancer).Citation16 Except for the light source, another factor that affects the efficiency of singlet oxygen is the photosensitizer itself. The traditional photosensitizers are hematoporphyrin derivatives, 5-aminolevulinic acid (5-ALA), and the phthalocyanine organic dye. Due to the issues of non-selectivity and biological toxicity, these photosensitizers have some disadvantages in clinical applications.Citation17 Thus, researchers focus on exploring new photosensitizers that have the ability to avoid or limit these harms.
Nanomedicines have been extensively investigated, and nanoscale photosensitizers have been explored extensively for cancer treatment.Citation17–Citation21 Recently, Chen et al. have reported a new type of nanoparticle photosensitizers, copper-cysteamine (Cu-Cy).Citation22,Citation23 Two striking characteristics of this new material are its strong red luminescence and its capability to produce singlet oxygen that is inducible by regular light,Citation24 X-rays,Citation23,Citation25 and microwave.Citation24 Therefore, Cu-Cy is a unique material that is being explored for treating cancers.
The development of Cu-Cy as a new sensitizerCitation24,Citation25 for cancer treatment is still at early stages, and many aspects of this new material are still unknown. Cu-Cy NPs have not been tested for liver cancer treatment, and the efficiency of Cu-Cy NPs on anti-liver cancer has not yet been evaluated. Here, for the first time, we report the PDT effects of Cu-Cy NPs on anti-HCC via in vivo and in vitro experiments.
Results
Characterization of solid and soluble Cu-Cy NPs
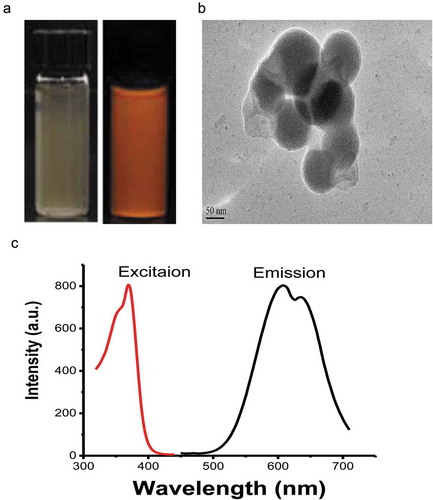
Cu-Cy NPs are highly crystalline. Cu-Cy NPs structure includes two different Cu atoms–Cu(1) and Cu(2) (they are both Cu+ ions), which bind to 4 and 3 other atoms, respectively. Cu-Cy NPs can display intense photoluminescence (showed in )) and X-ray luminescence. The detail of crystal structure and optical properties of Cu-Cy NPs were described in our previous paper.Citation24 For the samples used for this study, they were coated with polyethylene glycol, and their size is about 100 nm as judged from their TEM images as shown in ). They have strong red luminescence as displayed in ).
The production of singlet oxygen under UV radiation at a different time period
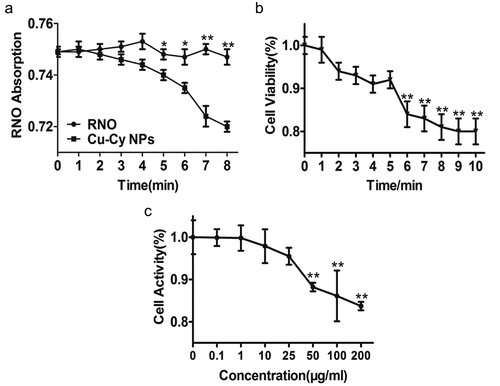
We used P-nitrosodimenthylaniline-imidazole to detect singlet oxygen being irradiated at different time duration under UV light. RNO is a water-soluble molecule that is quenched irreversibly by singlet oxygen when ID is present. Comparing to the quenching degree of RNO absorption with and without Cu-Cy NPs under UV irradiation, we observed that the amount of singlet oxygen generating by Cu-Cy NPs were dependent on the irradiation duration time. As shown in ), in the control group, RNO-ID only, the UV irradiation did not induce any singlet oxygen to quench the RNO absorption. However, after adding Cu-Cy NPs, the absorption of RNO was reduced obviously upon UV irradiation (0 min vs. 5 min: 0.749 ± 0.001 vs. 0.738 ± 0.006; P= 0.009). These results indicated that Cu-Cy NPs were a new type of photosensitizer that could be activated by UV to produce singlet oxygen for cancer treatment.
Figure 2. The generation of singlet oxygen, determination of UV dosage and the concentration of Cu-Cy NPs. (a) RNO absorption quenching indicates singlet oxygen production in control (RNO) and Cu-Cy NPs using UV light (365 nm). The data are expressed as the mean ± SD of three independent experiments. *P< 0.05 and **P< 0.005 compared with the control. (b) The viability of HepG2 cells treated with UV light at the indicated times for 24 h was determined using an MTT assay (0 min as control). The results are presented as the mean ± SD in triplicate from one experiment. *P < 0.05 and **P < 0.005 compared with the control. (c) The viability of HepG2 cells treated with Cu-Cy NPs at the indicated concentrations for 24 h was determined using an MTT assay (0 μg/ml as control). The data are expressed as the mean ± SD of three independent experiments. *P < 0.05 and **P < 0.005 compared with the control.
HepG2 cellular viability at different UV irradiated time durations
Ultraviolet light does not only sterilize, but it also influences cell growth. To evaluate the effect of UV radiation on the activity of cells, we used 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) to assay the cellular viability of HepG2 cells, which HepG2 cells were irradiated under UV at different time duration (0 min to 10 min) after cultured for 24 h. In ), the cellular viability was decreased after UV irradiation. It gradually decreased when the irradiation time was increased from 6 min to 10 min. UV irradiation had little effect on cells for less than 6 min. However, cells were significantly impacted when irradiation time was increased to 6 min (6 min vs. 0 min: 0.84 ± 0.03 vs. 1.00 ± 0.02; P = 0.001) or longer. Therefore, we chose 5 min for radiation (light dose = 6 J/cm2) in order to avoid the ‘side-effect’ from UV irradiation.
HepG2 cellular viability at different Cu-Cy NPs’ concentrations
Toxicity is an important factor to evaluate materials for biological application. To determine the cytotoxicity of Cu-Cy NPs, we utilized the MTT assay after Cu-Cy NPs were cultured with HepG2 cells for 24 h. As shown in ), the cellular activity was reduced when the concentration of Cu-Cy NPs increased. The cellular viability gradually decreased from ~100% to ~85% when the concentration of Cu-Cy NPs’ increased from 0 μg/ml to 200 μg/ml. We noticed that the cellular viability began to show effects at the concentration of 50 μg/ml (0 μg/ml vs. 50 μg/ml: 1.00 ± 0.02 vs. 0.88 ± 0.01; P < 0.001). This result indicated that Cu-Cy NPs had low toxicity at a concentration below 50 μg/ml, and 25 μg/ml was used for all the other experiments in our study to avoid the intrinsic cytotoxicity from the nanoparticles themselves.
Cu-Cy NPs’ binding HepG2 cells and the cellular viability
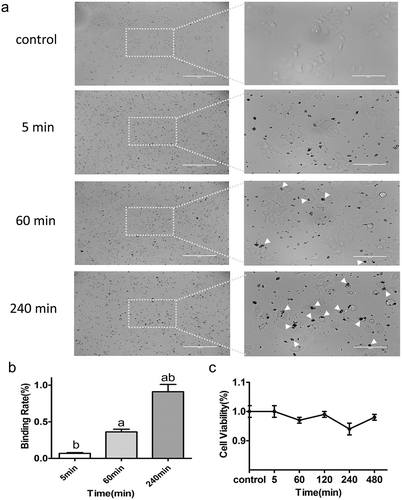
It has been reported that even Cu-Cy NPs do not enter the cells, but they are still effective for the destruction of cancer cells.Citation23 In our experiments, we adopted the MTT assay and fluorescence staining to evaluate the killing efficacy of Cu-Cy NPs on HepG2 cells for three different incubation periods. The degree of Cu-Cy NPs (25 μg/ml) binding to cells at three different stages were observed and shown in ). The proportion of Cu-Cy NPs binding to cells became larger when the incubation time increased. Comparing 5 min, 60 min, and 240 min, the binding degree was increased (60 min vs. 5 min: 0.36 ± 0.09 vs. 0.07 ± 0.02, P = 0.006; 240 min vs. 5 min: 0.91 ± 0.22 vs. 0.07 ± 0.02, P< 0.001; 240 min vs. 60 min: 0.91 ± 0.22 vs. 0.36 ± 0.09, P < 0.001) ()). There was no significant difference in the cellular viability for these different cultured stages ()). These observations indicated that there is no obvious toxic effect on cells at the concentration of 25 μg/ml.
Figure 3. (a) Images of HepG2 cells incubated for 5 min, 60 min, and 240 min with Cu-Cy NPs. As shown in picture 5 min, no cell uptake of Cu-Cy NPs, 60 min, and 240 min had varying degrees of binding. (b) The proportion of Cu-Cy NPs adhered by cells in different incubation time. The data are expressed as the mean ± SD of three independent experiments, a P < 0.05 compared with 5 min group, b P < 0.05 compared with 60 min group. (c) The viability of HepG2 cells at different binding degree of Cu-Cy NPs without UV light in 24 h was determined using an MTT assay. The data are expressed as the mean ± SD of three independent experiments.
However, we expect that the cellular viability would be different if UV was adopted to irradiate the nanoparticles. Here, the fluorescence images of HepG2 cells were taken before and after UV irradiation via an EVOS fluorescence microscope. Calcein and ethidiumhomodimer-1 (EthD-1) were used to stain the live cells green and the dead cells red after incubation for 30 min. As shown in ), the numbers of cellular death at different binding degrees of Cu-Cy NPs to cells are almost the same after 6 J/cm2 UV irradiation. Statistical analysis results showed that there was no significant difference among the three stages of incubation. According to the result of the control group, it was clear that UV irradiation alone was not able to kill the cells ()). The result of MTT assay also denoted that there was no difference for the cellular viability at three incubation stages of Cu-Cy NPs to cells after UV irradiation. This finding proved that the binding degree of Cu-Cy NPs to the cells was not a critical factor for their killing efficacy.
Figure 4. Destruction of HepG2 cells by Cu-Cy NPs (25 μg/ml) under different binding degree after UV irradiation. (a) Images of fluorescence staining show HepG2 live (green, calcein stain) and dead (red, EthD-1 stain) cells. Pictures (5 min), (60 min), and (240 min) show the damage of Cu-Cy NPs to cells in different binding degree (incubated with Cu-Cy NPs for 5 min, 60 min, and 240 min, respectively). (b) The proportion of dead and living cells in different incubation time after UV radiation. The data are expressed as the mean ± SD of three random pictures. a P < 0.05 compared with control. (c) The viability of HepG2 cells at the same binding degree of Cu-Cy NPs with UV light in 24 h was determined using an MTT assay. a P < 0.05 compared with control. The data are expressed as the mean ± SD of three independent experiments.
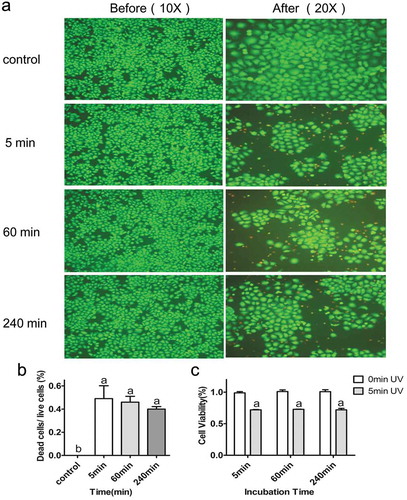
To evaluate the UV induced PDT effects, we compared the cellular viability at the same binding degree of Cu-Cy NPs before and after UV exposure. The results show that the cellular viability was significantly decreased after UV irradiation as compared to its corresponding control ()), and this indicated that Cu-Cy NPs had a significant inhibitory effect on cellular viability upon UV exposure. In general, whether Cu-Cy NPs are absorbed by cells or not, as soon as they are bound to the cells, a strong killing effect on cancer cells after 6 J/cm2 UV activation would appear.
Cu-Cy NPs induced the apoptosis of HepG2 cells
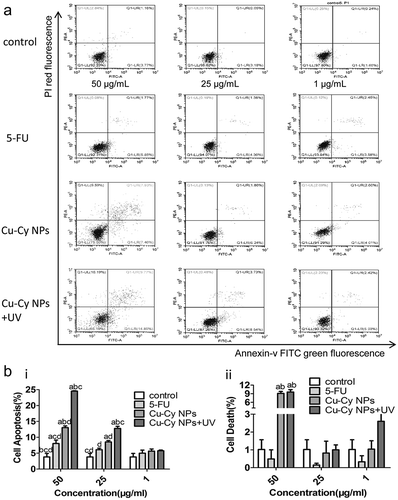
We evaluated the cell apoptosis induced by Cu-Cy NPs in comparison with 5-FU, which was a common drug for chemotherapy for HCC. The data in ) and () showed that, comparing to other three group, Cu-Cy NPs±UV induced obvious apoptosis at 25 μg/ml (Cu-Cy NPs±UV vs. control: 12.32 ± 0.53 vs. 3.35 ± 1.51, P< 0.001; Cu-Cy NPs+UV vs. 5-FU: 12.32 ± 0.53 vs. 4.86 ± 1.60, P = 0.001; Cu-Cy NPs+UV vs. Cu-Cy NPs: 12.32 ± 0.53 vs. 8.58 ± 0.89, P = 0.05. At a high dose (50 μg/ml), the rate of cellular death was improved in Cu-Cy NPs and Cu-Cy NPs+UV group (Cu-Cy NPs vs. control: 9.10 ± 0.69 vs. 1.09 ± 1.51, P< 0.001, Cu-Cy NPs vs. 5-FU: 9.10 ± 0.69 vs. 0.50 ± 0.51, P< 0.001, Cu-Cy NPs+UV vs. control: 6.09 ± 5.80 vs. 1.09 ± 1.51, P = 0.005, Cu-Cy NPs+UV vs. 5-FU: 6.09 ± 5.80 vs. 0.50 ± 0.51, P = 0.002) () and ()). These results indicated that both 5-FU and Cu-Cy NPs+UV could promote the apoptosis of HepG2 cells. Compared to 5-FU, UV irradiated Cu-Cy NP had a stronger effect on the apoptosis of HepG2 cells.
Figure 5. Comparison of Cu-Cy NPs and 5-FU induced HepG2 cells apoptosis. (a) Cell apoptosis profiles were measured by flow cytometry following treatment of different groups with the concentration of 25 μg/ml for 24 h. (b) Cellular consequence was quantified by apoptosis. The data are expressed as the mean ± SD of three independent experiments. a P < 0.05 compared with control, b P < 0.05 compared with 5-FU group, c P < 0.05 compared with Cu-Cy NPs group, and d P < 0.05 compared with Cu-Cy NPs+UV group. (c) The expression levels of the proteins including cleaved-caspase 3 and cleaved-PARP in HepG2 cells.
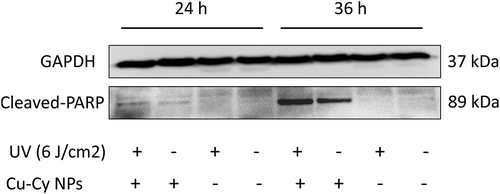
Further evidence was obtained to determine that apoptosis induced by Cu-Cy NPs. The expression of the apoptotic protein (cleaved-PARP) was analyzed by western blotting analyses in HepG2 cells that treated with Cu-Cy NPs + UV (6 J/cm2) and cultured for 24 h and 36 h. Consistent with the above results, compared to control, UV dose did not induce the expression of cleaved-PARP; however, increased expression levels of cleaved-PARP was found in the group which treated with Cu-Cy NPs. And Cu-Cy NPs led to more expression of cleaved-PARP after activated by UV (6 J/cm2). Furthermore, after adding Cu-Cy NPs and 6 J/cm2 UV irradiation, the expression of apoptosis-related protein increased more significantly at 36 h than 24 h (). This result indicated that apoptosis of cells increases gradually with the extension of action time.
Cu-Cy NPs anti-HepG2 tumors in vivo
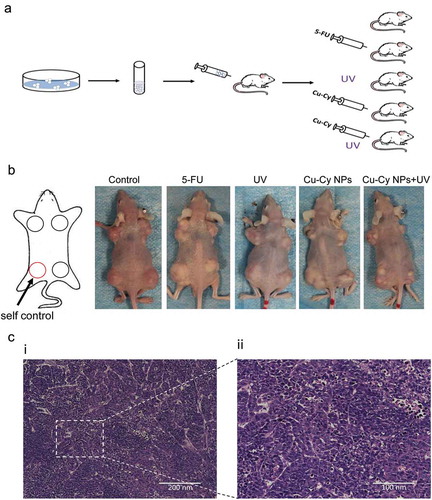
To further evaluate the potential of Cu-Cy NPs on cancer treatment, the UV irradiated Cu-Cy NPs were tested on in vivo subcutaneous tumor model. A suspension of 1 × 106 HepG2 cells was injected subcutaneously into the flank and shoulder of mice, and the tumor growth was monitored daily. Two weeks after injection, mice were divided randomly into five groups: control (saline), 5-FU, UV, Cu-Cy NPs and Cu-Cy NPs+UV as shown in ). In Cu-Cy NPs+UV group, the mice were irradiated with 6 J/cm2 UV each day for the first three days, and all trials were observed for one month. As shown in ), the tumor was successfully grown in each group of the nude mice. H&E staining was used to confirm the formation of tumor as seen in ) for the typical morphological characteristics of tumor cells: the volume and nucleus were larger than normal cells, the cellular shape and arrangement were irregular, the nuclei of cells were deeply stained and varied in shape, and a large number of multinucleated cells could be seen. These factors demonstrated that the nude mice model of the tumor was successfully established.
Figure 7. Establishment of a tumor model. (a) The process and grouping of an experiment in nude mice. (b) Images of nude mice with different sized HepG2 tumors after different treatment. The left leg of each mouse is the control. (c) HE staining of HepG2 tumor in mice.
As shown in ), there was no effect on tumor growth in the UV group, the Cu-Cy NPs alone have slightly inhibited the growth of the tumor, and the growth rates of tumors in the 5-FU and Cu-Cy NPs+UV groups were obviously slower than the control. These observations indicated that UV irradiated Cu-Cy NPs had the same inhibitory effect on tumor growth as 5-FU.
Figure 8. Images of tumors sized after treatment. (a) Image of the growth curve of tumor in different groups of mice. The results are presented as the mean of eight mice per group from the experiment. *P < 0.05 Cu-Cy NPs+UV compared with the control, +P < 0.05 5-FU compared with the control. (b) Images of tumor size in the different group after different treatment. (c) Scatter plots of tumor volume after treatment with different groups of mice. The results are presented as the mean ± SD of eight mice per group from the experiment. *P < 0.05 compared with the control.
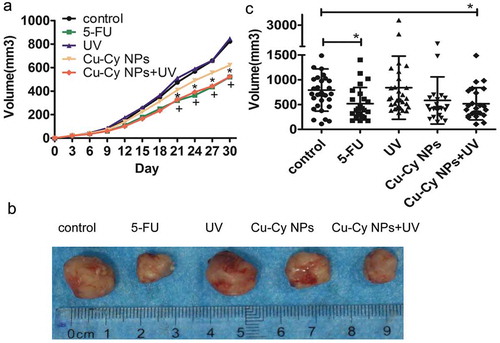
After 1 month treatment, the tumors were surgically dissected. Compared with the control, the tumor volumes in 5-FU were significantly smaller (control vs. 5-FU: 793.16 ± 425.33 vs. 518.40 ± 327.11, P = 0.026). The volumes in Cu-Cy NPs+UV were also smaller than control (control vs. Cu-Cy NPs+UV: 793.16 ± 425.33 vs. 515.99 ± 323.98, P = 0.004). However, the volumes in 5-FU and Cu-Cy NPs+UV groups showed no difference. Also, there were no differences among the control, UV alone, and Cu-Cy NPs alone () and ()). These observations confirmed that UV irradiated Cu-Cy NPs had the same inhibitory effect on tumor growth as 5-FU.
Exosome’s absorption of Cu-Cy-NPs for delivery
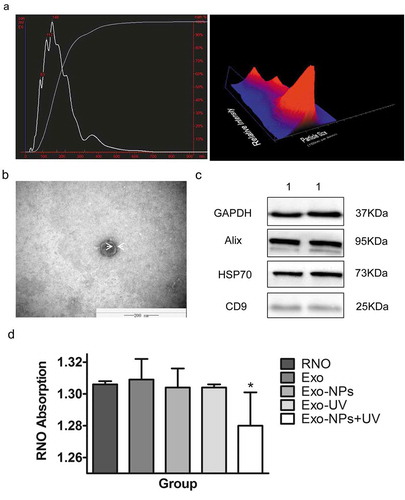
From the above results, we know that Cu-Cy NPs were effective sensitizers that could be activated by UV for HCC treatment. For effective treatment, Cu-Cy NPs should be delivered and targeted to tumor cells by drug carriers. Here, we explored the possibility of exosomes for delivery. For that, we first needed to figure out if Cu-Cy NPs could be embedded into exosomes. We added the water-soluble Cu-Cy NPs to HepG2 cells and isolated the exosomes of HepG2 cells by using the ExoQuick exosome precipitation kit, and the exosomes of Huh7 cells were collected in the same way. Then, the nanosight, transmission electron microscopy (TEM) and western blot were used to affirm the exosomes collected. As shown in ), the curve was smooth, the peak of particle size was 148 nm, and most of the particles were ranged from 30 nm to 150 nm, and this was consistent with the diameter of exosomes. The image of TEM presented a vesicle-like morphology of the particles. Their shapes were round or oval with a diameter of around 100 nm. We could also clearly see the bilayer membrane structure of the particles. These factors were the typical structure of exosomes ()). To further confirm whether the collected substance were exosomes, we used western blot to detect the specific protein of exosomes (Alix,Citation26 HSP70,Citation27 and CD9Citation28 were considered to be the marker protein of exosomes) derived from Huh7 cells (). The marker protein – Alix, HSP70, and CD9 – were expressed in Huh7 exosome. All these results proved that the particles we separated were exosomes of HCC cell lines. Next, we adopted the RNO decolorization test to detect if the soluble Cu-Cy NPs entered the exosomes. As shown in ), in the Exo, Exo-NPs, and Exo-UV groups, there was no singlet oxygen could be detected. However, singlet oxygen was surprisingly detected in the Exo-NPs+UV samples (RNO vs. Exo-NPs+UV: 1.31 ± 0.01 vs. 1.28 ± 0.02, P = 0.006). The finding showed that the soluble Cu-Cy NPs were absorbed into cells and encapsulated in exosomes, and Cu-Cy NPs could be delivered with exosomes to target tumor cells.
Figure 9. Identification of exosomes, and absorption of soluble Cu-Cy NPs by cells. (a) Nanosight analysis of the HepG2 exosomes. (b) TEM of HepG2 exosome. (c) The western blot of the exosomes protein: (1) Huh7 exosomes. (d) RNO absorption quenching indication of singlet oxygen production in different groups. The data are expressed as the mean ± SD of three independent experiments. *P < 0.05 compared with the control (RNO as control).
Discussion
As a new technology of cancer treatment, PDT has attracted increasing attention in recent years.Citation29,Citation30 It uses a photosensitizer that is activated by light to react with oxygen molecules present in tissues. This response will generate cytotoxic singlet oxygen and cause oxidative damage.Citation31,Citation32 In this process, the photosensitizer, which is one of the three components of PDT (photosensitizer, light source, and tissue oxygen),Citation33–Citation35 plays an important role in the production of singlet oxygen. In our study, Cu-Cy NPs effectively produced singlet oxygen when activated by UV light. More singlet oxygen was generated at higher UV doses. The killing effect of singlet oxygen on cells needed to exclude the influence of UV and Cu-Cy NPs itself. UV can be used for sterilization, and other studies have shown that nanoparticle has low toxicity to cells under a low concentration.Citation36–Citation38 Our MTT assay found that there was no significant effect on cellular viability at 5 min (6 J/cm2) UV irradiation, or at 25 μg/ml of Cu-Cy NPs incubation, and the combination of UV irradiation and Cu-Cy NPs provided a strong killing function for the cell.
Our previous study had investigated the sensitivity of three different cancer cells (MCF-7, Du145, and HepG2) with Cu-Cy NPs, and we found that Cu-Cy NPs had a significant impact on HepG2 cellular growth. Thus, we mainly studied the effect of Cu-Cy NPs in liver cancer cells.
Did Cu-Cy NPs have to go into hepatoma carcinoma cells to work? It has been reported that the insoluble nanoparticles had poor absorbance ability, but it still had a strong ability for killing cells.Citation23 Our MTT assay and fluorescent staining data demonstrated that as the time increased, the binding degree of Cu-Cy NPs was improved, but the killing ability of Cu-Cy NPs on HepG2 cells was the same. This means that the killing ability of combination was not relying on the binding of Cu-Cy NPs to cells. The effectiveness of killing ability depended on the concentration of Cu-Cy NPs.
Currently, there were three explanations for the anti-tumor mechanism of PDT: (i) reactive oxygen species (ROS) directly killing tumor cells by cells’ apoptosis or necrosis;Citation34,Citation39 (ii) the destruction of the blood vessels in the tumor stroma resulting in tumor ischemic necrosis;Citation40,Citation41 or (iii) inflammatory cell releasing immune mediator to enhance a specific immune function for promoting the anti-tumor effect.Citation42–Citation45 Most researchers believe that ROS directly kills tumor cells by inducing apoptosis and that singlet oxygen is the main cytotoxic substance.Citation46–Citation50 EmensCitation35,Citation51 found that the photosensitizer is accumulated in subcellular organelles such as the lysosome, mitochondria, or other membranous organelles. Singlet oxygen increases the permeability of the lysosome and releases many enzymes that cause cell damage.Citation52 It also damages mitochondria and releases apoptosis-related factors such as caspase-3.Citation53–Citation57 In our study, detected by flow cytometry, we found that both Cu-Cy NPs and 5-FU were able to induce cellular apoptosis. 5-FU is a traditional medicine for the treatment of liver cancer. It is taken alone or combined with other drugs in clinical liver cancer treatment.Citation8,Citation9 We also observed that the expression of apoptosis-related proteins increased as the duration of Cu-Cy NPs (after irradiated with UV) action increased. Pleasingly, in vitro experiment, we discovered that at the optimum concentration of Cu-Cy NPs (25 μg/ml), which was far away less than the IC50 value of 5-FU (IC50 value of HepG2 was calculated as 226.84 µg/mlCitation58), Cu-Cy NPs could initiate more cellular apoptosis compared to 5-FU, indicated that the mechanism of Cu-Cy NPs in tumor treatment was the induction of cellular apoptosis. In vivo study, we discovered that the anti-cancer function of Cu-Cy NPs+UV was effective as 5-FU, which proved that Cu-Cy NPs had potential on anti-cancer. Nevertheless, in vivo experiment, there was no difference between the Cu-Cy NPs group and the Cu-Cy NPs+UV group. The reason was that Cu-Cy NPs had a specific ability to kill cancer cells. Due to the limitation of this experiment, we adopted UV as the light source rather than X-ray, and UV was limited on the effect of penetrating the skin.Citation59 As a result, the outcome of Cu-Cy NPs+UV was influenced. According to the earlier result from Lun Ma’s study, the best light source to irradiate Cu-Cy NPs was X-ray.Citation23 If we used X-ray to replace UV, the in vivo study would provide a better outcome.
Two issues must be solved for practical applications of this new type of photosensitizer. One is that photosensitizer needs to reach the target tissue, and the other one is avoiding to be cleaned by the reticuloendothelial system. Exosomes are a natural carrier in cancer drug delivery systems.Citation60,Citation61 They form a protective layer between the tissue and the drug. This protective layer does not only help to avoid the reticuloendothelial system, but it also minimizes the tissue destruction by singlet oxygen that is produced by Cu-Cy NPs. According to the results of the singlet oxygen test, we suggested that the soluble Cu-Cy NPs were able to enter the exosomes that were secreted from HepG2 cells. This finding offered a possible way to solve these two issues that were mentioned above.
PDT is a highly effective therapy that can work as surgery or radiation therapy in treating certain kinds of cancers and pre-cancerous lesions.Citation62 Unlike some traditional therapy (such as radiotherapy, hormone therapy, or chemotherapy), PDT rarely leads to DNA damage, and it is effective in treating tumors that have developed resistance to other cytotoxic therapies. However, PDT still has some limitations in clinical application. One of the limitations is the need for direct light delivery for photosensitizer activation. Therefore, for some tumors that are deeply located anatomically, the delivery of light is the reason that the use of PDT is challenging for deep cancer treatment.Citation63 The other limitation is the biotoxicity of the photosensitizer. Many traditional photosensitizers such as 5-aminlevulinic acid (5-ALA) can cause damage to the human body during the treatment.Citation17 Cu-Cy NPs which were described in this paper might provide a new way to solve these two limitations. Cu-Cy NPs are new Cu-Cy complex which is different from traditional Cu-Cy complex and considered as a model compounds to increase the fundamental knowledge of copper-containing enzymes. These Cu-Cy NPs have several unique characteristics:Citation23 (i) low cytotoxicity; (ii) can be activated directly by UV light, X-rays, microwave and ultrasound; (iii) soluble Cu-Cy NPs were able to enter exosome and provides a new way for the targeted transport of nanoparticles. Also, Cu-Cy NPs are easy to synthesize at a low cost.
Conclusions
In summary, here we report the anti-HCC effects of copper-cysteamine nanoparticle sensitizers. The observation showed that Cu-Cy NPs could significantly reduce the activity of HepG2 cells at a very low dose after a short time of ultraviolet radiation, and the cell death was induced by Cu-Cy NPs, which is associated with cellular apoptosis. Furthermore, we found that Cu-Cy NPs obviously inhibited the tumor growth in vivo. Cu-Cy NPs can enter into exosomes secreted by tumor cells, and exosomes could be used to deliver Cu-Cy NPs to target tumor cells. All these observations suggest that Cu-Cy NPs as a new type of medicines have a good potential for cancer treatment.
Materials and method
Cell culture
The human HCC cell line HepG2 and Huh7 were obtained from the Chinese Academy of Sciences. The cells were maintained in a 95% air and 5% CO2 humidified atmosphere at 37°C. The Dulbecco’s modified Eagle’s medium (DMEM) (HyClong), supplemented with 10% fetal bovine serum (FBS) (HyClong) and 1% penicillin-streptomycin (PS) (HyClong) was used for routine sub-culturing and all experiments.
Cu-Cy NPs synthesis and characterization
CuCl2 · 2H2O (0.460 g) and cysteamine (0.636 g) were dissolved in distilled (DI) water followed by NaOH solution (2.5 M) (Sigma) to adjust the pH to 8. The solution was stirred for about 2 h at room temperature and heated to its boiling point. After boiling for 30 min, the Cu-Cy NPs were obtained by centrifuging and washing the crude product with a solution of DI water and ethanol (v/v = 5:4) five times followed by sufficient sonication. Finally, the particles were dried completely in a vacuum oven at room temperature overnight. The synthesis, size, structure and optical properties had been reported in detail in recent publications by Chen et al.Citation22,Citation23
Singlet oxygen measurement
We used a P-nitrosodimenthylaniline (RNO) (Sigma) decolorization test to prove that Cu-Cy NPs were capable to produce singlet oxygen. A quantity of 0.225 mg RNO and 16.34 mg imidazole (ID) (Sigma) was added in 30 ml DI water, which was air saturated by sufficient air bubbling. The sample solution was prepared by adding 1 mg of testing sample (Cu-Cy NPs) into 3 ml of RNO-ID solution. Next, the RNO-ID solution and sample solutions were exposed by ultraviolet (UV) lamp (irradiation peak at 360 nm, 20 mW/cm2) for 5 min (light dose = 6 J/cm2). This excitation source was placed 3 cm away from testing samples. Meanwhile, the intensity of RNO absorption was monitored by a Shimadzu UV-vis spectrophotometer.
Influence of UV-light on cell viability
The effect of ultraviolet rays on the cells was evaluated by 3-(4,5-dimethylthiazol- 2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Solarbio, Beijing, China) assay. About 3,000 cells/well HepG2 cells were seeded into 96-well plates and allowed to grow for 24 h in an incubator (5% CO2, 37°C, humidified atmosphere). On experiment day, cells were exposed under different amounts of ultraviolet rays (0 min to 10 min) by using a UV lamp (irradiation peak at 360 nm, 20 mW/cm2); the cellular viability was evaluated after 24 h of cell incubation. The intensity of UV light which affects cellular viability was measured, and then 6 J/cm2 was used for the experiment.
Cytotoxicity test
To determine the cytotoxicity of Cu-Cy NPs, the MTT assay was adopted. For this assay, HepG2 cells (3000 cells/well) were seeded into 96-well plates and grew for 24 h in an incubator (5% CO2, 37°C, humidified atmosphere). On the day of the experiment, the cultured medium was removed. One hundred μl fresh media that contained different concentrations of Cu-Cy NPs were added (0, 0.1, 1, 10, 25, 50, 100, and 200 μg/ml). A PBS solution was added to the negative control (cells that were not incubated with the Cu-Cy NPs). Cytotoxicity was evaluated after 24 h of cell incubation.
In vitro cell destruction using fluorescence imaging
We used fluorescence imaging to evaluate the cell’s killing ability of the Cu-Cy NPs before and after being bounded and adhered. HepG2 cells (200,000 cell/well) were seeded into four 6-well plates (control, 5 min, 60 min, and 240 min incubation group), and they were incubated for 24 h at 37°C in a humidified atmosphere of 5% v/v CO2 in dark. On the day of the experiment, the culture medium was removed. Two ml fresh medium was added to control. The other three groups had 2 ml of media with 50 μg (25 μg/ml) Cu-Cy NPs and were irradiated with 5 min under UV after 5 min, 60 min, and 240 min incubation, respectively. Control group was also treated with 6 J/cm2 UV. After another 24 h culturing, the cells were incubated with 500 μl of dye mixture – calcein AM and ethidiumhomodimer-1 (EthD-1) (Sigma) – for 30 min under standard conditions of cell culturing in the dark. The cells were observed under an EVOS fluorescence microscope (Thermo Fisher Scientific).
Cell apoptosis assay
HepG2 cells (200,000 cell/well) were seeded in four separated 6-well and grew for 24 h at 37°C in a humidified atmosphere with 5% v/v CO2 in the dark. This experiment was divided into four treatment groups (control, 5-FU, Cu-Cy NPs, and Cu-Cy NPs+UV). Each group had three treated concentrations (1 μg/ml, 25 μg/ml, and 50 μg/ml). (In this study, 5-FU was used as the positive control, which was a traditional medicine for a variety of cancers. The side effects include nausea, vomiting, diarrhea stomatitis, etc. 5-FU that we used was a white powder with the molecular weight of 130.08, purchased from Sigma, and the price was 375 RMB/G). At the day of the experiment, the media was removed and 2 ml of 5-FU and 2 ml Cu-Cy NPs (1 μg/ml, 25 μg/ml, and 50 μg/ml) were added into corresponding groups. Two ml fresh medium without drug was marked as the control. The cells in group Cu-Cy NPs+UV were treated with Cu-Cy NPs and irradiated with 6 J/cm2 UV immediately. After another 24 h, the supernatant and cells were collected in a 15 ml centrifuge tube. They were detected by a flow cytometry apoptosis assay kit (Beckman Coulter Inc., California, USA). This whole experiment was repeated three times.
Western blotting
HepG2 cells (200,000 cell/well) were seeded in 6-well and grew for 24 h at 37°C in a humidified atmosphere with 5% v/v CO2 in the dark. This experiment was divided into four groups: control, UV, Cu-Cy NPs, and Cu-Cy NPs+UV. On the day of the experiment, the media was removed and 2 ml of Cu-Cy NPs (25 μg/ml) were added into corresponding groups. Two ml of fresh medium without drug was the control and UV group. The cells in treated groups were irradiated with 6 J/cm2 UV after added Cu-Cy NPs immediately. The supernatant and cells were collected in a 2 ml centrifuge tube after reached their respective culture time. Protein of cells was extracted with RIPA buffer (Cell Signaling Technology) containing protease inhibitors (Roche). Equal amounts of protein were separated by electrophoresis on 10% SDS-PAGE gels and transferred onto polyvinylidene difluoride membranes (PVDF; Millipore). After blocking with 5% nonfat dry milk in Tris-buffered saline (TBST, 100 mM NaCl, 50 mM Tris, 0.1% Tween-20; pH 7.5), membranes were incubated with primary antibody for cleaved-PARP (Rabbit, 1:1000; Cell Signaling Technology), and GAPDH (Rabbit, 1:1000; Cell Signaling Technology) overnight at 4°C. After washing twice with TBST, the membranes were incubated with secondary anti-rabbit antibodies conjugated to horseradish peroxidase. The signals were detected by enhanced chemiluminescence (ECL; Bio-Rad).
Tumor xenograft in nude mice and anti-tumors in vivo
Animal subcutaneous xenograft models were used to measure therapeutic efficacy (n = 8 for each group). A suspension of 3 × 106 HepG2 cells was subcutaneously injected into both the shoulder and the flank of each nude mouse. These mice were monitored daily for tumor growth. When the tumors reached about 5 mm in diameter (approximately 2 weeks), the mice were randomly divided into five groups (control, 5-FU, UV, Cu-Cy NPs, Cu-Cy NPs+UV). Tumor-bearing mice were injected intratumorally with saline (as control), 5-FU (1 mg/ml; 100 μl/day; three consecutive days), Cu-Cy NPs (1 mg/ml; 100 μl/day; three consecutive days). In UV group, 5 min of UV was applied. The tumor on the lower left of each mouse was treated as self-control by injecting of saline. After injection, the mice in the Cu-Cy NPs+UV group were irradiated with 6 J/cm2 UV. The volume of the tumor was measured daily, and the mice were euthanized one month later. The tumors in mice were surgically dissected and measured.
Exosome absorption of nanoparticles
Singlet oxygen testing confirmed soluble Cu-Cy NPs were absorbed by exosomes. HepG2 cells were cultured and divided into five groups, which included: Exo, Exo-UV, Exo-NPs, Exo-NPs+UV, RNO. Group Exo and Exo-UV were treated without Cu-Cy NPs; group Exo-NPs and Exo-NPs+UV were treated with Cu-Cy NPs. RNO (reagent of singlet oxygen detection) was used as the control. The process was the following: after the concentration of HepG2 cells reached 80% to 90%, the media was removed, and 6 ml fresh medium was added to group Exo and Exo-UV. Six ml medium that contained 150 μg Cu-Cy NPs were added to group Exo-NPs and Exo-NPs+UV (25 μg/ml), respectively. After 48 h of culture, the supernatant from five groups were collected into centrifuge tubes and centrifuged to extract exosomes. At first, 3000 × g was used for 15 min at 4°C to remove cells and fragments. Secondly, the supernatant was collected with a filter (Millipore Amicon-ultra-15, 100KD) followed by 4000 × g for 30 min at 4°C for enrichment. Thirdly, the ExoQuick was added to the concentrated liquid according to the ratio of 1:5 and mixed them completely. After 12 h incubation at 4°C, 1500 × g was used for 30 min at 4°C. The white precipitate was found at the bottom of the tube. Then, the supernatant was removed and centrifuging was continued for 5 min by 1500 × g at 4°C. Then, carefully removed the remaining supernatant and washed with PBS which was filtered. Group Exo-UV and Exo-NPs+UV were irradiated with 6 J/cm2 UV. Singlet oxygen in five groups was measured at the same time.
Ethics statement
The animal study is approved by the institutional animal ethical committee of Guangxi Medical University with approval No. 201512150. The methods were carried out in accordance with the approved guidelines.
Statistical analyses
The data were presented as the mean ± standard deviation (SD). GraphPad Prism 5.0 (Graph Pad Software, La Jolla, CA, USA) was used for statistical analysis. Two-tailed Student’s t-test was used to evaluate the significance of the difference between two groups of data in experiments. One-Way analysis of variance (ANOVA) was used to determine the statistically significant difference among three or more different groups of data in the experiment. P≤ 0.05 was considered to be statistically significant.
Authors’ contributions
W.C. and M.H. conceived the research and led the project; X.H. designed the experiments. X.H., F.W., L.M., L.T., JB.P., RX.L., C.L., J.C., J.D., and Y.L. Performed the experiments. X.H. analyzed the data and wrote the paper. All authors reviewed the final manuscript.
Ethics approval and consent to participate
The animal study is approved by the institutional animal ethical committee of Guangxi Medical University with approval No. 201512150.
Disclosure of Potential Conflicts of Interest
The authors declare that they have no competing interests, and all authors should confirm its accuracy.
Acknowledgments
We also would also like to acknowledge the support from the US Army Medical Research Acquisition Activity (USAMRAA) under Contracts of W81XWH-10-1-0279 and W81XWH-10-1-0234.
Additional information
Funding
References
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. 87-10 doi:10.3322/caac.21262. PMID: 25651787.
- El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142(6):1264–1273. PMID: 22537432. doi:10.1053/j.gastro.2011.12.061.
- Padhy AK, Dondi M. A report on the implementation aspects of the international atomic energy agency’s first doctoral coordinated research project, “management of liver cancer using radionuclide methods with special emphasis on trans-arterial radio-conjugate therapy and internal dosimetry”. Semin Nucl Med. 2008;38(2):S5–12. PMID: 18243843. doi:10.1053/j.semnuclmed.2007.10.002.
- Wang K, Eguchi S, Hidaka M, Jin T, Soyama A, Kuroki T, Huang M, Wu L, Zou S, Shao J. Comparison of the outcomes of hepatocellular carcinoma after hepatectomy between two regional medical centers in China and Japan. Asian J Surg. 2017;40(5):380–388. PMID: 27236717. doi:10.1016/j.asjsur.2016.03.002.
- Nordenstedt H, White DL, El-Serag HB. The changing pattern of epidemiology in hepatocellular carcinoma. Dig Liver Dis. 2010;42(Suppl. 3):S206–214. PMID: 20547305. doi:10.1016/S1590-8658(10)60507-5.
- Shimozawa N, Hanazaki K. Longterm prognosis after hepatic resection for small hepatocellular carcinoma. J Am Coll Surg. 2004;198(3):356–365. PMID: 14992736. doi:10.1016/j.jamcollsurg.2003.10.017.
- Verhoef C, de Man RA, Zondervan PE, Eijkemans MJ, Tilanus HW, Ijzermans JN. Good outcomes after resection of large hepatocellular carcinoma in the non-cirrhotic liver. Dig Surg. 2004;21(5–6):380–386. PMID: 15523181. doi:10.1159/000081882.
- Zhang S, Xue H, Chen Q. Oxaliplatin, 5-fluorouracil and leucovorin (FOLFOX) as secondline therapy for patients with advanced urothelial cancer. Oncotarget. 2016;7(36):58579–58585. PMID: 27409828. doi:10.18632/oncotarget.10463.
- Chen JH, Kim SH, Fan PW, Liu CY, Hsieh CH, Fang K. The aqueous extract of chinese medicinal herb brucea javanica suppresses the growth of human liver cancer and the derived stem-like cells by apoptosis. Drug Des Devel Ther. 2016;10:2003–2013. PMID: 27382253. doi:10.2147/DDDT.S107909.
- Yang TS, Chang HK, Chen JS, Lin YC, Liau CT, Chang WC. Chemotherapy using 5-fluorouracil, mitoxantrone, and cisplatin for patients with advanced hepatocellular carcinoma: an analysis of 63 cases. J Gastroenterol. 2004;39(4):362–369. PMID: 15168248. doi:10.1007/s00535-003-1303-8.
- Juzenas P, Moan J. Singlet oxygen in photosensitization. J Environ Pathol Toxicol Oncol. 2006;25(1–2):29–50. PMID: 16566709.
- Barr H. Photodynamic therapy in gastrointestinal cancer: a realistic option? Drugs Aging. 2000;16 PMID: 10755325 (2):81–86. doi:10.2165/00002512-200016020-00001.
- Sibata CH, Colussi VC, Oleinick NL, Kinsella TJ. Photodynamic therapy in oncology. Expert Opin Pharmacother. 2001;2(6):917–927. PMID: 11585008. doi:10.1517/14656566.2.6.917.
- Mg V. Porphyrin-based sensitizers in the detection and treatment of cancer: recent progress. Curr Med Chem Anticancer Agents. 2001;1 PMID: 12678766 (2):175–194. doi:10.2174/1568011013354769.
- Bc W. Photodynamic therapy for cancer: principles. Can J Gastroenterol. 2002;16 PMID: 12096303 (6):393–396. doi:10.1155/2002/743109.
- Kalka K, Merk H, Mukhtar H. Photodynamic therapy in dermatology. J Am Acad Dermatol. 2000;42 PMID: 10688709 (3):389–413. doi:10.1016/S0190-9622(00)90209-3.
- Wu DQ, Li ZY, Li C, Fan JJ, Lu B, Chang C, Cheng SX, Zhang XZ, Zhuo RX. Porphyrin and galactosyl conjugated micelles for targeting photodynamic therapy. Pharm Res. 2010;27(1):187–199. PMID: 19888639. doi:10.1007/s11095-009-9998-8.
- Choi J, Kim H, Choi Y. Theranostic nanoparticles for enzyme-activatable fluorescence imaging and photodynamic/chemo dual therapy of briple-negative breast cancer. Quant Imaging Med Surg. 2015;5(5):656–664. PMID: 26682135. doi:10.3978/j.issn.2223-4292.2015.08.09.
- El-Hussein A, Mfouo-Tynga I, Abdel-Harith M, Abrahamse H. Comparative study between the photodynamic ability of gold and silver nanoparticles in mediating cell death in breast and lung cancer cell lines. J Photochem Photobiol B. 2015;153:67–75. PMID: 26398813. doi:10.1016/j.jphotobiol.2015.08.028.
- Cheng Y, Cheng H, Jiang C, Qiu X, Wang K, Huan W, Yuan A, Wu J, Hu Y. Perfluorocarbon nanoparticles enhance reactive oxygen levels and tumour growth inhibition in photodynamic therapy. Nat Commun. 2015;6:8785. PMID: 26525216. doi:10.1038/ncomms9785.
- Seong DY, Kim YJ. Enhanced photodynamic therapy efficacy of methylene blue-loaded calcium phosphate nanoparticles. J Photochem Photobiol B. 2015;146:34–43. PMID: 25794464. doi:10.1016/j.jphotobiol.2015.02.022.
- Lun M, Wei C, Gabriele S, Wei W, Alan GJ, Yining H, Ramaswami S, Marius H. A new Cu-cysteamine complex: structure and optical properties. J Mater Chem C. 2014;2:4239–4246. doi:10.1039/C4TC00114A.
- Ma L, Zou X, Chen W. A new X-ray activated nanoparticle photosensitizer for cancer treatment. J Biomed Nanotechnol. 2014; 10(8):1501–1508. PMID: 25016650 doi:10.1166/jbn.2014.1954.
- Yao M, Ma L, Li L, Zhang J, Lim RX, Chen W, Zhang Y. A new modality for cancer treatment—nanoparticle mediated microwave induced photodynamic therapy. J Biomed Nanotechnol. 2016;12 PMID: 29359896 (10):1835–1851. doi:10.1166/jbn.2016.2322.
- Liu Z, Xiong L, Ouyang G, Ma L, Sahi S, Wang K, Lin L, Huang H, Miao X. The investigation of copper cysteamine nanoparticles as a new type of radiosenstizers for colorectal carcinoma. Sci Rep. 2017;7(1):9290. PMID: 28839163. doi:10.1038/s41598-017-09375-y.
- Sun Z, Wang L, Dong L, Wang X. Emerging role of exosome signalling in maintaining cancer stem cell dynamic equilibrium. J Cell Mol Med. 2018;22:3719–3728. PMID: 29799161. doi:10.1111/jcmm.13676.
- Tucher C, Bode K, Schiller P, Claßen L, Birr C, Mm S-C, Blank N, Hm L, Schiller M. Extracellular vesicle subtypes released from activated or apoptotic t-lymphocytes carry a specific and stimulus-dependent protein cargo. Front Immunol. 2018;9:534. PMID: 29599781. doi:10.3389/fimmu.2018.00534.
- Warnecke-Eberz U, Chon SH, Hölscher AH, Drebber U, Bollschweiler E. Exosomal onco-miRs from serum of patients with adenocarcinoma of the esophagus: comparison of miRNA profiles of exosomes and matching tumor. Tumour Biol. 2015;36(6):4643–4653. PMID: 25631748. doi:10.1007/s13277-015-3112-0.
- Yn K, Gurny R, Allemann E. State of the art in the delivery of photosensitizers for photodynamic therapy. J Photochem Photobiol B. 2002;66(2):89–106. PMID: 11897509.
- Celli JP, Spring BQ, Rizvi I, Evans CL, Samkoe KS, Verma S, Pogue BW, Hasan T. Imaging and photodynamic therapy: mechanisms, monitoring, and optimization. Chem Rev. 2010;110(5):2795–2838. PMID: 20353192. doi:10.1021/cr900300p.
- Henderson BW, Dougherty TJ. How does photodynamic therapy work? Photochem Photobiol. 1992;55(1):145–157. PMID: 1603846.
- Dolmans DE, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer. 2003;3(5):380–387. PMID: 12724736. doi:10.1038/nrc1071.
- Oleinick NL, Evans HH. The photobiology of photodynamic therapy: cellular targets and mechanisms. Radiat Res. 1998;150(5 Suppl):S146–156. PMID: 9806617.
- Oleinick NL, Morris RL, Belichenko I. The role of apoptosis in response to photodynamic therapy: what, where, why, and how. Photochem Photobiol Sci. 2002;1(1):1–21. PMID: 12659143.
- Castano AP, Demidova TN, Hamblin MR. Mechanisms in photodynamic therapy: part three-photosensitizer pharmacokinetics, biodistribution, tumor localization and modes of tumor destruction. Photodiagnosis Photodyn Ther. 2005;2(2):91–106. PMID: 25048669. doi:10.1016/S1572-1000(05)00060-8.
- Feng L, Zhu J, Wang Z. Biological functionalization of conjugated polymer nanoparticles for targeted imaging and photodynamic killing of tumor cells. ACS Appl Mater Interfaces. 2016;8(30):19364–19370. PMID: 27406913. doi:10.1021/acsami.6b06642.
- Rene HD, Badireddy AR, José MS, Francisco CC, Israel MG, Isela SN, Chellam S, Claudio CR. Cytotoxic effect of lipophilic bismuth dimercaptopropanol nanoparticles on epithelial cells. J Nanosci Nanotechnol. 2016;16(1):203–209. PMID: 27398446.
- Mirzaie ZH, Irani S, Mirfakhraie R, Atyabi SM, Dinarvand M, R D, Varshochian R, Atyabi F. Docetaxel-chitosan nanoparticles for breast cancer cell treatment: cell viability and gene expression study. Chem Biol Drug Des. 2016;88(6):850–858. PMID: 27390258. doi:10.1111/cbdd.12814.
- Drews J, Ryser S. The role of innovation in drug development. Nat Biotechnol. 1997;15(13):1318–1319. PMID: 9415870. doi:10.1038/nbt1297-1318.
- Krammer B. Vascular effects of photodynamic therapy. Anticancer Res. 2001;21(6B):4271–4277. PMID: 11908681.
- Dolmans DE, Kadambi A, Hill JS, Waters CA, Robinson BC, Walker JP, Fukumura D, Jain RK. Vascular accumulation of a novel photosensitizer, MV6401, causes selective thrombosis in tumor vessels after photodynamic therapy. Cancer Res. 2002;62(7):2151–2156. PMID: 11929837.
- Kopbelik M. Induction of tumor immunity by photodynamic therapy. J Clin Laser Med Surg. 1996;14(5):329–334. PMID: 9612200. doi:10.1089/clm.1996.14.329.
- Hanlon JG, Adams K, Rainbow AJ, Gupta RS, Singh G. Induction of Hsp60 by photofrin-mediated photodynamic therapy. J Photochem Photobiol B. 2001;64(1):55–61. PMID: 11705730.
- Van Duijnhoven FH, Aalbers RI, Rovers JP, Terpstra OT, Kuppen PJ. The immunological consequences of photodynamic treatment of cancer, a literature review. Immunobiology. 2003;207(2):105–113. PMID: 12675268. doi:10.1078/0171-2985-00221.
- Castano AP, Mroz P, Hamblin MR. Photodynamic therapy and anti-tumour immunity. Na Rev Cancer. 2006;6(7):535–545. PMID: 16794636. doi:10.1038/nrc1894.
- Patterson MS, Madsen SJ, Wilson BC. Experimental tests of the feasibility of singlet oxygen luminescence monitoring in vivo during photodynamic therapy. J Photochem Photobiol B. 1990;5(1):69–84. PMID: 2111394.
- Weishaupt KR, Gomer CJ, Dougherty TJ. Identification of singlet oxygen as the cytotoxic agent in photoinactivation of a murine tumor. Cancer Res. 1976;36(7/1):2326–2329. PMID: 1277137.
- Krasnovsky AA Jr. Singlet molecular oxygen in photobiochemical systems: ir phosphorescence studies. Membr Cell Biol. 1998;12(5):665–690. PMID: 10379647.
- Niedre M, Patterson MS, Wilson BC. Direct near-infrared luminescence detection of singlet oxygen generated by photodynamic therapy in cells in vitro and tissues in vivo. Photochem Photobiol. 2002;75(4):382–391. PMID: 12003128. doi:10.1562/0031-8655(2002)075<0382:DNILDO>2.0.CO;2.
- Baier J, Maisch T, Maier M, Landthaler M, Bäumler W. Direct detection of singlet oxygen generated by UVA irradiation in human cells and skin. J Invest Dermatol. 2007;127(6):1498–1506. PMID: 17363921. doi:10.1038/sj.jid.5700741.
- Castano AP, Demidova TN, Hamblin MR. Mechanisms in photodynamic therapy: part one-photosensitizers, photochemistry and cellular localization. Photodiagnosis Photodyn Ther. 2004;1(4):279–293. PMID: 25048432. doi:10.1016/S1572-1000(05)00007-4.
- Ferreira SD, Tedesco AC, Sousa G, Zângaro RA, Silva NS, Pacheco MT, Pacheco-Soares C. Analysis of mitochondria, endoplasmic reticulum and actin filaments after PDT with AlPcS(4). Lasers Med Sci. 2004;18(4):207–212. PMID: 15042425. doi:10.1007/s10103-003-0282-6.
- Chiu SM, Oleinick NL. Dissociation of mitochondrial depolarization from cytochrome C release during apoptosis induced by photodynamic therapy. Br J Cancer. 2001;84(8):1099–1106. PMID: 11308261. doi:10.1054/bjoc.2000.1714.
- Granville DJ, Cassidy BA, Ruehlmann DO, Choy JC, Brenner C, Kroemer G, van Breemen C, Margaron P, Hunt DW, McManus BM. Mitochondrial release of aapoptosis-inducing factor and cytochrome C during smooth muscle cell apoptosis. Am J Pathol. 2001;159(1):305–311. PMID: 11438477. doi:10.1016/S0002-9440(10)61696-3.
- Kantaei C, Walczak H. Caspase-8 and bid: caught in the act between death receptors and mitochondria. Biochim Biophys Acta. 2011;1813(4):558–563. PMID: 21295084. doi:10.1016/j.bbamcr.2011.01.026.
- Yoo JO, Lim YC, Kim YM, Ha KS. Transglutaminase 2 promotes both caspase-dependent and caspase-independent apoptotic cell death via the calpain/bax protein signaling pathway. J Biol Chem. 2012;287(18):14377–14388. PMID: 22418443. doi:10.1074/jbc.M111.326074.
- Marino J, García Vior MC, Furmento VA, Blank VC, Awruch J, Roguin LP. Lysosomal and mitochondrial permeabilization mediates zinc(ii) cationic phthalocyanine phototoxicity. Int J Biochem Cell Biol. 2013;45(11):2553–2562. PMID: 23994488. doi:10.1016/j.biocel.2013.08.012.
- Liu Y, Bi T, Dai W, Wang G, Qian L, Gao Q, Shen G. 2016. Oxymatrine synergistically enhances the inhibitory effect of 5-fluorouracil on hepatocellular carcinoma in vitro and in vivo. Tumour Biol. 37:7589–7597. doi:10.1007/s13277-015-4642-1.
- Larue L, Ben Mihoub A, Youssef Z, Colombeau L, Acherar S, André JC, Arnoux P, Baros F, Vermandel M. Using X-rays in photodynamic therapy: an overview. Photochem Photobiol Sci. 2018;17:1612–1650. PMID: 29938265. doi:10.1039/c8pp00112j.
- Kooijmans SA, Achiffelers RM, Zarovni N, Vago R. Modulation of tissue tropism and biological activity of exosomes and other extracellular vesicles: new nanotools for cancer treatment. Pharmacol Res. 2016;111:487–500. PMID: 27394168. doi:10.1016/j.phrs.2016.07.006.
- Lunavat TR, Jang SC, Nilsson L, Park HT, Repiska G, Lässer C, Nilsson JA, Gho YS, Lötvall J. RNAi delivery by exosome-mimetic nanovesicles – implications for targeting c-Myc in cancer. Biomaterials. 2016;102:231–238. PMID: 27344366. doi:10.1016/j.biomaterials.2016.06.024.
- Pass HI. Photodynamic therapy in on1cology: mechanisms and clinical use. J Natl Cancer Inst. 1993;85 PMID: 8445672 (6):443–456. doi:10.1093/jnci/85.6.443.
- Moore CM, Pendse D, Emberton M. Photodynamic therapy for prostate cancer—a review of ccurrent status and future promise. Nat Clin Pract Urol. 2009;6(1):18–30. PMID: 19132003. doi:10.1038/ncpuro1274.